Published online Sep 28, 2013. doi: 10.3748/wjg.v19.i36.6069
Revised: May 30, 2012
Accepted: June 8, 2012
Published online: September 28, 2013
AIM: To investigate the effects of diammonium glycyrrhizinate (Gly) on portal hypertension (PHT) in isolated portal perfused rat liver (IPPRL) with carbon tetrachloride (CCl4)-induced chronic hepatitis.
METHODS: PHT model was replicated with CCl4 in rats for 84 d. Model was identified by measuring the ascetic amounts, hepatic function, portal pressure in vivo, splenic index, and pathological alterations. Inducible nitric oxide synthase (iNOS) in liver was assessed by immunohistochemistry. IPPRLs were performed at d0, d28, d56, and d84. After phenylephrine-induced constriction, Gly was geometrically used to reduce PHT. Gly action was expressed as median effective concentration (EC50) and area under the curve (AUC). Underlying mechanism was exploited by linear correlation between AUC values of Gly and existed iNOS in portal triads.
RESULTS: PHT model was confirmed with ascites, splenomegaly, serum biomarkers of hepatic injury, and elevated portal pressure. Pathological findings had shown normal hepatic structure at d0, degenerations at d28, fibrosis at d56, cirrhosis at d84 in PHT rats. Pseudo lobule ratios decreased and collagen ratios increased progressively along with PHT development. Gly does dose-dependently reduce PHT in IPPRLs with CCl4-induced chronic hepatitis. Gly potencies were increased gradually along with PHT development, characterized with its EC50 at 2.80 × 10-10, 3.03 × 10-11, 3.77 × 10-11 and 4.65×10-11 mol/L at d0, d28, d56 and d84, respectively. Existed iNOS was located at hepatocyte at d0, stellate cells at d28, stellate cells and macrophages at d56, and macrophages in portal triads at d84. Macrophages infiltrated more into portal triads and expressed more iNOS along with PHT development. AUC values of Gly were positively correlated with existed iNOS levels in portal triads.
CONCLUSION: Gly reduces indirectly PHT in IPPRL with CCl4-induced chronic hepatitis. The underlying mechanisms may relate to rescue NO bioavailability from macrophage-derived peroxynitrite in portal triads.
- Citation: Zhao X, Deng B, Xu XY, Yang SJ, Zhang T, Song YJ, Liu XT, Wang YQ, Cai DY. Glycyrrhizinate reduces portal hypertension in isolated perfused rat livers with chronic hepatitis. World J Gastroenterol 2013; 19(36): 6069-6076
- URL: https://www.wjgnet.com/1007-9327/full/v19/i36/6069.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i36.6069
Portal hypertension (PHT) is a fetal complication in patients with advanced chronic hepatitis[1]. Some changes are reversible in PHT pathogenesis, such as an increase of hepatic vascular resistance and an elevation of portal hyperemia[2]. Therefore, it is possible to develop drugs for PHT therapy.
Insufficient intrahepatic nitric oxide (NO) bioavailability is involved in PHT development[3]. Macrophages in portal triads generate peroxynitrite via inducible nitric oxide synthase (iNOS)[4] and superoxide via NADPH oxidases[5]. Under chronic hepatitis, macrophages infiltrated more into portal triads and expressed more iNOS[6,7]. Peroxynitrite was derived from NO and superoxide, to reduce NO bioavailability[3,4]. The decreased NO availability leads to an increase in intrahepatic portal resistance, resulting in PHT[3,4].
Diammonium glycyrrhizinate (Gly), a molecule derived from a medical plant of Radix glycyrrhizae, is effective in treatment of PHT patients[8] and animals[9,10], but the underlying mechanisms are still unclear[9-11]. In our previous studies, we demonstrated that Gly lower PHT in isolated portal perfused rat livers (IPPRLs) at physiological status[12].
Suitable perfuse velocities were designated as anatomic preloads in IPPRLs with CCl4-induced chronic hepatitis at four stages[13]. A pharmacodynamic model of PHT had been developed in IPPRLs with chronic hepatitis, as median effective concentration (EC50) values of phenylephrine and acetylcholine[14]. This model makes it possible to evaluate candidates for PHT therapy. Furthermore, several studies have shown that Gly enhances NO generation from inflammatory macrophages[15,16], limits superoxide release and increases NO bioavailability[16].
In this study, we investigated the effects of Gly on PHT in the IPPRLs with CCl4-induced chronic hepatitis, and on NO bioavailability from the macrophages in portal triads.
Male Wistar rats (200 ± 13 g) were obtained from Animal Centre of Chinese Academy of Medical Sciences. Rats were maintained in a Special Pathogen Free laboratory, with a 25.0 ± 0.2 °C, a 12-h/12-h light/dark photoperiod and 45% ± 2% humidity. All rats were fed standard rodent pellets and allowed free access to filtered water. All experiment procedures were performed in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China.
Carbon tetrachloride (CCl4, CAS 56-23-5), olive oil (CAS 8001-25-0) and heparin sodium (MW 12000, CAS 9041-08-1) were obtained from Sinopharm Chemical Reagent Company; Acetylcholine chloride (CAS 60-31-1) and phenylephrine hydrochloride (CAS 61-76-7) were from Sigma (United States); Diammonium Gly (CAS 79165-06-3) were from Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
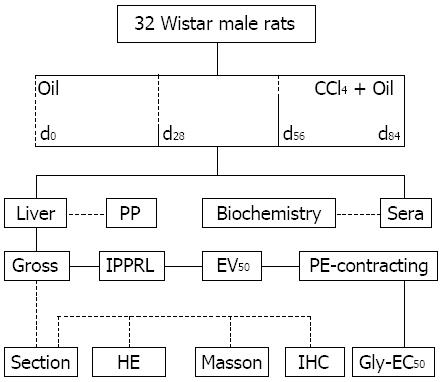
As described previously[13,14,17], PHT model was replicated by subcutaneous injection with 3 mL/kg mixture of 40% (v/v) CCl4 in olive oil, twice weekly for 0, 4, 8 and 12 wk; age-matched animals did with olive oil along as vehicle control (Figure 1). Forty-eight hours after last injection, rat was weighted (Wb) and anesthetized with pentobarbital sodium (50 mg/kg) subcutaneously, a midline incision was made to open abdominal cavity, soak up ascitic samples, expose liver vessels, measure portal pressure, collect blood sample, and canalize hepatic artery, portal vein, hepatic vein. The remained blood in the isolated livers was eliminated with perfusate containing 20.0 μg/mL of heparin sodium through hepatic artery.
As described previously[13,14,17], the velocity in each IPPRL was finely controlled by a quantified roller pump. The perfuse pressure was continuously recorded with the Powerlab linked to a computer with a pressure transducer immediately ahead of the portal inlet cannula. The global viability of portal perfused livers was assessed with gross appearance, a stable perfusate pH (7.40 ± 0.10), a stable perfuse pressure, and active response to acetylcholine.
As described previously[13,14,17], exudative liquid was soaked up by dried soft paper in a tube (W0). Before (W1) and after (W2) the wet paper with exudation was dried enough, the paper with tube was weighed exactly. Basing on the body weight (Wb), exuded water (EWR) and dried mass ratios (EDMR) were calculated as EWR = (W1 - W2)/Wb× 1000 and EDMR = (W2 - W0)/Wb× 1000, expressed as exudative weight (g) per kilogram body weight (g/kg).
Sera were separated by centrifugation at 300 g for 5 min and were stored at -80 °C. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein (TP) and albumin (Alb) were determined by an autoanalyzer (Hitachi 7060; Hitachi, Japan) with commercial kits (Biosino Biotechnology and Science, China) according to the manufacturer’s instructions.
Organ indexes: Liver, spleen and kidneys were weighed (Wi) and the organ indexes (OIi) were calculated for each rat (OIi = Wi/Wb× 100).
Histological morphometry: After perfusion as described previously[13,14], a portion of left lobe from each liver was fixed in 10% buffered formaldehyde, embedded in paraffin, cut as 6 μm sections, and stained with haematoxylin-eosin (HE) and Masson’s trichrome (Masson). Images were acquired with a Digital Pathology system (Hamamatsu, Japan). Ten fields from each liver were randomly selected, the percentage of lobules (ratio of lobule area per total analyzed field area × 100, under × 10, in HE) and one-ten thousandth of collagen (ratio of collagen area per total analyzed field area × 10000, under × 40, in Masson) were measured using Image Pro Plus analysis system 7.0.1 (No41N70000-60555, Media cybernetics, United States); the average of values from ten random fields generated a datum.
Immunohistochemical morphometry: Formalin-fixed, paraffin-embedded, 6 μm sections were used. Rat iNOS was stained with a rabbit polyclonal antibody (1:200, Santa Cruz Biotechnology) and an avidin-biotin peroxidase immunostaining kit with diaminobenzidine (Boster, Wuhan, China). One set of sections was counterstained with hematoxylin for observing cellular location; the other set did not for quantifying existed levels. Microscopic images under × 40 were acquired with Digital Pathology System. Mean optical density (OD), positive staining area (AP) and total observed area (AT) were measured using Image Pro Plus Analysis System. Levels of existed iNOS were represented as diaminobenzidine-OD average per volume [OD × (AP/AT)3/2]; the average of values from ten random fields generated a datum.
As described previously[13,14], pharmacodynamical model in IPPRLs with chronic hepatitis was modified delicately with structural and functional preloads at d0, d28, d56 or d84 in PHT development. Perfused velocity as structural preload was defined as 3935.50, 4720.63, 4753.35, or 5164.16 μL/min[13]; Concentration of phenylephrine as functional preloads was designated as 1.69 × 10-10, 2.64 × 10-10, 5.82 × 10-10 or 8.24 × 10-10 mol/L[14].
Portal pressure in each IPPRL was initially maintained at defined velocity for 30 min[13]. Maintained pressure was near to portal pressure in vivo. Elevated pressure was stabilized for 10 min after phenylephrine-induced constriction at designated concentration[14]. Elevated pressure was considered as the baseline for analyzing Gly to relax hepatic portal venules. Cumulative geometric concentrations of Gly (10-12-10-6 mol/L, k = 0.10) were finally used in recirculating perfusate to reduce elevated portal pressure. Gly concentration-response curve was regressed from the cumulative concentrations and the changed percentage of perfused pressure from the baseline of phenylephrine constriction.
Data are expressed as mean ± SD in each stage. Unpaired t test was used, P < 0.05 was considered significance. Equation, EC50 with its 95%CI and area under the curve (AUC) of Gly were calculated by regression analysis using Graph-Pad Prism 4 (Graph-Pad Software) in non-linear fit and various slopes. EC50 values of Gly were regressed linearly with the duration (0, 4, 8 and 12 wk) in chronic hepatitis; AUC values of Gly did with the levels of existed iNOS in portal triads.
Ascites: As one of PHT consequences, exuded watery and dried mass ratios increased progressively along with PHT development (P < 0.01, Table 1).
| Groups | Exudations [g/(100g)] | Organ indexes [g/(100g)] | |||
| Water | Dried mass | Liver | Spleen | Kidneys | |
| d00 | 0.223 ± 0.091 | 0.490 ± 0.260 | 2.801 ± 0.347 | 0.163 ± 0.040 | 0.731 ± 0.096 |
| d28 | 0.372 ± 0.127b | 1.250 ± 0.210b | 5.936 ± 1.081b | 0.236 ± 0.037b | 0.854 ± 0.095a |
| d56 | 0.791 ± 0.134bd | 1.540 ± 0.150bd | 4.472 ± 0.909bd | 0.292 ± 0.103b | 0.861 ± 0.117a |
| d84 | 2.267 ± 0.732bdf | 3.590 ± 1.610bdf | 3.037 ± 0.349df | 0.409 ± 0.095bde | 1.071 ± 0.117bdf |
Organ indexes: Hepatic indexes were the lowest at d0, the highest at d28, higher at d56 and lower at d84. Splenic or renal indexes increased gradually from d0 to d84 (P < 0.01, Table 1).
Hepatic function: ALT and AST activities in sera at d28 increased from that at d0, then relieved at d56 and d84. ALP activities at d28 and d56 increased from that at d0, then relieved at d84; TP and Alb levels at d28 and d56 decreased from that at d0, then relieved at d84 (P < 0.05, Table 2).
| Groups | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | TP (g/L) | Alb (g/L) |
| d00 | 49.63 ± 14.03 | 124.13 ± 20.20 | 72.88 ± 14.58 | 63.63 ± 4.26 | 33.12 ± 1.97 |
| d28 | 237.13 ± 107.91b | 503.25 ± 235.12b | 318.75 ± 147.81b | 56.84 ± 5.43b | 25.93 ± 3.16b |
| d56 | 160.25 ± 42.39bc | 411.63 ± 143.51b | 363.50 ± 170.36b | 54.91 ± 5.27b | 24.13 ± 4.25b |
| d84 | 230.00 ± 58.58bf | 475.50 ± 201.02b | 303.00 ± 225.94b | 58.36 ± 7.92 | 26.98 ± 6.86a |
PHT: The portal pressures in vivo increased progressively from d0 to d84 in PHT development. Elevated portal pressures (> 20 mmHg) at d56 and d84 reached to the diagnostic criteria of clinic PHT (P < 0.01, Table 3).
| Groups | PP (mmHg) | Lobule | Collagen | iNOS-OD/V |
| d00 | 6.648 ± 2.235 | 0.564% ± 0.022% | 0.048% ± 0.013% | 0.165 ± 0.011 |
| d28 | 9.225 ± 2.114b | 0.511% ± 0.031%b | 0.248% ± 0.120%b | 0.197 ± 0.005b |
| d56 | 24.724 ± 3.368bd | 0.230% ± 0.024%bd | 1.974% ± 0.637%bd | 0.132 ± 0.010bd |
| d84 | 26.752 ± 3.263bdf | 0.134% ± 0.009%bdf | 5.925% ± 1.761%bdf | 0.236 ± 0.040bdf |
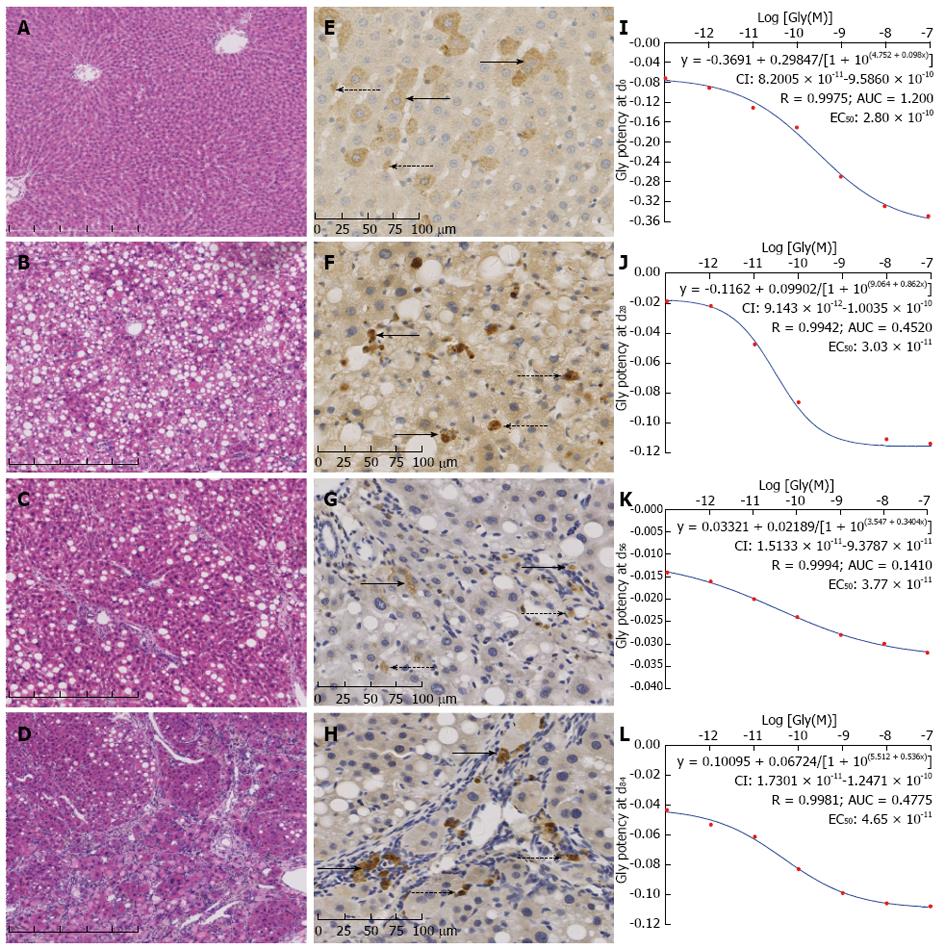
Pathologic changes: At d0, histological observation showed normal hepatic structure; some droplet steatosis appeared in hepatocytes, collagen located only at portal triads (Figure 2A). At d28, hepatic steatosis and cellular swellings were observed pathologically; hepatic sinusoids were severely narrowed by enlarged hepatic cords (Figure 2B). At d56, hepatic fibrosis had been demonstrated with more deposited collagen, relieved enlarged hepatic cords, and widened hepatic sinusoids. Deposited collagen had extended into lobules from portal triads, separated lobules incompletely; therefore circulative directions had not changed in hepatic sinusoids (Figure 2C). At d84, hepatic cirrhosis had been demonstrated with deposited collagen separated through the lobules, pseudo lobules formed all over, circulative directions had completely deranged in hepatic sinusoids (Figure 2D).
Pseudo lobule ratio: Pseudo lobule ratios decreased progressively from d0 to d84 in PHT development (P < 0.01, Table 3).
Collagen ratio: Collagen ratios increased progressively from d0 to d84 in PHT development (P < 0.01, Table 3).
Localization: At d0, iNOS was mainly located in hepatocytes and scattered stellate cells of lobules (Figure 2E). At d28, positive granules were thinner in scattered hepatocytes, thicker in the stellate cells in lobules (Figure 2F). At d56, granules in hepatocytes were completely quenched; positive granules limited within macrophages in portal triads and stellate cells in lobules (Figure 2G). At d84, thick positive granules located in macrophages in portal triads and stellate cells in pseudo lobules (Figure 2H).
Quantification: Levels of existed iNOS per volume in portal triads at d28, d56 and d84 were significantly increased by 19.39%, -20.00%, and 43.03% from that at d0 (P < 0.01, Table 3); these at d56 and d84 were decreased by 32.99% and increased by 19.80% from that at d28, respectively (P < 0.01). So did it increased by 78.79% at d84 from that at d56 (P < 0.01).
Dose-effective relation: Gly had the same shape of dose-effective curves at d0 (Figure 2I), d28 (Figure 2J), d56 (Figure 2K), d84 (Figure 2L), respectively, in PHT development. The equations of effective potency to reduce PHT were y = -0.3691 + 0.29847/[1 + 10(4.75212 + 0.09841x)] (R = 0.9975, P < 0.01), y = -0.1162 + 0.09902/[1 + 10(9.064 + 0.8616x)] (R = 0.9942, P < 0.01), y = -0.03321 + 0.02189/[1 + 10(3.547 + 0.3404x)] (R = 0.9994, P < 0.01), and y = 0.10095 + 0.06724/[1 + 10(5.5121 + 0.5336x)] (R = 0.9981, P < 0.01); EC50 values with their 95%CIs were 2.80 × 10-10 (8.20 × 10-11 - 9.59 × 10-10), 3.03 × 10-11 (9.14 × 10-12 - 1.00 × 10-10), 3.77 × 10-11 (1.51 × 10-11 - 9.38 × 10-11), and 4.65 × 10-11 (1.73 × 10-11 - 1.25 × 10-10) mol/L, respectively.
Time-effective relation: Pathological development affected on Gly potency to reduce PHT. Therefore, EC50 values of Gly (Y × 10-11 mol/L) related linearly with durations of PHT (y = 0.0289x + 2.1967, R = 0.9985, P < 0.05).
Mechanic connection: Existed iNOS was involved in Gly potency to reduce PHT. Therefore, AUC values of Gly regressed linearly with existed iNOS levels in portal triads at d28, d56, d84 in PHT development (y = 0.2669x + 0.0931, R = 0.9517, P < 0.05).
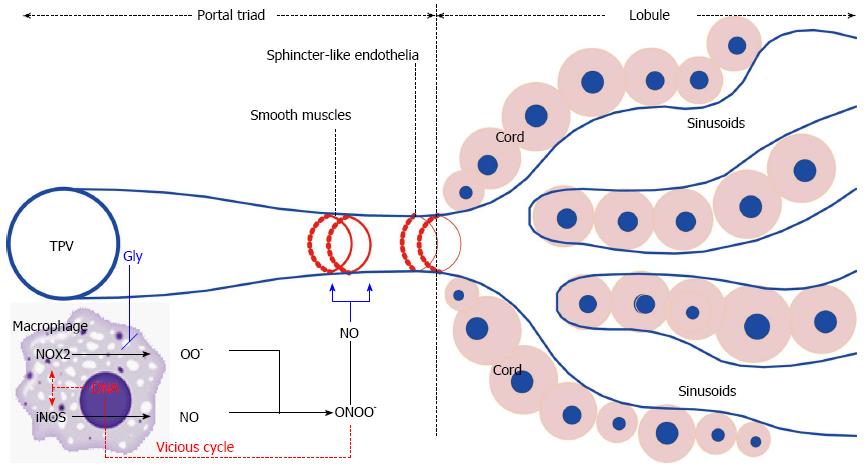
PHT is a common complication in patients with advanced chronic hepatitis[1]. It is possible to develop therapeutic candidates against reversible mechanisms in PHT pathogenesis[1,2]. In this study, we use PHT model in IPPRL with CCl4-induced chronic hepatitis to investigate the potential effects of Gly on PHT, and to explore further the possible underlying mechanisms. NO does directly decrease PHT[2,18]. However, eNOS-derived NO precedes systemic hyperemia[19], and iNOS-derived NO reduces itself bioavailability via peroxynitrite[4]. Extracellular superoxide dismutase in cirrhosis inhibits peroxynitrite generation, increases NO bioavailability, and reduces PHT degree[3]. In this regard, local NO bioavailability could be drug targets for PHT. Intrahepatic resistance is mainly originated from terminal portal venules[5]. Macrophage-derived iNOS in portal triads contributed to more this resistance. Gly is a molecular from a medical plant[6] used for treating PHT[7-12], it can increase NO bioavailability[12,16].
Three findings were obtained in this study. (1) A rat model of PHT with chronic hepatitis was confirmed by ascites, portal pressure, splenic index, serum biomarkers and pathological changes. Four stages of CCl4-induced chronic hepatitis were hepatic degenerations, fibrosis, cirrhosis only, cirrhosis with PHT[13,14,17]. CCl4 is transformed into trichloromethyl via cytochrome P450 2E1[17,20], trichloromethyl initiated lipid peroxidation consequently. Therefore, free radicals injury is the major mechanism involved in CCl4-induced chronic hepatitis, which is associated with the pathogenesis of human disease; (2) As a drug candidate for PHT, Gly had the similar S shapes of dose-effective curves to reduce PHT in IPPRLs with chronic hepatitis at various stages. EC50 value of Gly at d0 was nearly 10 times higher than these at d28, d56, and d84, indicating that Gly may be more effective on PHT than on physiological portal pressure. EC50 values of Gly were geometrically increased at d28, d56, and d84, suggesting that the portal responsibility in pathological status may be gradually decreased along with PHT development; and (3) Existed iNOS disappeared gradually in lobules, and strengthened continuously in portal triads in PHT development. AUC values of Gly were positively correlated with levels of existed iNOS in portal triads.
Gly had been shown to cause renal and central systemic hypertension via inhibiting type 2 11β-hydroxysteroid dehydrogenase[21]. Possible mechanisms of Gly to relax directly portal vein included inhibiting gap junction intercellular communications[22] and activating peroxisome proliferator-activated receptor[23]. As hepatic artery being ligated in this study, IPPRL was made to evaluate the effects of Gly on portal venules alone. Under this condition, portal resistance is mainly originated from both of smooth muscle cells in terminal portal venules and sphincter-like endothelia at hepatic sinusoid inlets[24]. Different from extrahepatic vasodilators[25], Gly relaxes indirectly portal vein via improving intrahepatic NO bioavailability[26]. In this study, AUC values of Gly depend on macrophage-derived iNOS in portal triads. Macrophages infiltrated in portal triads expressed more iNOS and oxidative enzymes, consequently generated more peroxynitrite to decrease NO bioavailability[15]. Gly reduces superoxide[27], decreases peroxynitrite, increases NO bioavailability[4], and relieves PHT (Figure 3)[24].
Our results suggested that Gly reduces PHT, which might explain therapeutic effectiveness of Gly-contained prescriptions for patients with PHT ascites. It is also a clue to find more effective candidates related to macrophage-generated iNOS or NADPH oxidase, which partially contributes to the effects of Gly to reduce PHT. Gly or its more effective derivates should be exploited as candidates to maintain NO bioavailability in terminal portal venules, especially against free radical injury.
Portal hypertension (PHT) is a common complication of chronic hepatitis, with significant morbidity and mortality in clinic. It is important to develop new drugs for this disease. A sensitive pharmacological model has been established for PHT in the isolated perfused rat livers, which supplies suitable methods for evaluating candidates for PHT.
Diammonium glycyrrhizinate (Gly) is one of the representative candidates for PHT in experiment and clinic. It relaxes portal veins in isolated portal perfused rat livers at physiological status. It is also believed that the inducible nitric oxide synthase (iNOS) expressed from infiltrated macrophages in portal triads is important to increase portal resistance. The sensitive pharmacological model was used here to investigate the effect of Gly on PHT, and to exploit further the possible mechanisms of its actions.
The intrahepatic portal resistance of PHT originates mainly from the smooth muscle cells in terminal portal venules and the sphincter-like endothelia at hepatic sinusoid inlets. Gly is effective for reducing PHT in isolated portal perfused rat livers with chronic hepatitis, with the similar S shape and different potency from its dose-effective curves. As PHT advanced, it was found that more macrophages infiltrated in portal triads and expressed more iNOS. Therefore, macrophage iNOS in portal triads involves in the pathogenesis, on which with Gly acted. The mechanisms of Gly for PHT in isolated portal perfused rat liver with chronic hepatitis are related to increase of nitric oxide (NO) bioavailability.
Gly reducing PHT might explain the actions of medical plants in Chinese prescriptions. More effective derivate might be generated from Gly molecule structure. NO bioavailability is a candidate target for PHT.
Peroxynitrite is the anion with the formula ONOO-, an unstable structural isomer of nitrate. It derives from both of NO and O2- in activated macrophages at portal triads with chronic hepatitis. It damages all biomolecules in cells, such as DNA and proteins. Peroxynitrite could let NO availability decrease severely.
The authors investigated the effect of Gly on PHT. They found that Gly reduce PHT in isolated portal perfuse rat livers with chronic hepatitis. Such action may explain Chinese medical herbs for PHT. It also suggests that NO availability involve in the pathogenesis of PHT.
P- Reviewers Chowdhury P, Ciaccio EJ, Maeda S, Takabe K S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Pinzani M, Vizzutti F. Fibrosis and cirrhosis reversibility: clinical features and implications. Clin Liver Dis. 2008;12:901-913, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Laviña B, Gracia-Sancho J, Rodríguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, García-Pagán JC. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Kajita M, Murata T, Horiguchi K, Iizuka M, Hori M, Ozaki H. iNOS expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H1021-H1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: Part I. General considerations and redox biology in hepatitis. J Surg Res. 2010;162:95-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 979] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 7. | Robinson MA, Baumgardner JE, Otto CM. Oxygen-dependent regulation of nitric oxide production by inducible nitric oxide synthase. Free Radic Biol Med. 2011;51:1952-1965. [PubMed] [DOI] [Full Text] |
| 8. | Vilar Gomez E, Sanchez Rodriguez Y, Torres Gonzalez A, Calzadilla Bertot L, Arus Soler E, Martinez Perez Y, Yasells Garcia A, Abreu Vazquez Mdel R. Viusid, a nutritional supplement, increases survival and reduces disease progression in HCV-related decompensated cirrhosis: a randomised and controlled trial. BMJ Open. 2011;1:e000140. [PubMed] |
| 9. | Jiang J, Du Q, Han L, Mashoufi A, Li P. Effect of Glytan on the Expression of Nicotinic Acetylcholine Receptor a7 in Portal Hypertension Rats. World Science and Technology. 2010;12:383-386. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Liu SF, Cai DY, Li PT, Xiang PR. Study on compatibility of Ganshen decoction in moderateing Hepatic Fibrosis. Zhonghua Zhongyiyao Zazhi. 2005;20:373-375. |
| 11. | Ren L, Zang X, Deng X, Liu S, Liu Y, Cai D. Wave characteristics of portal pressure and their affected factors. Beijing Zhongyiyao Daxue Xuebao. 2006;29:840-843. |
| 12. | Zhou H, Wang SX, Zhang T. Effects of salvianolic acid B and diammonium glycyrrhizinate on portal pressure in rats. Zhongguo Zhongxiyi Jiehe Zazhi. 2010;30:1084-1086. [PubMed] |
| 13. | Xu X, Zhang T, Zhou H, Zhao X, Zhang T, Yin H, Li T, Li P, Cai D. Portal pressure determined by perfusion velocity in isolated portal perfused rat livers with chronic hepatitis. Shijie Huaren Xiaohua Zazhi. 2010;18:2745-2749. |
| 14. | Zhang T, Xu XY, Zhou H, Zhao X, Song M, Zhang TT, Yin H, Li T, Li PT, Cai DY. A pharmacodynamic model of portal hypertension in isolated perfused rat liver. World J Gastroenterol. 2012;18:472-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 16. | Kato T, Horie N, Hashimoto K, Satoh K, Shimoyama T, Kaneko T, Kusama K, Sakagami H. Bimodal effect of glycyrrhizin on macrophage nitric oxide and prostaglandin E2 production. In Vivo. 2008;22:583-586. [PubMed] |
| 17. | Xu Y, Cai D, Tang C. Pathogenesis of cirrhostic portal hypertension induced with CCl4. Shijie Huaren Xiaohua Zazhi. 2005;13:235-238. |
| 18. | Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829-837, 837a-837d. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2678] [Cited by in RCA: 2901] [Article Influence: 223.2] [Reference Citation Analysis (0)] |
| 19. | Theodorakis NG, Wang YN, Wu JM, Maluccio MA, Sitzmann JV, Skill NJ. Role of endothelial nitric oxide synthase in the development of portal hypertension in the carbon tetrachloride-induced liver fibrosis model. Am J Physiol Gastrointest Liver Physiol. 2009;297:G792-G799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [PubMed] |
| 21. | Gomez-Sanchez EP, Gomez-Sanchez CE. Central hypertensinogenic effects of glycyrrhizic acid and carbenoxolone. Am J Physiol. 1992;263:E1125-E1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Dhein S. Pharmacology of gap junctions in the cardiovascular system. Cardiovasc Res. 2004;62:287-298. [PubMed] [DOI] [Full Text] |
| 23. | Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S. Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor {gamma} agonists in colon cancer cells. Mol Cancer Ther. 2007;6:1588-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 196] [Reference Citation Analysis (0)] |
| 24. | Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 25. | Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 782] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 27. | Oh HM, Lee S, Park YN, Choi EJ, Choi JY, Kim JA, Kweon JH, Han WC, Choi SC, Han JK. Ammonium glycyrrhizinate protects gastric epithelial cells from hydrogen peroxide-induced cell death. Exp Biol Med (Maywood). 2009;234:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |