Published online Sep 28, 2013. doi: 10.3748/wjg.v19.i36.6110
Revised: January 16, 2013
Accepted: March 8, 2013
Published online: September 28, 2013
Processing time: 302 Days and 7.6 Hours
Hepatic vein stenosis is a rare but serious complication following liver transplantation. Multiple modalities can be utilized to image the hepatic vasculature. Magnetic resonance venography (MRV) provides certain advantages over ultrasound, computed tomography angiography and digital subtraction venography. MRV utilizes the same imaging principles of magnetic resonance angiography in order to image the venous system. Blood pool contrast agents, specifically gadofosveset trisodium, allow for steady state imaging up to 1 h following injection, with improved visualisation of vital venous structures by utilising delayed steady state imaging. Additionally, the inherent physics properties of magnetic resonance imaging also provide excellent soft tissue detail and thus help define the extent of complications that often plague the post-liver transplant patient. This case report describes the use of gadofosveset trisodium in a patient with hepatic venous stenosis following liver transplantation. Initial venography failed to outline the stenoses and thus MRV using a blood pool contrast agent was utilised in order to delineate the anatomy and plan a therapeutic endovascular procedure.
- Citation: Strovski E, Liu D, Scudamore C, Ho S, Yoshida E, Klass D. Magnetic resonance venography and liver transplant complications. World J Gastroenterol 2013; 19(36): 6110-6113
- URL: https://www.wjgnet.com/1007-9327/full/v19/i36/6110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i36.6110
The inherent physics principles of magnetic resonance imaging (MRI) make it extremely sensitive to blood flow. MRI investigations of blood flow have been targeted at investigation of the arterial system; the same principles can be applied to the venous system for the diagnosis and planning of therapy targeted at improving, re-establishing or decreasing venous flow, depending on the underlying diagnosis. Digital subtraction venography (DSV) is regarded as the most accurate method for diagnosis of venous thrombosis as well as venous stenosis[1]. However, DSV has associated morbidity related to radiation dose, intravenous contrast injection and venous puncture.
Magnetic resonance angiography (MRA) uses specific acquisition sequences to highlight blood flow and is widely used to assist in the management of patients with vascular disease[2].
Magnetic resonance venography (MRV) utilizes the same imaging principles as MRA in order to image the venous system. Blood pool contrast agents such as gadofosveset trisodium (Ablavar, Lantheus, N Bellerica, Mass, United States) allow improved first pass imaging due to the increased relaxivity of the contrast agent[3]. The important clinical property that makes it ideal for venous imaging is the longer intravascular dwell time, allowing for steady state acquisitions up to 1 h following injection. Delayed, steady state imaging facilitates improved visualization of vital venous structures with a small decay in signal.
This case report presents a novel application of a blood pool contrast agent in planning an intervention in a liver transplant patient with perianastomotic hepatic vein stenosis.
A 47-year-old female was referred to the interventional radiology department with refractory ascites following a live donor liver transplant 10 mo previously for papillary cholangiocarcinoma.
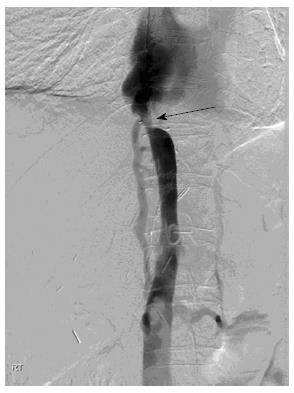
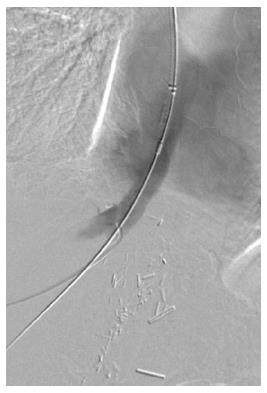
A transjugular cavogram demonstrated an inferior vena cava (IVC) stenosis, however we were unable to reflux contrast into the transplant hepatic veins (Figure 1). A decision was made to abandon the procedure and perform further imaging in order to demonstrate and delineate the hepatic venous anastomosis. An US study was originally performed but the result was deemed insufficient to delineate the anatomy prior to further intervention.
A contrast enhanced MR venogram (GE Healthcare 1.5T, Giles, United Kingdom) was performed using Gadofosveset Trisodium (Ablavar, Lantheus, N Bellerica, Mass, United States). Spoiled gradient echo sequences were obtained using first pass imaging (Coronal acquisition, Flip angle: 30°, FoV: 40 cm, section thickness 2.8 mm, frequency coding: 320, phase coding: 192, NEX: 0.75, K-space filling: centric, spatial resolution: 1.25/2.1) at 25 s and steady state imaging (Coronal acquisition, Flip angle: 20°, FoV: 40 cm, section thickness 2.8 mm, frequency coding: 320, phase coding: 320, NEX: 1, K-space filling: centric, spatial resolution: 1.25/1.25) at 2 min and 5 min after injection of 10 mL Gadofosveset Trisodium (1 mL/s) followed by a 30 mL saline flush (1 mL/s).
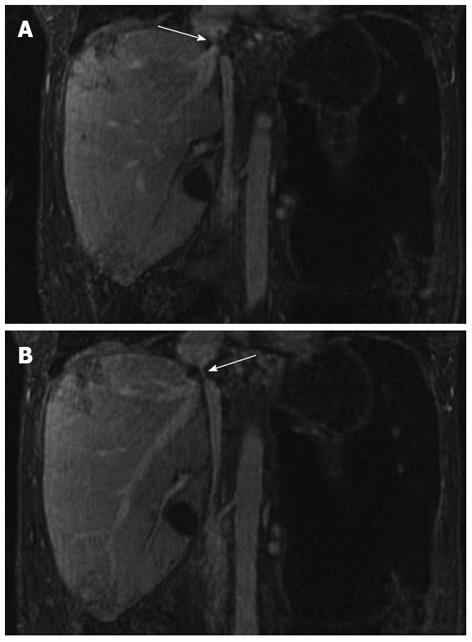
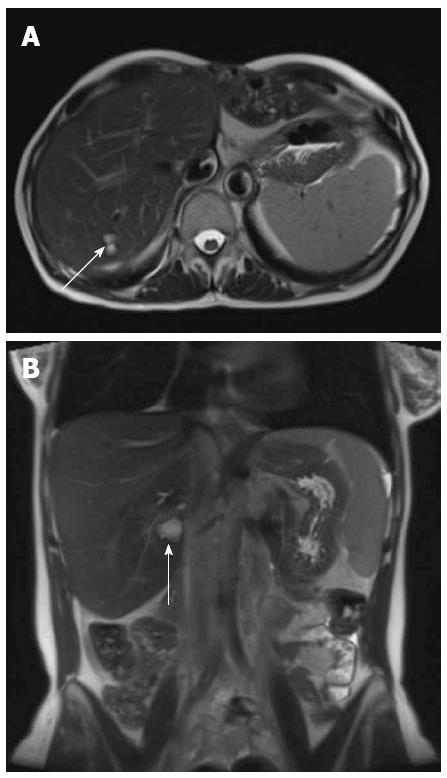
The first pass images demonstrated a patent hepatic arterial anastomosis, with no focal arterial pathology seen. The 5-min steady state imaging demonstrated a patent hepatic venous stenosis with a significant perianastomotic stenosis (Figure 2A) and an IVC stenosis (Figure 2B). No hepatic or portal venous thrombosis was seen.
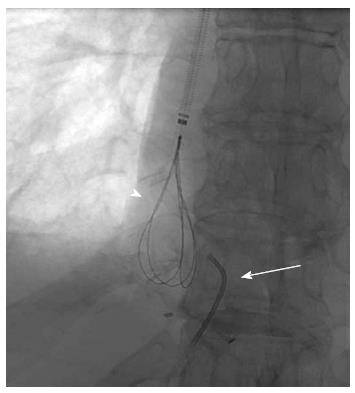
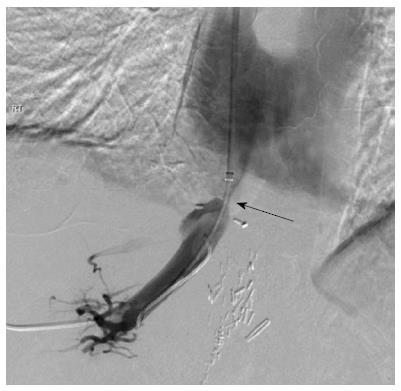
A combined transhepatic and transjugular hepatic venoplasty was performed following review of the imaging. Initially, an ultrasound guided transhepatic puncture of the right hepatic vein was performed and a guidewire inserted across the perianastomotic stenosis into the right atrium (Figure 3). In order to obtain through and through access to the hepatic venous and IVC stenosis, a loop snare was introduced from a jugular venous access site and the guidewire was snared and retracted through the jugular venous access site (Figure 4). The stenoses were dilated using a high-pressure balloon (Atlas, Bard Medical, Tempe, AZ, United States), with satisfactory angiographic appearances post venoplasty (Figure 5). Both stenoses were treated concurrently due to their close proximity. The hepatic venous/right atrial gradient decreased from 15 to 8 mmHg following the procedure; due to the close proximity of the hepatic venous and IVC stenoses, a separate gradient across the IVC stenosis was not felt to be practical given a second catheter would need to placed in the infra-hepatic IVC, in order to maintain the hepatic venous access. The patient was monitored post-procedure for four hours as per departmental protocol and discharged with no peri-procedural complications.
The patient was reviewed three months post treatment at our transplant clinic, where the ascites had completely resolved and she had resumed her normal daily activities. Doppler ultrasound demonstrated normal flow in the hepatic vein with no evidence of recurrent stenosis. A follow-up MRI was performed 1-year post venoplasty as part of annual surveillance to exclude tumor recurrence. The MRI demonstrated no evidence of ascites (Figure 6), which together with the patient’s normal liver function parameters and clinical examination, confirmed a satisfactory result. Follow-up venography was not felt to be necessary given the clinical response.
This technical report demonstrates the benefit of steady-state imaging using a blood pool contrast agent, Gadofosveset Trisodium. MRI provides exquisite soft tissue contrast and allows for the delineation of liver transplant anatomy either prior to embarking on a therapeutic intervention or if conventional angiography is unsuccessful as in this case. Cannulation of the stenosed hepatic vein proved technically challenging and without adequate reflux of contrast into the hepatic veins, cross-sectional imaging was required in order to plan a safe combined transhepatic and transjugular approach.
Hepatic vein stenosis is a rare but serious complication after liver transplantation[4]. It has been reported to occur from 1% to 6% of the cases. It can occur both acutely or in a delayed fashion. Acute hepatic vein stenosis is thought to be due to surgical technique whereas delayed stenosis is caused by intimal hyperplasia or perianastomotic fibrosis[4]. Clinical signs of hepatic vein stenosis include hepatic dysfunction, liver engorgement, ascites, and occasionally variceal bleeding. If the stenosis occurs at the level of the suprahepatic IVC as in this case, renal dysfunction and lower extremity edema may occur.
Gadofosveset Trisodium (Ablavar, Lantheus, N Bellerica, Mass, United States) reversibly binds to serum albumin, providing significantly higher relaxivity and prolonged intravascular enhancement compared to existing extracellular MR contrast agents[2]. The optimal dose is 0.03 mmol/kg, however in our experience greater than 10mL of contrast is not required in the majority of patients. The safety and efficacy of the contrast medium has been studied using a variety of clinical scenarios including aorto-iliac occlusive disease and pedal arterial disease which demonstrated the contrast agent to improve the rate of interpretability and improved diagnostic confidence[2].
The significantly longer intravascular time allows equilibrium imaging due to a prolongation of the T1 relaxivity, despite the inevitable dilution of the injected contrast medium after first pass imaging[5]. Prolonged T1 reduction thus allows diagnostic imaging of the vascular tree 45-60 min after injection of contrast. The increased imaging window can be used to acquire images with much higher spatial resolution without a significant loss of vessel to background contrast.
A rapid injection rate is not required. A saline chaser of at least 30 mL at a rate similar to the injection rate of the contrast medium ensures the full dose of contrast medium enters the central circulation without affecting the signal to noise ratio (SNR)[6].
The use of Gadofosveset Trisodium for problem solving in liver transplant patients, to our knowledge has not been described. The same parameters utilized for our traditional abdominal first pass and steady state imaging were utilized for image acquisition and provided the interventional radiology team with the anatomical detail required to perform the necessary venoplasty.
The favourable safety profile and the low dose required to obtain diagnostic images (0.03 mmol/kg) makes this agent preferable as to date, there have been no reported cases of NSF associated with gadofosveset trisodium[2,7].
In conclusion, MRV of transplant livers using a blood pool contrast agent is an accurate, noninvasive method of evaluating the vascular anastomoses (arterial, hepatic and portal venous systems). The longer intravascular dwell time of Gadofosveset Trisodium allows imaging with little loss of SNR. MRV with a blood pool contrast agent is a robust MRI technique, which eliminates contrast bolus timing, provides good temporal and spatial resolution and allows for dynamic image acquisition whilst compensating for differences in patient blood flow dynamics.
P- Reviewer Ong JP S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Lensing AW, Prandoni P, Büller HR, Casara D, Cogo A, ten Cate JW. Lower extremity venography with iohexol: results and complications. Radiology. 1990;177:503-505. [PubMed] |
| 2. | Goyen M. Gadofosveset-enhanced magnetic resonance angiography. Vasc Health Risk Manag. 2008;4:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1275] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 4. | Darcy MD. Management of venous outflow complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Hartmann M, Wiethoff AJ, Hentrich HR, Rohrer M. Initial imaging recommendations for Vasovist angiography. Eur Radiol. 2006;16 Suppl 2:B15-B23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Nissen JC, Attenberger UI, Fink C, Dietrich O, Rohrer M, Schoenberg SO, Michaely HJ. Thoracic and abdominal MRA with gadofosveset: influence of injection rate on vessel signal and image quality. Eur Radiol. 2009;19:1932-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Janus N, Launay-Vacher V, Karie S, Clement O, Ledneva E, Frances C, Choukroun G, Deray G. Prevalence of nephrogenic systemic fibrosis in renal insufficiency patients: results of the FINEST study. Eur J Radiol. 2010;73:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |