Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6214
Revised: July 24, 2013
Accepted: August 4, 2013
Published online: October 7, 2013
Processing time: 174 Days and 2.2 Hours
AIM: To investigate characteristics of hepatitis B virus (HBV) implicated in HBV reactivation in patients with hematological malignancies receiving immunosuppressive therapy.
METHODS: Serum samples were collected from 53 patients with hematological malignancies negative for hepatitis B surface antigen (HBsAg) before the start of and throughout the chemotherapy course. HBV reactivation was diagnosed when the HBsAg status changed from negative to positive after the initiation of chemotherapy and/or when HBV DNA was detected by real-time detection polymerase chain reaction (RTD-PCR). For detecting the serological markers of HBV infection, HBsAg as well as antibodies to the core antigen (anti-HBc) and to the surface antigen were measured in the sera by CEIA. Nucleic acids were extracted from sera, and HBV DNA sequences spanning the S gene were amplified by RTD-PCR. The extracted DNA was further subjected to PCR to amplify the complete genome as well as the specific genomic sequences bearing the enhancer II/core promoter/pre-core/core regions (nt 1628-2364). Amplicons were sequenced directly.
RESULTS: Thirty-five (66%) of the 53 HBsAg-negative patients were found to be negative serologically for anti-HBc, and the remaining 18 (34%) patients were positive for anti-HBc. Five of the 53 (9.4%) patients with hematologic malignancies experienced HBV reactivation. Genotype D1 was detected in all five patients. Four types of mutant strains were detected in the S gene product of HBV strains and were isolated from 3 patients with HBV reactivation: T/S120, L143, and I126. HBV DNA was detected in the pretreatment HBsAg-negative samples in one of the five patients with HBV reactivation. In this patient, sequences encompassing the HBV full genome obtained from sera before the start of chemotherapy and at the time of de novo HBV hepatitis were detected and it showed 100% homology. Furthermore, in the phylogenetic tree, the sequences were clustered together, thereby indicating that this patient developed reactivation from an occult HBV infection.
CONCLUSION: Past infection with HBV is a risk factor for HBV reactivation in Egypt. Mandatory anti-HBc screening prior to chemotherapy in patients with hematological malignancies is recommended.
Core tip: The study aimed to investigate characteristics of hepatitis B virus (HBV) implicated in HBV reactivation in patients with hematological malignancies receiving immunosuppressive therapy in Egypt. Fifty-three hepatitis B surface antigen (HBsAg)-negative patients treated with chemotherapy were included in the study. The incidence of HBV reactivation was 9.4% among the studied cohort, and all of the affected individuals were positive for HBsAg as well as antibodies to the hepatitis B core antigen. The present study provides further evidence via molecular evolutionary analysis of the development of HBV reactivation from an occult HBV infection. Past infection with HBV is a risk factor for HBV reactivation in Egypt. Mandatory antibodies to the core antigen screening prior to chemotherapy in patients with hematological malignancies is suggested.
- Citation: Elkady A, Aboulfotuh S, Ali EM, Sayed D, Abdel-Aziz NM, Ali AM, Murakami S, Iijima S, Tanaka Y. Incidence and characteristics of HBV reactivation in hematological malignant patients in south Egypt. World J Gastroenterol 2013; 19(37): 6214-6220
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6214
Infection with hepatitis B remains one of the major causes of acute and chronic liver disease. An estimated 350-400 million people are chronically infected with hepatitis B virus (HBV) worldwide[1].
The reactivation of hepatitis B infection has been recorded in many clinical settings: chronic HBV infection after the cessation of HBV treatment, patients with malignant disease who receive immunosuppressant or chemotherapy, patients with end stage renal failure, and patients co-infected with human immunodeficiency virus (HIV)[2-6]. Patients with resolved HBV infection are diagnosed serologically by clearance of serum hepatitis B surface antigen (HBsAg) and the appearance of the hepatitis B core antibody (anti-HBc), with or without antibodies to hepatitis B surface antigen (anti-HBs)[7]. These patients are at risk of hepatitis B reactivation due to any factor that can suppress the immune system[8,9]. De novo hepatitis B is of particular concern in this subset of patients because it commonly leads to severe liver dysfunction and fatal hepatitis[10,11].
Occult hepatitis B is defined by the presence of HBV DNA in the serum or the liver in the absence of HBsAg, with or without anti-HBc or anti-HBs. In these patients, a low level of HBV replication has been shown to persist in the liver and in peripheral blood mononuclear cells for decades[12]. Occult HBV infection is observed worldwide, and its prevalence is related closely to the endemicity of HBV infection.
Large scale geographic heterogeneity in the prevalence of HBV had been reported worldwide. Africa is one of the highly endemic regions of HBV, and an intermediate endemicity of HBV infection had been recorded in Egypt[13,14].
The aim of this study was to investigate the incidence of HBV reactivation and the underlying risk factors of hepatitis B reactivation in Egyptian patients who received cytotoxic chemotherapy for hematological malignancies.
Fifty-nine consecutive patients with hematological malignancies were admitted to the oncology department of Sohag Faculty of Medicine and South Egypt Cancer Institution from November 2010 to October 2011. After admission, all patients underwent physical examination and blood and serum biochemistry analyses. All of patients received chest computed tomography and ultrasonography of the abdomen as an initial evaluation.
In clinical practice, patients are monitored during chemotherapy using liver function tests. HBsAg and HBV DNA are tested in patients with elevated liver enzymes. For the purpose of this study, serum samples were collected before and after the start of the chemotherapy course. The collected sera were stored at -80 °C for future examination of HBsAg, anti-HBs, and anti-HBc. HBV reactivation was diagnosed when the HBsAg status changed from negative to positive after the initiation of chemotherapy and/or when HBV DNA was detected as measured by real-time detection polymerase chain reaction (RTD-PCR) using stored samples from patients, as described latter.
HBsAg was measured by enzyme immunoassay (EIA) (AxSYM; Abbott Japan, Tokyo, Japan) or chemiluminescence enzyme immunoassay (CLEIA) (Fujirebio, Tokyo; Japan). Anti-HBc of the IgG class was determined by radioimmunoassay (Abbott Japan). All serologic assays were performed according to the manufacturer’s instructions.
HBV-DNA sequences spanning the S gene were amplified by RTD-PCR according to the previously described protocol with a slight modification and a detection limit of 100 copies/mL (equivalent to 20 IU/mL)[15].
Nucleic acids were extracted from serum samples (200 μL) using the QIAamp DNA extraction kit (Qiagen, Hilden, Germany).
Extracted DNA was subjected to PCR for amplifying the complete genome and the specific genomic sequences bearing enhancer II/core promoter/pre-core/core regions (nt 1628-2364), as described previously[16].
Amplicons were sequenced directly using the ABI Prism Big Dye ver. 3.1 kit in the AMI 3100 DNA automated sequencer (Applied Biosystems; Foster City, CA, United States).
All sequences were analyzed in both the forward and reverse directions. HBV genotypes were determined by molecular evolutionary analysis. Reference HBV sequences were retrieved from the DDBJ/EMBL/GenBank database and aligned by CLUSTALX, and genetic distances were estimated with the 6-parameter method in the Hepatitis Virus Database (http://s2as02.genes.nig.ac.jp/)[17]. Based on the obtained distances, phylogenetic trees were constructed by the neighbor-joining (NJ) method with the mid-point rooting option. To confirm the reliability of the phylogenetic trees, bootstrap resampling tests were performed 1000 times for analysis by the ODEN program of the National Institute of Genetics.
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and its subsequent amendments, and informed consent was obtained from all patients.
Statistical analysis was performed with the Fisher’s exact probability test and the independent t test for the continuous variables using the SPSS software package (SPSS, Chicago, IL, United States). P values (two-tailed) less than 0.05 were considered statistically significant.
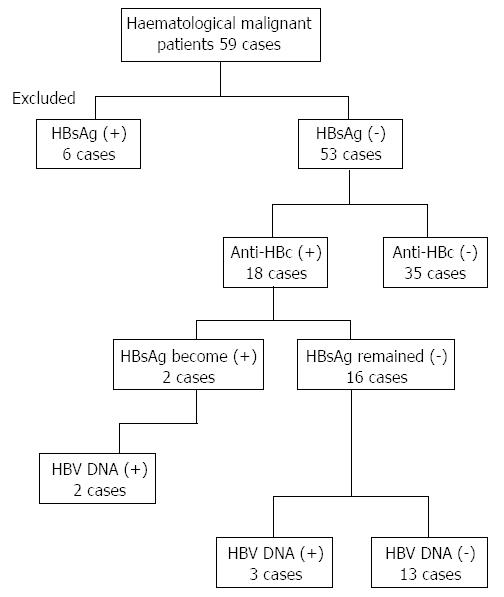
Six of the 59 patients with hematologic malignancies were found to be HBsAg positive and were excluded from the analysis. Therefore, a total of 53 HBsAg-negative patients were checked for the serological markers of infection with hepatitis B. The background general characteristics of the 53 HBsAg-negative patients are presented in Table 1. The mean age of the analyzed cohort was 27.8 ± 26.2 years old. Thirty-five (66%) of 53 HBsAg-negative patients were found to be anti-HBc-negative, and 18 (34%) patients were serologically positive for anti-HBc. The predominance of male patients was observed in both the anti-HBc-positive and -negative patient groups. Twenty-six patients (40.1%) were diagnosed with malignant lymphoma, whereas 25 patients (47.2%) were diagnosed with acute leukemia. Solitary cases of chronic leukemia and multiple myeloma were also included in the studied cohort. An insignificantly higher incidence of acute leukemia cases was observed in the anti-HBc-positive patients (9/18; 50%) compared with the anti-HBc-negative patients (15/35; 42.9%).
| Characteristics | Total | Anti-HBc positive | Anti-HBc negative | P value |
| (n = 53) | (n = 18) | (n = 35) | ||
| Age yr, mean ± SD | 27.8 ± 26.2 | 34.4 ± 27.9 | 27.7 ± 25.4 | 0.42 |
| Gender (male) | 26 (49.1) | 10 (55.6) | 16 (45.7) | 0.56 |
| Diagnosis | ||||
| Malignant lymphoma | 26 (40.1) | 9 (50.0) | 17 (48.6) | 1.00 |
| Acute leukemia | 25 (47.2) | 9 (50.0) | 15 (42.9) | 0.77 |
| Chronic leukemia | 1 (1.9) | 0 (0.0) | 1 (2.9) | 1.00 |
| Multiple myeloma | 1 (1.9) | 0 (0.0) | 1 (2.9) | 1.00 |
After the initiation of systemic chemotherapy, examination of the HBV serology revealed that two (3.8%) of the HBsAg-negative patients became serologically positive for HBsAg. In addition, 3 more patients (5.8%) exhibited detectable HBV DNA in their sera after the start of the anticancer therapy (Figure 1). Interestingly, none of the serologically negative patients for anti-HBc became serologically positive for HBsAg or molecularly detectable for HBV DNA. In contrast, 2 of the 18 anti-HBc-positive patients (11.1%) became serologically positive for the HBsAg, and 3 (16.7%) became molecularly detectable for the HBV DNA. In brief, 5 of the 53 HBsAg negative patients (9.4%), representing 27.8% (5/18) of the anti-HBc-positive patients in the studied cohort, manifested the criteria of HBV reactivation (Figure 1).
Five of the 53 patients (9.4%) treated for hematologic malignancies manifested HBV reactivation throughout the anti-cancer therapy regimen. The demographic, clinical and virological criteria of the HBV infection of the five patients who experienced HBV reactivation are summarized in Table 2 (cases 1-5). The mean age of the five patients was 24.6 ± 30.9 years old. Three of the patients were males (cases 2, 4 and 5), and two were females. Four patients were diagnosed with acute leukemia (cases 2-5), and only one patient (case 1) was diagnosed with malignant lymphoma. All of the 5 patients received a steroid regimen as a part of their anticancer therapy. All 5 patients were positive for anti-HBc. Three patients were positive for anti-HBs (cases 1, 2 and 4), and only one patient was serologically negative for the anti-HBs (case 3). Because of small volume of serum sample obtained from case 5, anti-HBs could not be tested. After HBV reactivation, two cases (cases 2 and 4) exhibited abnormal ALT levels, and one patient (case 2) experienced a more than 3-fold increase in the ALT level, indicating the emergence of hepatitis in this patient. None of the 5 cases who experienced had the HBV reactivation after cancer chemotherapy received an antiviral treatment for HBV.
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
| Age (yr)/gender | 79/F | 8/M | 11/F | 5/M | 20/M |
| Diagnosis | NHL (stage III) | AML | ALL | ALL | ALL |
| Treatment1 | CVP | St Jude protocol | St Jude protocol | St Jude protocol | St Jude protocol |
| HBV serology and DNA prior to chemotherapy | |||||
| HBsAg/anti-HBs/HBV DNA (log copy/mL) | (-)/(+)/1.8 | (-)/(+)/negative | (-)/(-)/negative | (-)/(+)/negative | (-)/(nt)/negative |
| HBV reactivation months after anti-cancer therapy | 12 | 4 | 5 | 6 | 4 |
| HBV serology and DNA after chemotherapy | |||||
| HBsAg/anti-HBs/HBV DNA (log copy/mL) | (+)/(nt)/7.6 | (+)/(+)/5.8 | (-)/(-)/3.1 | (-)/(+)/2.9 | (-)/(nt)/2.0 |
| ALT (IU/mL) | 35 | 195 | 27 | 86 | 17 |
| Total bilirubin (mg/dL) | 1 | 1.1 | 1.3 | 1.1 | 0.2 |
| Outcome | Died | Died | Died | Alive | Alive |
| HBV genotype | D1 | D1 | D1 | D1 | D1 |
| Core promoter mutation | Wild | T1764/G1766 | A1764 | Wild | - |
| Pre-core A1896 | Mutant | Wild | Wild | Wild | - |
| Amino acid mutation in S gene product | P120S/S143L | P120T | - | T126I | - |
The virological and molecular criteria are summarized in Table 2. The infecting genotype of the HBV strains was HBV genotype D, subtype D1 in all five cases. Two core promoter HBV variants were detected in 2 patients. The two variants were T1764/G1766 and A1764 in cases 2 and 3, respectively. The stop codon pre-core HBV mutant (A1896) was detected in one patient (case 1).
Infection with HBV mutant strains in the S gene product was detected in 3 patients. The amino acid escape mutant strains are as follows: S120 and L143 (case 1), T120 (case 2) and I126 (case 4). Four types of mutant strains (T/S120, L143, and I126) were detected in the S gene strains of 3 patients (cases 1, 2 and 4, respectively).
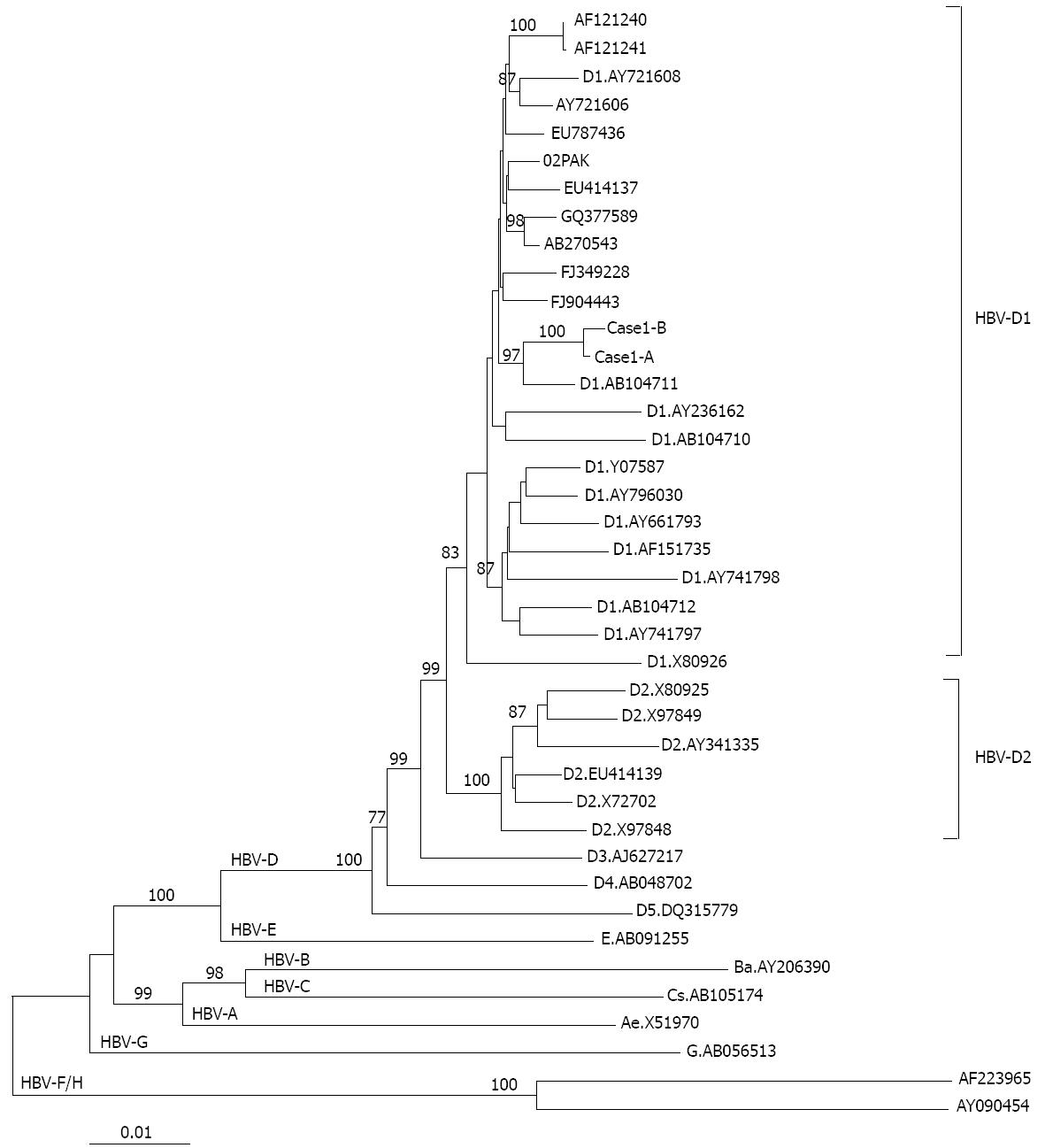
HBV DNA was quantified retrospectively by RTD-PCR in the stored samples of the five patients with HBV reactivation. Evidence of occult HBV infection at the time of the HBsAg-negative status (before the start of anticancer therapy) was detected by RTD-PCR in one patient (case 1). To determine the source of HBV infection, sera from case 1 before (case 1-A) and at the time of HBV reactivation (case 1-B) were subjected to HBV full genome amplification and sequencing. Sequences encompassing the HBV full genome obtained from sera before the start of chemotherapy and at the time of de novo HBV hepatitis revealed 100% homology, and the two sequences clustered together in the phylogenetic tree (Figure 2). These results demonstrate that case 1 developed reactivation from an occult HBV infection.
This study is considered the first step in documenting and characterizing the reactivation of hepatitis B in Egypt among patients negative for the HBsAg who received immunosuppressive therapy. The current study presented further evidence that resolved hepatitis B infection and occult HBV infection may represent a hidden risk factor for the development of de novo hepatitis B.
The incidence of hepatitis B reactivation in the HBsAg-negative group was 9.4%, and all cases of reactivation occurred in patients with resolved or past infection with hepatitis B, as evidenced by the absence of HBsAg and the serological detection of anti-HBc. The patients who had HBV reactivation represent 27% of the HBsAg-negative/anti-HBc-positive patients. This incidence was comparable to the incidence that was described by Hui et al[18]. In their study, Hui et al[18] described an HBV reactivation incidence of 3.3% (8/244) in their studied cohort, which included HBsAg-negative lymphoma patients receiving systemic chemotherapy. Of note, all 8 patients were seropositive for either anti-HBc or anti-HBs antibody. Recently, Matsue et al[19] conducted a retrospective study on consecutive patients with CD20-positive B cell lymphoma before and after rituximab-containing treatment. In the latter study, 5 out of 230 patients negative for HBsAg (2.2%) experienced HBV reactivation, representing an incidence of 8.9% of the anti-HBc-positive patients[19]. In a prospective observational study of patients with hematological malignancies (a study cohort similar to the current study), Francisci et al[20] reported the incidence of HBV reactivation was (18%), which is close to that detected in the present study. The reasons for the difference in the incidence in HBV reactivation among different studies remain to be elucidated. However, the intensity of treatment, patient characteristics, and geographic differences in HBV prevalence and its genotypes may account for these differences[21]. Furthermore, the lack of a clear definition of HBV reactivation should not be ignored as a possible explanation for this variation in the incidence. In this study, the inclusion of patients who had detectable HBV DNA after cancer chemotherapy plus patients who exhibited HBsAg seroconversion after receiving the anticancer therapy dramatically increased the incidence of HBV reactivation among the studied cohort. This criterion of including cases with detectable HBV DNA after cancer chemotherapy as a sign of HBV reactivation was not used to define cases with HBV reactivation in the related studies[18,19]. The variations in the cohort size among the different studies cannot be ignored as a possible factor that may be implicated in such discrepancy.
Occult HBV infection is defined by the detection of HBV DNA in the sera or in the livers of serologically HBsAg-negative patients[14]. Until recently, the clinical effects of occult HBV infection were unclear regarding the influence on the progression of liver disease, the development of hepatocellular carcinoma, the risk for HBV reactivation, and the transmission of HBV infection[22]. The underlying mechanisms for the pathogenesis of occult HBV infection may be due to either viral or host factors[23]. One of the important viral factors is the presence of mutations in the HBV DNA sequence, which may interfere with the detection of HBsAg by the commercial assays, i.e., “escape mutations”[24]. In the present study, 4 types of possible escape mutants were detected in 3 of the 5 patients who experienced HBV reactivation[25]. Previous in vitro studies have reported that escape mutations are associated with an increased immune evasive capacity and are capable of causing symptomatic flare up and high viral loads[26]. Furthermore, studying the viral genome isolated from case 1 revealed a complete match of the sequences obtained before the start of chemotherapy and at the time of reactivation. The present study provides further evidence of the emergence of HBV reactivation of occult hepatitis B as confirmed by the molecular evolutionary analysis[27]. Furthermore, two amino acid escape mutations in the S gene product, P120S and S143L, were detected in the HBV viral genome isolated from case 1.
Patients with malignancies in Egypt are monitored only by testing ALT levels throughout the chemotherapy course. Therefore, the present study, which is the first to explore HBV reactivation in Egypt, suggests mandatory serological screening for anti-HBc and anti-HBs in patients planning to receive immunosuppressant therapy. Patients found to be positive for anti-HBc, particularly patients who are negative for anti-HBs, should be closely monitored with HBsAg, HBV DNA and serum biochemistry during chemotherapy and for at least 6 mo after the completion of therapy. Further prospective multicenter studies are needed to explore the incidence and risk factors of HBV reactivation in Egypt. Further studies are recommended to determine whether specific genomic mutations are implicated in de novo hepatitis in this subset of patients infected with HBV genotype D1.
The reactivation of hepatitis B is a syndrome characterized by an abrupt appearance or rise of the hepatitis B virus (HBV) DNA in the sera of patients with resolved or inactive hepatitis B infection. Reactivation can be spontaneous but is typically triggered by cancer chemotherapy, immune suppression or alterations in immune system function. Hepatitis B reactivation is of special clinical concern in immunocompromised patients because it leads to severe liver dysfunction and hepatic failure. However, hepatitis B reactivation is easy to prevent by introducing a prophylactic oral antiviral therapy. Occult hepatitis B is defined by the presence of HBV DNA in the serum or the liver in the absence of Hepatitis B surface antigen (HBsAg) with or without hepatitis B core antibody (anti-HBc) or antibodies to HBV surface antigen (anti-HBs). These patients are at risk of developing hepatitis B reactivation due to any factor suppressing the immune system. In Egypt, patients receiving cancer chemotherapy are typically monitored by liver function tests, with no screening for HBsAg or HBV DNA except in cases with elevated liver enzymes. This study aimed to investigate the incidence of HBV reactivation and the underlying risk factors of reactivation in Egyptian patients with hematological malignancies who were receiving cancer chemotherapy.
In a cohort of 53 patients with hematological malignancies receiving cancer chemotherapy who were negative for HBsAg, 18 patients (34%) were found to be positive for the anti-HBc, and five of the 53 (9.4%) patients with hematologic malignancies experienced HBV reactivation. All five patients were positive for anti-HBc. HBV DNA was detected in pretreatment HBsAg-negative samples in one of the five patients with HBV reactivation. In this patient, sera were obtained before the start of chemotherapy and at the time of de novo HBV hepatitis; the molecular evolutionary analysis of the sequences encompassing the HBV full genome obtained from the sera revealed that this patient developed reactivation from an occult HBV infection.
This study is the first in Egypt to characterize HBV reactivation in Egypt. The study introduces more evidence through molecular evolutionary analysis that occult HBV infection is a risk factor for reactivation of hepatitis B in patients with hematological malignancies receiving cancer chemotherapy.
The study strongly recommends mandatory serological screening for anti-HBc and anti-HBs in this subset of patients before the commencement of chemotherapy. Patients found to be positive for anti-HBc, particularly patients who are negative for anti-HBs, should be closely observed for signs of HBV reactivation through the regular monitoring of HBsAg and HBV DNA.
In the study, performance of sequencing and molecular analysis of HBV genomes seems relevant in characterization of the strains associated with HBV reactivation. Their findings are significant and beneficial for the readers.
P- Reviewers Jang JW, Suzukawa K, Zhou YH S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | Freudiger H, Sitavanc R. Reverse seroconversion of hepatitis B in a haemodialysis patient. Nephrol Dial Transplant. 2004;19:238-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Schnepf N, Sellier P, Bendenoun M, Zini JM, Sanson-le Pors MJ, Mazeron MC. Reactivation of lamivudine-resistant occult hepatitis B in an HIV-infected patient undergoing cytotoxic chemotherapy. J Clin Virol. 2007;39:48-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Chakvetadze C, Bani-Sadr F, Le Pendeven C, Lamontagne F, Vincensini JP, Pialoux G. Reactivation of hepatitis B virus replication during peginterferon-ribavirin therapy in an HIV/hepatitis C virus-co-infected patient with isolated anti-hepatitis B core antibodies. AIDS. 2007;21:393-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Clark SJ, Creighton S, Horner M, Smith HM, Portmann B, Taylor C, Cramp ME. Reactivation of latent hepatitis B virus infection with HIV-related immunosuppression. Int J STD AIDS. 2006;17:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Bortolotti F, Crivellaro C, Brunetto MR, Cadrobbi P, Bertolini A, Alberti A. Selection of a precore mutant of hepatitis B virus and reactivation of chronic hepatitis B acquired in childhood. J Pediatr. 1993;123:583-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Lowell JA, Howard TK, White HM, Shenoy S, Huettner PC, Brennan DC, Peters MG. Serological evidence of past hepatitis B infection in liver donor and hepatitis B infection in liver allograft. Lancet. 1995;345:1084-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Nordbø SA, Skaug K, Holter E, Waage A, Brinch L. Reactivation of hepatitis B virus infection in an anti-HBc and anti-HBs positive patient after allogeneic bone marrow transplantation. Eur J Haematol. 2000;65:86-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Chamorro AJ, Casado JL, Bellido D, Moreno S. Reactivation of hepatitis B in an HIV-infected patient with antibodies against hepatitis B core antigen as the only serological marker. Eur J Clin Microbiol Infect Dis. 2005;24:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Kitano K, Kobayashi H, Hanamura M, Furuta K, Ueno M, Rokuhara A, Tanaka E, Umemura T, Kiyosawa K. Fulminant hepatitis after allogenic bone marrow transplantation caused by reactivation of hepatitis B virus with gene mutations in the core promotor region. Eur J Haematol. 2006;77:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Lin PC, Poh SB, Lee MY, Hsiao LT, Chen PM, Chiou TJ. Fatal fulminant hepatitis B after withdrawal of prophylactic lamivudine in hematopoietic stem cell transplantation patients. Int J Hematol. 2005;81:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepat. 2002;9:243-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Ozaslan E, Purnak T. Controversies about occult hepatitis B virus infection. World J Gastroenterol. 2009;15:4986-4987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 385] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 15. | Sugiyama M, Tanaka Y, Kurbanov F, Maruyama I, Shimada T, Takahashi S, Shirai T, Hino K, Sakaida I, Mizokami M. Direct cytopathic effects of particular hepatitis B virus genotypes in severe combined immunodeficiency transgenic with urokinase-type plasminogen activator mouse with human hepatocytes. Gastroenterology. 2009;136:652-662.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Ueda R. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J Virol. 2002;76:5985-5992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 233] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Shin-I T, Tanaka Y, Tateno Y, Mizokami M. Development and public release of a comprehensive hepatitis virus database. Hepatol Res. 2008;38:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, Leung N, Luk JM, Lie AK, Kwong YL. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 362] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Matsue K, Kimura S, Takanashi Y, Iwama K, Fujiwara H, Yamakura M, Takeuchi M. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer. 2010;116:4769-4776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Francisci D, Falcinelli F, Schiaroli E, Capponi M, Belfiori B, Cecchini E, Baldelli F. Reactivation of hepatitis B virus replication due to cytotoxic therapy: a five-year prospective study. Tumori. 2012;98:220-224. [PubMed] |
| 21. | Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol. 2005;43:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Romero M, Madejón A, Fernández-Rodríguez C, García-Samaniego J. Clinical significance of occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1549-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Said ZN. An overview of occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1927-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (3)] |
| 24. | Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25:142-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Zaaijer HL, Torres P, Ontañón A, Ponte LG, Koppelman MH, Lelie PN, Hemert FJ, Boot HJ. Multiple surface antigen mutations in five blood donors with occult hepatitis B virus infection. J Med Virol. 2008;80:1344-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Henke-Gendo C, Amini-Bavil-Olyaee S, Challapalli D, Trautwein C, Deppe H, Schulz TF, Heim A, Tacke F. Symptomatic hepatitis B virus (HBV) reactivation despite reduced viral fitness is associated with HBV test and immune escape mutations in an HIV-coinfected patient. J Infect Dis. 2008;198:1620-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Sugauchi F, Tanaka Y, Kusumoto S, Matsuura K, Sugiyama M, Kurbanov F, Ueda R, Mizokami M. Virological and clinical characteristics on reactivation of occult hepatitis B in patients with hematological malignancy. J Med Virol. 2011;83:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |