Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6258
Revised: July 21, 2013
Accepted: August 8, 2013
Published online: October 7, 2013
Processing time: 144 Days and 16.7 Hours
AIM: To investigate the stress-induced apoptosis of natural killer (NK) cells and the changes in their killing activity in mouse livers.
METHODS: A restraint stress model was established in mice. Flow cytometry was employed to measure the percentage of NK cells and the changes in their absolute number in mouse liver. The cytotoxicity of hepatic and splenic NK cells was assessed against YAC-1 target cells via a 4 h 51Cr-release assay.
RESULTS: The restraint stress stimulation induced the apoptosis of NK cells in the liver and the spleen, which decreased the cell number. The number and percentage of NK cells in the spleen decreased. However, the number of NK cells in the liver decreased, whereas the percentage of NK cells was significantly increased. The apoptosis of NK cells increased gradually with prolonged stress time, and the macrophage-1 (Mac-1)+ NK cells were more susceptible to apoptosis than Mac-1- NK cells. Large numbers of Mac-1- NK cells in the liver, which are more resistant to stress-induced apoptosis, were observed than the Mac-1- NK cells in the spleen. The stress stimulation diminished the killing activity of NK cells in the spleen was significantly decreased, but the retention of numerous Mac-1- NK cells in the liver maintained the killing ability.
CONCLUSION: Significant stress-induced apoptosis was observed among Mac-1+ NK cells, but not Mac-1- NK cells in the mouse liver. Stress stimulation markedly decreased the killing activity of NK cells in the spleen but remained unchanged in the liver.
Core tip: Hepatic natural killer (NK) cells are classified into macrophage-1 (Mac-1)+ and Mac-1- cells, and the different functional characteristics of Mac-1+ or Mac-1- NK cells in response to stress stimulation are confirmed. This study further proves the heterogeneity of NK cell function, and the results provide a reference for preventing the immune system damage caused by stress.
- Citation: Ma Z, Liu Y, Zhou X, Yu HL, Li MQ, Tomiyama-Miyaji C, Abo T, Bai XF. Research on stress-induced apoptosis of natural killer cells and the alteration of their killing activity in mouse liver. World J Gastroenterol 2013; 19(37): 6258-6264
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6258
In the modern society, the acceleration of work and life style and the deterioration of the environment, as well as natural disasters and frequent traffic accidents, expose people to increasing stress. Prolonged or intense stress that overwhelms autoregulation, causes nervous, endocrine, and immune system dysfunction[1-3], as well as the apoptosis of lymphocytes such as natural killer (NK) cells, B cells and T cells. Immune system dysfunction is the direct cause of infectious diseases, cancers and self-deterioration[4-6].
NK cells are an important type of lymphocytes, accounting for 10% to 15% of total lymphocytes and play a crucial role in body resistance to against infections and tumours, as well as immune and hematopoietic regulation[7,8].
This study aims to determine the effects of persistent and intense stress on the apoptosis of hepatic and splenic NK cells and change in their function in mice.
Eight-week-old clean grade C57BL/6 mice were purchased from the Animal Centre of The 3rd Affiliated Hospital of Harbin Medical University.
The protocol used has been described in the published literature[9]. The mice were placed in a 50 mL Falcon tube with 4 to 5 drilled holes at the bottom to maintain ventilation. Sufficient amounts of absorbent cotton were placed inside the tube to immobilise the mouse, and then the lid was screwed shut. The tube was kept at room temperature for 24 h, and the mouse was not fed any food and water. The mice in the control group were left in the original cage without any disturbance.
The protocol used has been described in the published literature[10]. The mouse was anaesthetised with ether and sacrificed via heart puncture. Subsequently, the mouse liver and spleen were collected and minced. The tissue sample was washed with phosphate-buffered saline (PBS) and filtered with 200 mesh strainer, and then the cell suspension was collected. After gradient centrifugation, the cells were lysed with 0.83% NH4Cl-Tris buffer (pH 7.6). The resulting cell suspension was collected and the concentration was adjusted to 1.0 × 106/mL.
Lymphocytes were isolated from mouse liver and spleen, and then double or triple immunofluorescence staining was performed to identify the CD3-NK1.1+ cells as NK cells. Fluorescein-isothiocyanate (FITC)-labelled antibodies: CD3 (145-2C11 clone); PE-labelled antibody: NK1.1 (PK136 clone); Biotin-labelled antibody: macrophage-1 (Mac-1) (M1/70 clone) CD69 (H1.2F3 clone), Ly49C/I (5E6 clone). All the monoclonal antibodies were purchased from BD Biosciences Pharmingen in San Diego, United States.
Cell suspension was transferred in centrifuge tube (cell number < 2 × 106). After 2 min of centrifugation at 2500 r/min and 4 °C, the supernatant was removed, followed by vibration. Then, 10 μL of 2.4 G2 was added (anti-FcγR II/III). After incubation at 4 °C for 10 min, 10 μL of various monoclonal antibodies (CD3, NK1.1, Mac-1, CD69, and Ly49C) were added, accordingly. After vortex and incubation at 4 °C for 20 min, the cells were washed once with PBS (2500 r/min at 4 °C). For double staining, the cells were diluted with 0.5 mL of PBS and filtered with nylon mesh, and then 5 μL of propidium iodide (PI) was added for flow cytometry analysis. When subjected for triple staining, cells were incubated with 10 μL of biotin-labelled secondary antibody at 4 °C for 20 min, and then washed with PBS once (2500 r/min at 4 °C). After diluting with 0.5 mL of PBS and filtration with a nylon mesh, the stained cells were analyzed via flow cytometry[11]. Flow Cytometer was FAC sort from BD-United States and software was Cell Quest 3.0.
The cytotoxicity of NK cells was assessed against YAC-1 target cells. Target cells were continuously cultured for 24 h in RPMI 1640 containing 200 mL/L FCS. YAC-1 cells were collected at exponential phase and counted through trypan blue staining. Viable cells were considered as targets cells when their percentage exceeded 95%. The cell concentration was adjusted to 1 × 105 /mL with RPMI 1640. After incubation with 51Cr for 2 h, the cells were washed three times with RPMI 1640 to remove free 51Cr. The target cell concentration was adjusted to 1.0 × 104/mL or 2.0 × 104/mL. The cells were divided into three groups: NK cell group, target cell maximum release group, and target cell spontaneous release group. Subsequently, the cells were seeded in U-bottom microplates (96-well). Lymphocytes in the mouse liver and spleen were utilized as effector cells, which were added into the U-bottom microplates at an effectoritarget ratio of 50:1, 25:1, and 12.5:1 in a volume of 100 μL/well. The cells were incubated at 37 °C with 5% CO2 for 4 h and the microplates were centrifuged at 1500 r/min for 5 min. About 100 μL of supernatant was collected from each well, and its radioactivity (CPM) was measured with gamma counter[12].
The specific killing rate was calculated using the following formula: specific killing rate (%) = (experimental cell release - target cell spontaneous release)/(target cell maximum release - target cell spontaneous release) × 100%.
The data are presented as mean ± SD and percentage. Significant differences between two samples were analyzed with Student’s t test.
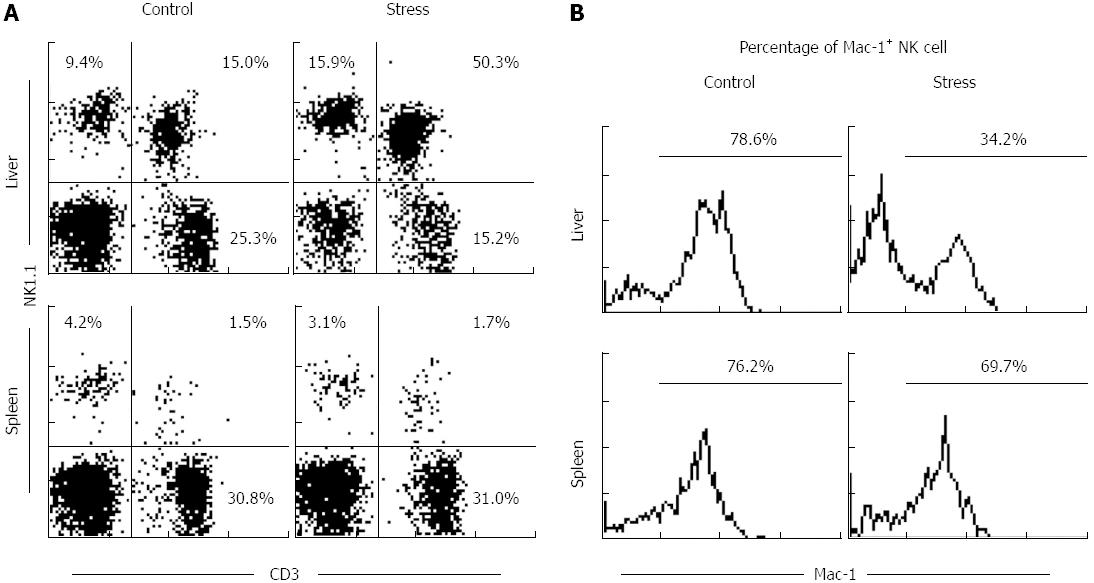
The percentage of splenic NK cells in total lymphocytes did not change significantly (3.9% ± 1.2% vs 2.6% ± 1.1%, P > 0.05), whereas the percentage of hepatic NK cells increased (8.6% ± 1.3% vs 14.9% ± 1.5%, P < 0.05; Figure 1).
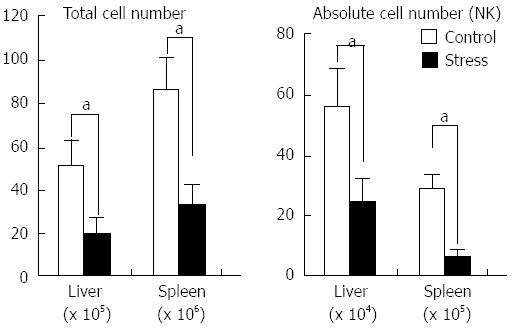
After restraint stress stimulation, the numbers of lymphocytes in the experimental and control groups were (53.1 ± 9.7) × 105vs (19.7 ± 4.6) × 105 (n = 6, P < 0.05) in liver, (87.7 ± 9.6) × 106vs (36.4 ± 7.1) × 106 (n = 6, P < 0.05) in spleen. The number of NK cells in the experimental and control groups were (57.4 ± 8.9)× 105vs (24.6 ± 7.3 )× 105 (n = 6, P < 0.05) in the liver, (29.7 ± 6.5) × 106vs (8.6 ± 1.4 )× 106 (n = 6, P < 0.05) in the spleen (Figure 2).
NK cells were isolated from mouse liver and spleen after 24 h of restraint stress stimulation, followed by immunofluorescence staining with FITC: Mac-1 and PE: NK1.1. The cells were analyzed via flow cytometry. The results show that the percentage of hepatic Mac-1+ NK cells in the experimental group was significantly higher than that of the control group (77.2% ± 1.7% vs 33.9% ± 1.1%, P < 0.05, Figure 1B), whereas the percentage of hepatic Mac-1- NK cells relatively increased. The percentage of splenic Mac-1+ NK cells was slightly decreased (75.1% ± 1.1% vs 68.5% ± 1.6%, P > 0.05).
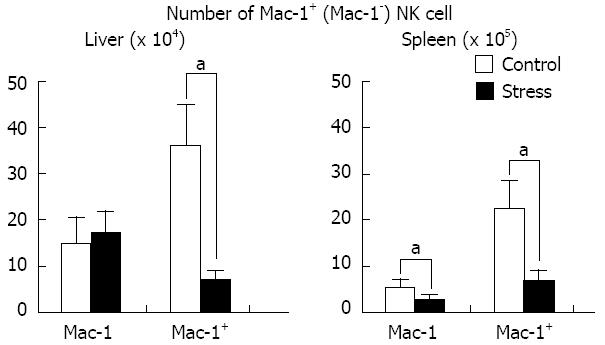
After 24 h of stress stimulation, the number of Mac-1+ NK cells in the liver and spleen in the stress group was significantly lower than those in the control group: liver, (37.7 ± 9.8) × 104vs (8.4 ± 1.7) × 104 (n = 6, P < 0.05); spleen, (23.5 ± 6.3) × 105vs (8.7 ± 1.9) × 105 (n = 6, P < 0.05). The results are shown in Figure 3.
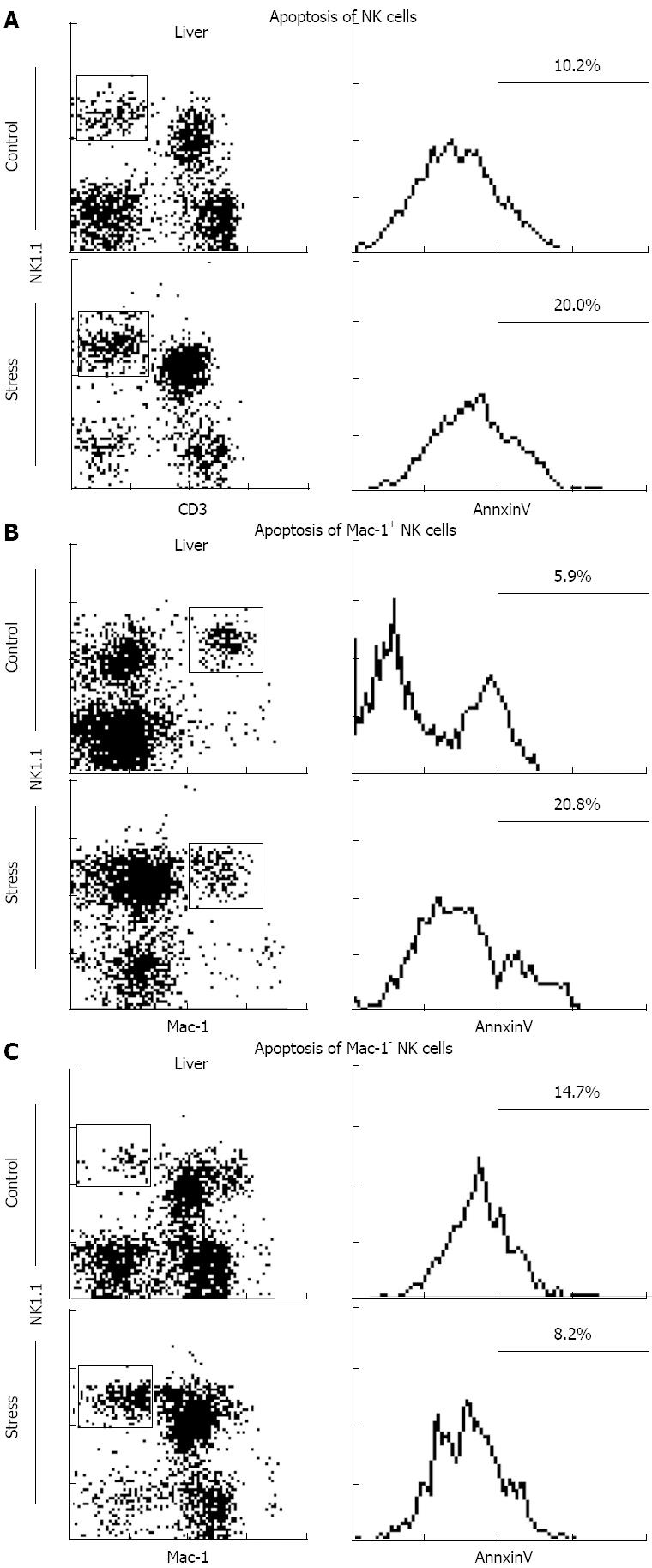
NK cells were isolated from mouse liver after 24 h of restraint stress stimulation, followed by immunofluorescence staining with Annexin V-FITC and PI. The cells were analyzed via flow cytometry. The results revealed significant apoptosis of NK cells (9.5% ± 1.4% vs 19.3% ± 1.3%, P < 0.05), especially the Mac-1+ NK cells (5.2% ± 1.8% vs 19.3% ± 1.4%, P < 0.05), whereas the apoptosis of Mac-1- NK cells did not significantly change (13.4% ± 1.3% vs 7.4% ± 1.7%, P > 0.05; Figure 4).
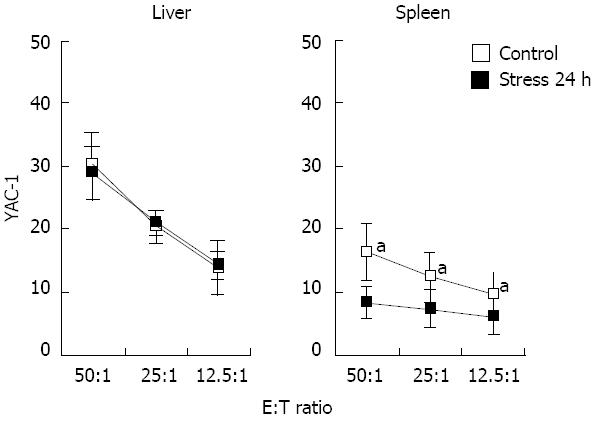
The killing activity of NK cells was markedly decreased in the spleen (16.7% ± 1.4% vs 8.9% ± 1.1%, P < 0.05), but was sustained in the liver even after 24 h of restraint stress stimulation (Figure 5).
With the development of modern medical technology, increasing attention has been focused on the relationship between stress and health. The response of the body to stress is a dynamic balance, which allows the body to recover to its original state through autoregulation after stress reaction. However, persistent stress may overwhelm autoregulation and cause psychosomatic damage[13,14].
Numerous studies have demonstrated that acute stress stimulation causes distinctly reduced number of lymphocytes in the thymus, spleen, peripheral blood, and liver, as well as disrupt the function of T, B and NK cells. Moreover, the decrease in lymphocytes mainly results from apoptosis.
Our research shows that the number of splenic lymphocytes was significantly decreased, but their percentage did not change significantly. We speculate that various lymphocytes in spleen decreased with the same percentage, which is consistent with the results of previous studies[10,15]. Moreover, the results showed that the number of hepatic lymphocytes significantly declined but the percentage of NK cells dramatically increased (Figures 1A and 2). Therefore, the number of NK cells in the liver and the spleen were determined. Although both the liver and the spleen had fewer NK cells, the number of NK cells was relatively high in the liver, dramatically increasing the percentage of NK cells in the liver (Figure 2). These results indicate that the hepatic NK cells differed from splenic NK cells after stress stimulation. A recent study reported that NK cells have organ specificity, which allows liver to be considered as immune organ and NK cells possess unique functional characteristics[16,17].
Mac-1 was employed to distinguish the subtypes of NK cells with different functions. Mac-1 (CD11b/CD18) is an adhesion molecule of the integrin family, highly expressed in most myeloid hematopoietic cells, such as neutrophils, monocytes/macrophages, eosinophils and B cells. Mac-1 is closely correlated with cell phagocytosis and adhesion, as well as a marker for myeloid and lymphoid hematopoietic cells. NK cells are the only lymphoid cells that express Mac-1. Some researchers have considered Mac-1 as a marker for mature NK cells, which have cell killing activity and are able to secrete cytokines, whereas Mac-1- NK cells are immature, with limited cell killing activity and cytokine production[18-20]. Mac-1 is expressed by 80% to 90% of mature NK cells in the liver, spleen, and peripheral blood. Therefore, current research on NK cells is mainly focused on Mac-1+ NK cells. Our previous study discovered numerous Mac-1- NK cells in the liver and demonstrated that Mac-1+ NK cells and Mac-1- NK cells have different functional characteristics and cell phenotypes[11]. Therefore, hepatic NK cells were classified into Mac-1+ and Mac-1- subtypes according to Mac-1 expression.
Our study shows that the number of Mac-1+ and Mac-1- NK cells in the spleen deceases with same percentage after stress stimulation, which results in sustained Mac-1 expression in splenic NK cells. The number of hepatic Mac-1+ NK cells significantly decreased, whereas the number of Mac-1- NK cells did not change significantly, which accounts for the reduced Mac-1 expression in hepatic NK cells (Figures 1B and 3). Therefore, we speculate that Mac-1- hepatic NK cells are resistant to the apoptosis induced by stress stimulation.
A large number of studies have demonstrated that acute stress stimulation causes distinctly reduced number of lymphocytes in the thymus, spleen, peripheral blood, and liver, as well as disrupts the function of T, B and NK cells. Moreover, the decrease in lymphocytes is mainly caused by the apoptosis of lymphocytes.
Intracellular Annexin V expression was measured to assess the degree of apoptosis of hepatic NK cells induced by stress stimulation[21]. The results revealed significant NK cell apoptosis, especially Mac-1+ NK cells, whereas Mac-1- NK cell apoptosis remained unchanged (Figure 4).
To determine the effects of stress on NK cell function, we employed YAC-1 as target cells to measure killing activity of NK cells. The results imply that stress stimulation decreases the killing activity of splenic NK cells, which accords with the results of previous research. Our study also discovered that stress stimulation does not affect the killing activity of hepatic NK cells, which contrasts with the conclusion of previous investigations. We concluded that Mac-1+ NK cells had stronger killing activity than that of Mac-1- NK cells[11]. We also believed that the number of hepatic Mac-1+ NK cells declined because of cell apoptosis, which allowed apoptosis-resistant Mac-1- NK cells to survive, and to exhibit relatively strong cell killing activity.
In conclusion, our research preliminarily demonstrates that intense stress stimulation induces the apoptosis of Mac-1+ hepatic NK cells instead of Mac-1- NK cells, which requires further investigation to understand the underlying mechanisms and the role of the liver in stress-triggered immune function.
Stress refers to non-specific systemic reactions to strong stimulus on the body. Intense and prolonged stress stimulation causes immune function disorders, volume shrinkage, and dysfunction of immune organs such as the liver, spleen, and thymus gland. In addition, stress stimulation causes the apoptosis and dysfunction of lymphocytes such as natural killer (NK), T, and B cells in the peripheral blood and immune organs. Most studies have demonstrated that strong stress stimulation may decreases NK cell killing activity in the peripheral blood and spleen, which weakens the immune system.
NK cells are a class of lymphocytes in the innate immune system that account for about 10% to 15% of all lymphocytes. They have anti-infective, anti-tumour, and immunomodulatory functions, and they regulate hematopoiesis. NK cells mainly function in killing cells and cytokine secretion. NK cells are divided into two subtypes according to surface antigens and functional cell expression, namely, NK1 and NK2. Previous investigations have studied NK cells in the peripheral blood and the spleen. The results confirm that the liver generates NK cells during embryogenesis and the function of NK cells in the liver is different from that in the peripheral blood and the spleen.
This study confirms that stress stimulation significantly decreases the number of splenic NK cells, with significantly decreased killing activity, whereas some NK cells survive in the liver. Further research proves that these surviving cells are macrophage-1 (Mac-1)- NK cells that resist stress-induced cell apoptosis. By contrast, the killing activity of Mac-1- NK cells is unaffected by stress stimulation.
This study proves the anti-stress ability of Mac-1- hepatic NK cells. This finding suggests that Mac-1- NK cells maintain immune functional stability under stress conditions. Further studies should investigate how to characterize Mac-1- NK cells and utilize them for preventing the immune dysfunction caused by stress.
NK cells are important immune cells with anti-tumour, anti-viral, and immune regulation function, but also participate in the hypersensitivity and occurrence of autoimmune diseases in some cases. Mac-1 (CD11b/CD18) is an adhesion molecule (integrins), and is expressed in most myeloid hematopoietic cells such as neutrophils, monocyte-macrophages, eosinophils, and B cells.
Authors investigated the stress-induced apoptosis of NK cells and the changes in their killing activity in mouse livers. NK cell is an important type of lymphocytes. The authors made an interesting research on NK cell. This study proves the anti-stress ability of Mac-1-hepatic NK cells. This finding suggests that Mac-1-NK cells maintain immune functional stability under stress conditions. Further studies should investigate how to characterize Mac-1-NK cells and utilize them for preventing the immune dysfunction caused by stress.
P- Reviewers Bell E, Ordi J S- Editor Wang JL L- Editor A E- Editor Ma S
| 1. | Gupta MA. Review of somatic symptoms in post-traumatic stress disorder. Int Rev Psychiatry. 2013;25:86-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 2. | Irwin MR. Human psychoneuroimmunology: 20 years of discovery. Brain Behav Immun. 2008;22:129-139. [PubMed] |
| 3. | Yokoyama M, Itano Y, Katayama H, Morimatsu H, Takeda Y, Takahashi T, Nagano O, Morita K. The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy. Anesth Analg. 2005;101:1521-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Nakata A. Psychosocial job stress and immunity: a systematic review. Methods Mol Biol. 2012;934:39-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Seth R, Tai LH, Falls T, de Souza CT, Bell JC, Carrier M, Atkins H, Boushey R, Auer RA. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann Surg. 2013;258:158-168. [PubMed] |
| 6. | Reiche EM, Morimoto HK, Nunes SM. Stress and depression-induced immune dysfunction: implications for the development and progression of cancer. Int Rev Psychiatry. 2005;17:515-527. [PubMed] |
| 7. | Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 639] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 8. | French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45-51. [PubMed] |
| 9. | Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 625] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 10. | Sagiyama K, Tsuchida M, Kawamura H, Wang S, Li C, Bai X, Nagura T, Nozoe S, Abo T. Age-related bias in function of natural killer T cells and granulocytes after stress: reciprocal association of steroid hormones and sympathetic nerves. Clin Exp Immunol. 2004;135:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Bai X, Wang S, Tomiyama-Miyaji C, Shen J, Taniguchi T, Izumi N, Li C, Bakir HY, Nagura T, Takahashi S. Transient appearance of hepatic natural killer cells with high cytotoxicity and unique phenotype in very young mice. Scand J Immunol. 2006;63:275-281. [PubMed] |
| 12. | Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, Hatakeyama K, Abo T. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Harlan JM, Vedder NB, Winn RK, Rice CL. Mechanisms and consequences of leukocyte-endothelial interaction. West J Med. 1991;155:365-369. [PubMed] |
| 14. | Ross GD, Vetvicka V, Yan J, Xia Y, Vetvicková J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42:61-74. [PubMed] |
| 15. | Shimizu T, Kawamura T, Miyaji C, Oya H, Bannai M, Yamamoto S, Weerasinghe A, Halder RC, Watanabe H, Hatakeyama K. Resistance of extrathymic T cells to stress and the role of endogenous glucocorticoids in stress associated immunosuppression. Scand J Immunol. 2000;51:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Michel T, Poli A, Domingues O, Mauffray M, Thérésine M, Brons NH, Hentges F, Zimmer J. Mouse lung and spleen natural killer cells have phenotypic and functional differences, in part influenced by macrophages. PLoS One. 2012;7:e51230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 18. | Vĕtvicka V, Hanikýrová M, Vĕtvicková J, Ross GD. Regulation of CR3 (CD11b/CD18)-dependent natural killer (NK) cell cytotoxicity by tumour target cell MHC class I molecules. Clin Exp Immunol. 1999;115:229-235. [PubMed] |
| 19. | Werfel T, Witter W, Götze O. CD11b and CD11c antigens are rapidly increased on human natural killer cells upon activation. J Immunol. 1991;147:2423-2427. [PubMed] |
| 20. | Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 485] [Article Influence: 21.1] [Reference Citation Analysis (0)] |