Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6265
Revised: July 15, 2013
Accepted: August 20, 2013
Published online: October 7, 2013
Processing time: 137 Days and 20.5 Hours
AIM: To investigate the mechanisms of how cyclooxygenase-2 (COX-2) regulates E-cadherin in gastric cancer cells.
METHODS: COX-2 expression in human gastric cancer cell lines SGC-7901, BGC-823, MGC-803 and AGS were measured at the mRNA and protein level. COX-2 rich cell line SGC-7901 was chosen for subsequent experiments. siRNA mediated gene knockdown was used to investigate the impact of COX-2 on nuclear factor-κB (NF-κB), Snail, and E-cadherin in gastric cancer cells. Gene expression was determined by Western blot and real-time polymerase chain reaction. To analyze whether NF-κB inhibition could interrupt the modulatory effect of COX-2 or prostaglandin E2 (PGE2) on E-cadherin, gastric cancer cells were treated with celecoxib or PGE2, in the presence of NF-κB specific siRNA.
RESULTS: Highest expression level of COX-2 was found in SGC-7901 cells, both at mRNA and protein levels. siRNA mediated down-regulation of COX-2 led to a reduced expression of NF-κB and Snail, but an increased expression of E-cadherin in SGC-7901 cells. siRNA mediated down-regulation of NF-κB also led to a reduced expression of E-cadherin and Snail in SGC-7901 cells. However, COX-2 expression did not alter after cells were treated with NF-κB specific siRNA in SGC-7901 cells. Treatment of SGC-7901 cells with celecoxib led to a reduced expression of Snail but an increased expression of E-cadherin. In contrast, treatment of SGC-7901 cells with PGE2 led to an increased Snail and a decreased E-cadherin. However, siRNA-mediated knockdown of NF-κB partially abolished the effect of celecoxib and PGE2 on the regulation of E-cadherin and Snail in SGC-7901 cells.
CONCLUSION: COX-2 likely functions upstream of NF-κB and regulates the expression of E-cadherin via NF-κB/Snail signaling pathway in gastric cancer cells.
Core tip: Cyclooxygenase-2 (COX-2) plays an important role in transcriptional regulation of E-cadherin in gastric cancer and other malignancies. On the contrary, prostaglandin E2 (PGE2) promotes invasion of tumor cells through down-regulating the expression of E-cadherin. Our study has provided further evidence that COX-2 functions upstream of nuclear factor-κB in the regulation of Snail and E-cadherin in gastric cancer cells. Blockade of COX-2 activity or inhibition of PGE2 production may offer some benefit in the chemoprevention and treatment of gastric cancer.
- Citation: Liu XJ, Chen ZF, Li HL, Hu ZN, Liu M, Tian AP, Zhao D, Wu J, Zhou YN, Qiao L. Interaction between cyclooxygenase-2, Snail, and E-cadherin in gastric cancer cells. World J Gastroenterol 2013; 19(37): 6265-6271
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6265.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6265
Cyclooxygenase-2 (COX-2) is an inducible isozyme of cyclooxygenase and catalyzes prostaglandin E2 (PGE2) formation in response to various inflammatory stimuli or growth factors[1]. PGE2 plays an important role in regulating diverse cellular functions under physiological and pathological conditions[2]. Overexpression of COX-2 is related to invasion and metastasis of tumor cells[3-5]. To further support the role of COX-2 in tumor promotion, it was reported that PGE2 was able to facilitate the invasion of tumor cells through down-regulation of E-cadherin[6]. On the other hand, celecoxib, a selective inhibitor of COX-2, could inhibit migration and metastasis of tumor cells by up-regulating E-cadherin[7]. Many studies have suggested that COX-2 is generally overexpressed in gastric cancer tissues, and it was thought to play a crucial role in the development and invasion of gastric cancers[8]. In contrast, the expression of E-cadherin, an important cell adhesion molecule, is usually low, mutated, or even lost in gastric cancer tissues[9,10]. Thus, COX-2 and E-cadherin appear to exhibit totally different expression patterns. Our group had previously reported an inverse correlation between COX-2 and E-cadherin and suggested that Snail is likely to be responsible for the regulation of COX-2 on the expression and function of E-cadherin in gastric cancer tissues[11,12].
Snail is a transcription factor and was reported to down-regulate the expression of E-cadherin, causing disruption to cell-to-cell adhesion and thereby facilitates tumor progression and metastases[13,14]. Meanwhile, it was reported that nuclear factor-κB (NF-κB) promotes tumor cell migration and invasion in many human cancers through up-regulating Snail and subsequent suppression of E-cadherin[15-17].
Therefore, it is very likely that the interaction between COX-2, Snail, and E-cadherin may play a key regulatory role in invasion and metastasis of gastric cancer. Our group is interested in understanding the possible interaction between COX-2, Snail, and E-cadherin during the development, progression, invasion, and metastasis of gastric cancer. Thus, the aim of this study is to investigate if COX-2 modulates E-cadherin expression via Snail and NF-κB in gastric cancer cells.
RPMI 1640 medium and PGE2 were purchased from Sigma-Aldrich (St. Louis; MO, United States). Opti-MEM I Reduced Serum Medium, Lipofectamine 2000, BLOCK-iT™ Fluorescent Oligo, and negative control for RNAi were purchased from Invitrogen (Carlsbad, CA, United States). Fetal calf serum was purchased from Hyclone Laboratories (Logan, UT, United States). Reverse transcription kit and quantitative polymerase chain reaction (qPCR) kit were purchased from Takara Biotechnology Co. Ltd. (Dalian, China). celecoxib was purchased from Cayman Chemical (Ann Arbor, MI, United States). Polyclonal antibodies against COX-2, NF-κB P65, E-cadherin, and β-actin were from BioWorld Corporation (CA, United States). Polyclonal antibody against human Snail was purchased from Abcam (Cambridge, United Kingdom). All primers were synthesized by Takara Biotechnology Co. Ltd. (Dalian, China). Double strand (ds) RNAi Stealth™ oligos, the specific siRNA against COX-2 and NF-κB (p65) were designed and synthesized by Invitrogen (Carlsbad, CA, United States).
Human gastric cancer cell lines SGC-7901, BGC-823, MGC-803 and AGS were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China).
Gastric cancer cell lines (SGC-7901, BGC-823, MGC-803 and AGS) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin, and maintained at 37 °C in a humidified atmosphere containing 50 mL/L CO2. Before transfection, the culture medium RPMI 1640 was replaced by Opti-MEM I.
SGC-7901, BGC-823, MGC-803 and AGS were plated respectively at a concentration of 105 cells/ well in a 6- well plate and incubated overnight. Total RNA and protein were exacted to determine the basal expression level of COX-2 at the mRNA by PCR and protein level by Western blot, respectively.
As the SGC-7901 cells showed the highest expression level of COX-2, we used siRNA knockdown approach to investigate the impact of COX-2 on NF-κB, Snail, and E-cadherin in this cell line. Three pairs of siRNA oligos against COX-2 and NF-κB p65, and a control (scrambled) siRNA were initially designed and commercially synthesized. The sequences of these siRNAs were shown in Table 1.
| siRNA against | Forward | Reverse |
| COX-2 | AAUAGGAGAGGUUAGAGAAGGCUUC | GAAGSCUUCUCUAACCUCUCCUAUU |
| NF-κB p65 | UCACUAGGCGAGUUAUAGSCUCAGG | CCUGAGGCUAUAACUCGCCUAGUGA |
For transfection, cells were seeded into a 6-well plate at a density of 3 × 105 cells per well and incubated overnight. Cells were then transfected with siRNA oligos using Lipofectamine 2000 and incubated for 24 to 72 h before further analysis. Transfection efficiency was determined by transfecting the cells with FITC labeled Oligo and counting the number of positive cells under the fluorescent microscopy. More than 80% of cells were routinely successfully transfected. The expression of COX-2, NF-κB, Snail and E-cadherin were analyzed by qPCR and Western blot in successfully transfected cells.
To analyze whether NF-κB inhibition could interrupt the modulation effect of COX-2 or PGE2 on E-Cadheirn, SGC-7901 cells were treated with 40 μmol/L celecoxib for 24 h alone or with NF-κB specific siRNA. Cells were also treated with 10 μmol/L PGE2 for 4 h alone or with NF-κB specific siRNA. The optimal dosages for celecoxib and PGE2 were based on our preliminary study. The expression of NF-κB, Snail and E-cadherin were measured by qPCR and Western blot.
Whole-cell extracts were prepared from the treated cells with 2 mL of RIPA buffer containing protease inhibitors. Cell lysates were centrifuged at 8000 rpm for 10 min and the supernatant was collected. Cell lysates were electrophoretically separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels. Proteins were transferred to nitrocellulose membrane and the membrane was blocked with 5% fat-free milk in TBS plus 0.1% Tween-20 (TBST). The membranes were then incubated with respective primary antibodies (rabbit polyclonal COX-2, NF-κB p65, Snail, E-cadherin, all at 1:1000 dilution) and the corresponding horseradish peroxidase-conjugated secondary antibody for 1 h. The membranes were incubated with enhanced chemiluminescence system and exposed to X-ray film for signal detection. β-actin was used as a control for equal loading of samples.
Total RNA was extracted with Trizol reagent according to the manufacturer’s instructions. Approximately 30 ng of total RNA was transcribed into cDNA. The synthesized cDNA samples were subjected to qPCR using SYBR® Green Quantitative PCR kit. Amplification was carried out in a total volume of 20 μL for 40 cycles of 15 s at 95 °C, 20 s at 60 °C, and 30 s at 72 °C. Samples were run in triplicate and their relative expression was determined by normalizing expression of each target to β-actin. The amplification was monitored on a Roter-Gene realtime PCR apparatus (Roter-Gene, Australia). Primers used in these experiments were shown in Table 2.
| Primers | Sense primer | Antisense primer |
| COX-2 | 5'-GCCTGAATGTGCCATAAGACTGAC-3' | 5'-AAACCCACAGTGCTTGACACAGA-3' |
| E-cadherin | 5'-TACACTGCCCAGGAGSCAGA-3’ | 5'-TGGCACCAGTGTCCGGATTA-3’ |
| Snail | 5'-GACCACTATGCCGCGCTCTT-3’ | 5'-TCGCTGTAGTTAGGCTTCCGATT-3’ |
| NF-κB p65 | 5'-TCAGTCAGSGCATCCAGACC-3’ | 5'-CAGAGSCGCACAGSATTCA-3’ |
| β-actin | 5'-TGGCACCCAGSACAATGAA-3' | 5'-CTAAGTCATAGTCCGCCTAGAAGSA-3' |
Data analysis was performed using SPSS11.0. All data were expressed as mean ± SD. Comparison of the differences between each group was performed by χ2 test. A P value of < 0.05 was considered statistically significant.
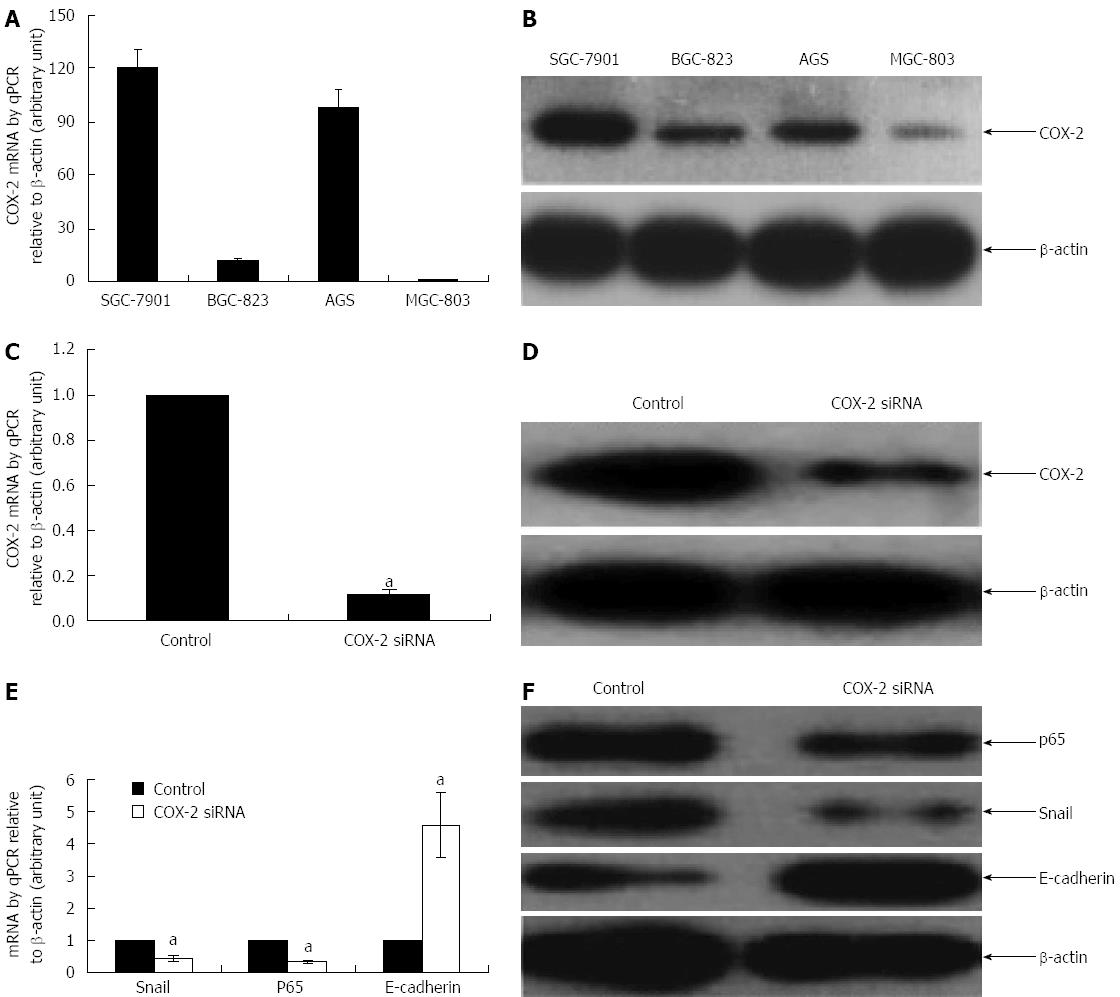
We first examined the basal level of COX-2 expression in several human gastric cancer cell lines using qPCR and Western blot. The cell lines tested include SGC-7901 (moderately differentiated), BGC-823 (poorly differentiated), MGC803 (undifferentiated), and AGS (well differentiated). Highest expression level of COX-2 was found in SGC-7901 cells, both at mRNA and protein levels (Figure 1A and B) (P < 0.05). Thus, the subsequent experiments were performed in SGC-7901 unless otherwise stated.
In order to test the effect of siRNA-mediated down-regulation of COX-2 on NF-κB, Snail and E-cadherin, SGC-7091 cells were incubated with COX-2 specific siRNA (COX-2-siRNA) and the target gene expression was examined by qPCR and Western blot. As shown in Figure 1, knockdown of COX-2 (Figure 1C and D) led to a 3-fold and 2.3-fold decrease but a 4.6-fold increase in the mRNA expression of NF-κB, Snail, and E-cadherin, respectively (Figure 1E) (P < 0.05, compared to their respective controls). These changes were confirmed at the protein level: COX-2-siRNA led to 2.3-fold and 2.8-fold decrease but a 2.5-fold increase in the expression of NF-κB, Snail, and E-cadherin, respectively (Figure 1F) (P < 0.05, compared to their respective controls).
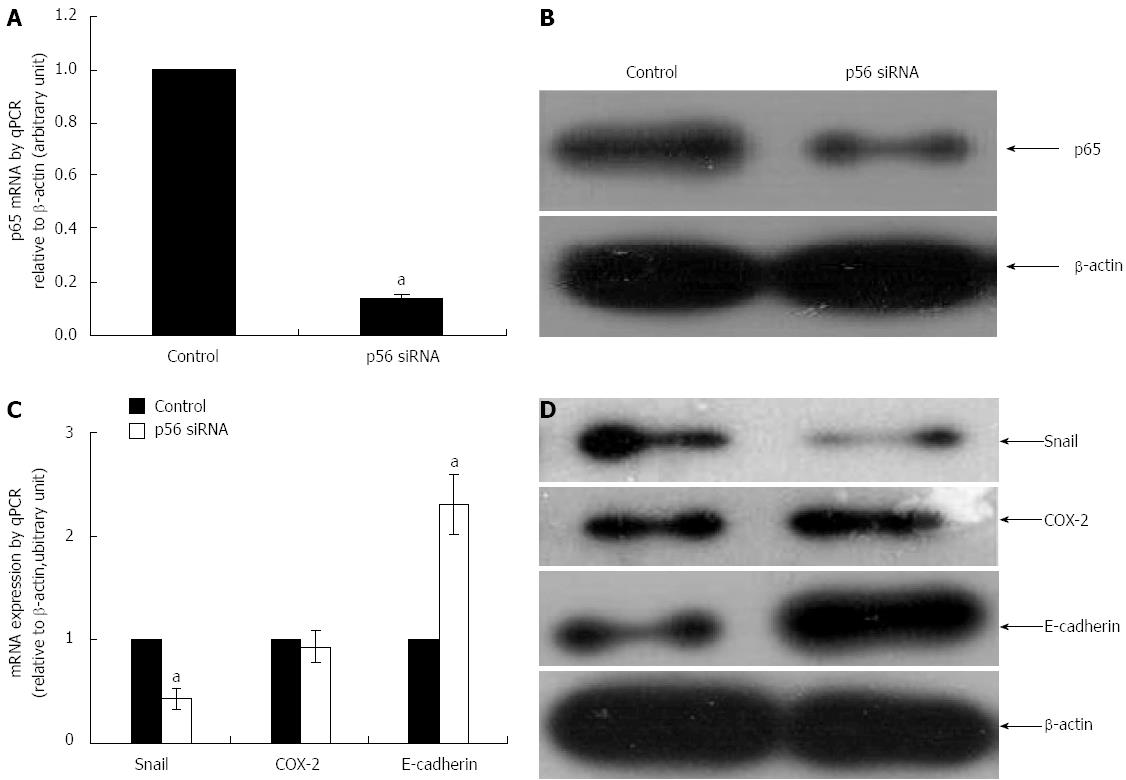
As noted above, siRNA mediated down-regulation of COX-2 led to a reduced expression of NF-κB and Snail in SGC-7901 cells. In order to confirm if COX-2 functions upstream of NF-κB, we examined if NF-κB was able to modulate COX-2 expression in SGC-7901 cells. As shown in Figure 2, knockdown of NF-κB subunit p65 using its specific siRNA (p65-siRNA) (Figure 2A and B) did not affect the expression of COX-2 at both mRNA and protein levels (Figure 2C and D) (P < 0.05, compared to their respective controls).
We then proposed that NF-κB could regulate the expression of E-cadherin via the transcription factor Snail. Therefore, the effect of NF-κB silencing on Snail and E-cadherin were further examined in SGC-7091 cells. As shown in Figure 2, knockdown of NF-κB (Figure 2A and B) was associated with a reduced expression of Snail at both mRNA and protein levels (Figure 2C and D) (P < 0.05, compared to their respective controls). On the other hand, blockade of NF-κB with p65-siRNA rendered an increase in the expression of E-cadherin at both mRNA and protein levels (Figure 2C and D) (P < 0.05, compared to their respective controls).
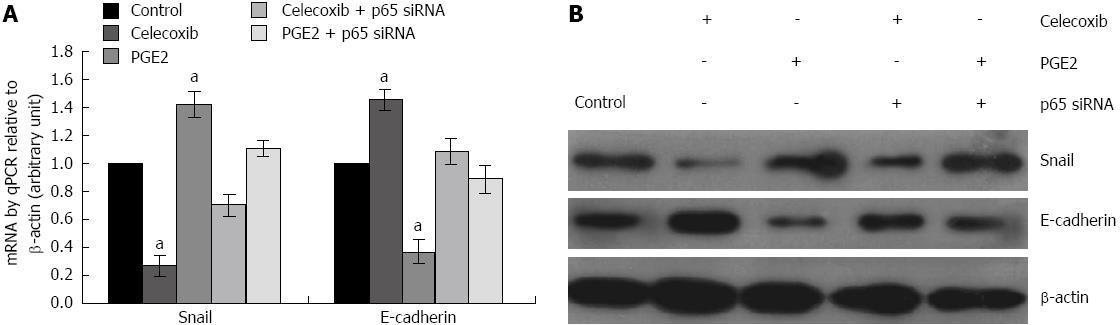
To further determine the regulatory role of NF-κB on E-cadherin, we used celecoxib, a potent COX-2 inhibitor, and PGE2, a principal COX-2 substrate with reported role in promoting cell migration and invasion in tumors, to treat SGC-7901 cells in the presence or absence of p65-siRNA.
As shown in Figure 3, treatment of SGC-7901 cells with celecoxib led to a reduced expression of Snail but an increased expression of E-cadherin both at mRNA (Figure 3A) and protein (Figure 3B) levels. In contrast, treatment of SGC-7901 cells with PGE2 led to an increased Snail and a decreased E-cadherin at mRNA (Figure 3A) and protein levels (Figure 3B). However, when the cells were pre-treated with p65-siRNA, the observed effects of celecoxib and PGE2 were reversed (Figure 3A and B) (P < 0.05, compared to their respective controls).
Abnormal down-regulation of E-cadherin is an important event involved in epithelial-mesenchymal transition, a critical process in the malignant transformation of epithelial cancers including gastric cancer[10,18]. Previous studies, including our own, have demonstrated that COX-2 has a modulatory effect on expression of E-cadherin in gastric cancer and other malignancies[12]. The regulatory role of COX-2 on the expression of E-cadherin is also reflected by the observed chemopreventive effect of the selective COX-2 inhibitor celecoxib which was shown to inhibit the migration and metastasis of tumor cells by up-regulating E-cadherin[19], and further supported by the fact that PGE-2 was able to promote the tumor invasion through down-regulating E-cadherin[20]. However, the mechanisms responsible for the regulatory effect of COX-2 on E-cadherin have not been well defined.
E-cadherin is usually lost in gastric cancer tissues and this appeared to be mediated by COX-2[21]. In our previous study, we found that inhibition of COX-2 activity by celecoxib was not only associated with a reduced expression of Snail, but also a marked reduction in NF-κB subunit p65[12]. In the current study, we explored the same regulatory effect based on RNAi technique. The results showed that COX-2 mediated down-regulation of E-cadherin appeared to be dependent on a functional NF-κB pathway, as blockade of COX-2 activity, either by COX-2-siRNA or celecoxib, restored the expression of E-cadherin. This was associated with a marked down-regulation of NF-κB and Snail expression. These findings are in agreement with previous reports that NF-κB up-regulates Snail and consequently represses E-cadherin in tumor cells[21,22]. Snail has been firmly established as a repressor of E-cadherin and it down-regulates E-cadherin transcription through an interaction with proximal E-boxes of the E-cadherin promoter[23]. In our current study, we further revealed that blockade of NF-κB by p65-siRNA did not alter the expression of COX-2 in SGC-7901 cells. However, the effect of celecoxib and PGE2 on Snail and E-cadherin was reversed by p65-siRNA, suggesting that a functional COX-2 was necessary for regulating NF-κB and Snail signaling in gastric cancer.
The regulatory role of NF-κB on COX-2 has been reported in other human tumors[24]. For example, NF-κB was found to enhance the expression of COX-2 and promote cells proliferation in human colorectal carcinoma cells[25]. In our study, NF-κB p65 was not found to regulate the expression of COX-2. This inconsistency may reflect a cell type specific difference. Additionally, the regulatory role of NF-κB on COX-2 in gastric cancer through other subunits could not be excluded. More studies are needed to unveil the possible mechanisms of how COX-2 and NF-κB interact during gastric cancer formation.
In conclusion, this study has provided further evidence that COX-2 functions upstream of NF-κB in the regulation of Snail and E-cadherin in gastric cancer cells. Blockade of COX-2 activity or inhibition of PGE2 production may offer some benefit in the chemoprevention and treatment of gastric cancer.
We also thank Drs. Li Qiong Wei, Jiao Jia, Hao Yuan and Xiao-Guang Liu from the same institution for their assistance in Western-blot and qPCR.
Cyclooxygenase-2 (COX-2) plays an important role in transcriptional regulation of E-cadherin in gastric cancer and other malignancies. celecoxib, a selective inhibitor of COX-2, inhibits migration and metastasis of tumor cells by up-regulation of E-cadherin. On the contrary, prostaglandin E2 (PGE2) promotes invasion of tumor cells through down regulating the expression of E-cadherin. This study aims to explore how COX-2/PGE2 regulates E-cadherin expression and further to determine whether COX-2/PGE2 reduces the expression of E-cadherin via nuclear factor-κB (NF-κB)/ Snail signal pathway in gastric cancer cells.
Although the correlation between COX-2 and E-cadherin is always inverse in tumor cells, the mechanism of how COX-2 regulates E-cadherin is not clear yet.
The authors firstly found COX-2 baseline expression was significantly higher in SGC-7901 cells in comparison to that in BGC-823, MGC-803 and AGS cells. celecoxib or COX-2 specific RNAi both down-regulated NF-κB and Snail expression, and up-regulated E-cadherin expression, in contrast to PGE2, in SGC-7901 cells. Next, they found that NF-κB specific RNAi did not influence the expression of COX-2 in SGC-7901 cells. Therefore, they can conclude preliminarily that NF-κB and Snail are the downstream molecules in COX-2 modulated E-cadherin signaling pathway in SGC-7901 cells.
This study has provided further evidence that COX-2 functions upstream of NF-κB in the regulation of Snail and E-cadherin in gastric cancer cells. Blockade of COX-2 activity or inhibition of PGE2 production may offer some benefit in the chemoprevention and treatment of gastric cancer.
Epithelial-mesenchymal transition (EMT): The epithelial-mesenchymal transition is a process by which epithelial cells lose their cell polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal cells. EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation. EMT has also been shown to occur in wound healing, in organ fibrosis and in the initiation of metastasis for cancer progression. COX-2: COX-2 is an inducible isozyme of cyclooxygenase and catalyzes PGE2 formation in response to various inflammatory stimuli or growth factors; E-cadherin: E-cadherin is an important cell adhesion molecule and is usually low, mutated, or even lost in gastric cancer tissues. COX-2 and E-cadherin appear to exhibit totally different expression patterns in tumor cells.
This is a very interesting paper on the molecular biology of COX-2 and E-cadherin via the NF-κB and Snail pathways. The methodology and reasoning is sound along with the results and logical discussion at the end.
P- Reviewers Lewis CJ, Yang AG S- Editor Wen LL L- Editor A E- Editor Ma S
| 1. | Nakao S, Ogtata Y, Shimizu E, Yamazaki M, Furuyama S, Sugiya H. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Mol Cell Biochem. 2002;238:11-18. [PubMed] |
| 2. | Takehara H, Iwamoto J, Mizokami Y, Takahashi K, Ootubo T, Miura S, Narasaka T, Takeyama H, Omata T, Shimokobe K. Involvement of cyclooxygenase-2--prostaglandin E2 pathway in interleukin-8 production in gastric cancer cells. Dig Dis Sci. 2006;51:2188-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Costa C, Soares R, Reis-Filho JS, Leitão D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429-434. [PubMed] |
| 4. | Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317-1322. [PubMed] |
| 5. | Huang DS, Shen KZ, Wei JF, Liang TB, Zheng SS, Xie HY. Specific COX-2 inhibitor NS398 induces apoptosis in human liver cancer cell line HepG2 through BCL-2. World J Gastroenterol. 2005;11:204-207. [PubMed] |
| 6. | Hu Z, Liu X, Tang Z, Zhou Y, Qiao L. Possible regulatory role of Snail in NF-κB-mediated changes in E-cadherin in gastric cancer. Oncol Rep. 2013;29:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Zhou Y, Ran J, Tang C, Wu J, Honghua L, Xingwen L, Ning C, Qiao L. Effect of celecoxib on E-cadherin, VEGF, Microvessel density and apoptosis in gastric cancer. Cancer Biol Ther. 2007;6:269-275. [PubMed] |
| 8. | Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol. 2003;37:28-33. [PubMed] |
| 9. | Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115-124. [PubMed] |
| 10. | Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645-3654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1157] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 11. | Zhou Y, Li G, Wu J, Zhang Z, Wu Z, Fan P, Hao T, Zhang X, Li M, Zhang F. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol. 2010;31:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Chen Z, Liu M, Liu X, Huang S, Li L, Song B, Li H, Ren Q, Hu Z, Zhou Y. COX-2 regulates E-cadherin expression through the NF-κB/Snail signaling pathway in gastric cancer. Int J Mol Med. 2013;32:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Bolós V, Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 902] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306-319. [PubMed] |
| 15. | Baranwal S, Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M, Katano M. Increased nuclear factor-kB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675-681. [PubMed] |
| 17. | De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F, Berx G. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 2005;65:6237-6244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008;14:3792-3797. [PubMed] |
| 19. | Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338-5345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Jee YS, Jang TJ, Jung KH. Prostaglandin E(2) and interleukin-1β reduce E-cadherin expression by enhancing snail expression in gastric cancer cells. J Korean Med Sci. 2012;27:987-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Rao DS, Gui D, Koski ME, Popoviciu LM, Wang H, Reiter RE, Said JW. An inverse relation between COX-2 and E-cadherin expression correlates with aggressive histologic features in prostate cancer. Appl Immunohistochem Mol Morphol. 2006;14:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445-7456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 23. | Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Zhang K, Zhaos J, Liu X, Yan B, Chen D, Gao Y, Hu X, Liu S, Zhang D, Zhou C. Activation of NF-B upregulates Snail and consequent repression of E-cadherin in cholangiocarcinoma cell invasion. Hepatogastroenterology. 2011;58:1-7. [PubMed] |
| 25. | Kojima M, Morisaki T, Izuhara K, Uchiyama A, Matsunari Y, Katano M, Tanaka M. Lipopolysaccharide increases cyclo-oxygenase-2 expression in a colon carcinoma cell line through nuclear factor-kappa B activation. Oncogene. 2000;19:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |