Published online Sep 21, 2013. doi: 10.3748/wjg.v19.i35.5828
Revised: June 24, 2013
Accepted: July 9, 2013
Published online: September 21, 2013
Processing time: 142 Days and 20.7 Hours
AIM: To investigate whether transforming growth factor-β1 (TGF-β1) signaling pathway is involved in the pathogenesis of primary biliary cirrhosis (PBC).
METHODS: A murine model of PBC was developed by injection of polyinosinic polycytidylic acids (poly I: C) in C57BL/6 mice, and the liver expressions of TGF β1, TGF-β receptor I (TβRI), TGF-β receptor II (TβRII), p-Smad2/3, monoclonal α-smooth muscle actin antibody (α-SMA) and α1 (I) collagen in the mouse model and control mice were evaluated by immunohistochemistry, immunoblotting and real-time polymerase chain reaction (RT-PCR). Lymphocyte subsets in liver were analyzed using flow cytometry.
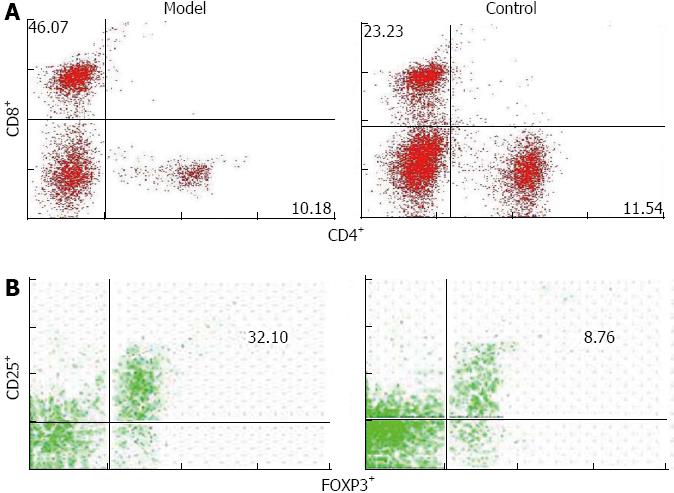
RESULTS: The mouse model had several key phenotypic features of human PBC, including elevated levels of alkaline phosphatase, antimitochondrial antibodies, portal bile ducts inflammation, and progressive collagen deposition. Compared with control mice, protein and mRNA levels of TGF β1, TβRI, TβRII, p-Smad2/3, α-SMA and α1 (I) collagen in liver (1.7 ± 0.4 vs 8.9 ± 1.8, 0.8 ± 0.2 vs 5.1 ± 1.5, 0.6 ± 0.01 vs 5.1 ± 0.1, 0.6 ± 0.3 vs 2.0 ± 0.3, 0.9 ± 0.4 vs 3.4 ± 0.6, 0.8 ± 0.4 vs 1.7 ± 0.3, 1.1 ± 1.2 vs 11.8 ± 0.6, P < 0.05), and the total number and percentage of CD4+ CD25+ FOXP3+ and CD8+ lymphocytes (0.01 ± 0.001 vs 0.004 ± 0.00, 0.12 ± 0.04 vs 0.52 ± 0.23, P < 0.01) were higher in the mouse model.
CONCLUSION: TGFβ1 might play a dual role in the development of PBC: it suppresses inflammatory response but operates to enhance fibrogenesis. The aberrant activity of TGF-β1 signaling contributes to the development of PBC.
Core tip: Primary biliary cirrhosis (PBC) is an autoimmune liver disease. Recent studies suggest that transforming growth factor-β1 (TGF-β1) signaling pathway might play an important role in the pathogenesis of PBC. However, whether TGF-β1 signaling pathway is involved in the development of PBC is still unknown. The studies have provided new data of TGF-β1 signaling pathway involving the pathogenesis of PBC, which will pose significant impact on our understanding of the pathogeneses of PBC. TGF-β1 signaling pathway is a potential target for PBC treatment.
- Citation: Liu B, Zhang X, Zhang FC, Zong JB, Zhang W, Zhao Y. Aberrant TGF-β1 signaling contributes to the development of primary biliary cirrhosis in murine model. World J Gastroenterol 2013; 19(35): 5828-5836
- URL: https://www.wjgnet.com/1007-9327/full/v19/i35/5828.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i35.5828
Primary biliary cirrhosis (PBC) is a progressive autoimmune liver disease characterized by portal inflammation and immune-mediated destruction of the intrahepatic bile ducts. Damage of bile ducts is associated with cholestasis, and eventually leads to liver failure[1].
Cytokines are involved in cell-to-cell interaction via specific receptors, inflammatory response amplification, immune regulation and fibrogenesis. Transforming growth factor-β1 (TGF-β1) is a prominent antiproliferative and profibrogenic cytokine that signals through TGF-β receptor II (TβRII), and receptor I (TβRI), that in turn phosphorylate Smads at the mad homology 2 domain[2]. Perturbation of TGF-β1 signaling has been implicated in several developmental disorders and in various human diseases including cancer, fibrosis and autoimmune disease[3-5]. Mice transgenic of a dominant negative form of TβRII, under the CD4 promoter lacking the CD8 silencer[6], spontaneously developed features characteristic of PBC[7]. A compromised cytoarchitecture and polarized trafficking of TGF-β1 signaling molecules including embryonic liver fodrin and Smad3 were also noted in the pathogenesis of PBC[8]. Moreover, TGF-β1 was a marker for fibrosis and reflected severity of disease in patients with PBC[9,10]. Therefore, aberrant TGF-β1 signaling contributes to a loss of self tolerance to autoantigens in the liver, which in turn leads to autoimmunity.
We developed an animal model of PBC by polyinosinic polycytidylic acids (poly I: C) injection in genetically susceptible C57BL/6 female mice that would allow the analysis of the early cellular events of PBC[11,12]. We found that TGFβ1 played a dual role in the development of PBC: it suppressed inflammatory response but operated to enhance fibrogenesis. The aberrant TGF-β1 signaling contributed to the development of PBC.
Adult 6-8 wk-old C57BL/6J (H-2b) mice were purchased from Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC, Beijing, China). They were maintained separately at the Department of Laboratory Animal, Peking Union Medical College Hospital (PUMCH), China, under controlled conditions (22 °C, 55% humidity, and 12 h day/night). All animals received adequate care according to good laboratory practice guidelines. The study protocol was approved by Committee of Animal Experimentation, PUMCH and CAMS. Female C57BL/6 mice were injected with 5 mg/kg poly I: C (Invivogen Co. San Diego, United States) or normal saline (NS) as controls twice a week for 24 consecutive weeks, according to the protocol of Okada[11].
At weeks 8 and 24, six mice of each group were sacrificed by cervical dislocation. Livers were fixed in buffered formalin (10%). Sera and tissue specimens were stored at -80 °C. The serum levels of alkaline phosphatase (ALP) and alanine amino-transferase (ALT) were measured by commercially available kit (WAKO Pure Chemical Industry, Osaka, Japan) exactly according to the manufacturer’s protocol.
Antimitochondrial antibodies (AMA) and M2 were detected by the commercial immunofluorescence (IF), enzyme-linked immunosorbent assay (ELISA) kits (EUROIMMUN, Germany) and immunoblotting kits (IMTEC Corporation, Germany), according to the manufacturer’s protocol. Fluorescein isothiocyanate (FITC) or horseradish peroxidase (HRP)-conjugated monoclonal goat anti-mouse IgM or IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, United States) was used as the secondary antibody. Plates were read at 450 nm with a microplate reader (Bio-RAD Model 550, Tokyo, Japan). Sera with optical density (OD) values greater than the mean ± 2SD from the negative controls were regarded as AMA positive.
Formalin-fixed, paraffin-embedded tissue sections were cut into 5 μm slices for routine hematoxylin and eosin staining. Tissues were also stained with Azan to detect collagen deposition[13]. Briefly, sections were deparaffinized in xylene, dehydrated, rehydrated in distilled water, immersed in 5% potassium dichromate solution for 30 min, and stained with azocarmine G for 30 min. Sections were then immersed in 3% 12 tungsto (IV) phosphoric acid n-hydrate solution for 1 h and stained with aniline blue-orange G for 20 min.
Antibodies against CD4 (1/200; L3T4, eBioscience) and CD8 (1/100; 53-6.7; Biolegend) were used for immunohistochemical staining of the portal tract infiltrates. Anti-cytokeratin 7 (1/50; RCK 105; BD Bioscience, San Jose, CA, United States) was used to detect biliary cell. Antibodies against TGF-β1 (1/200; V), TβRI (1/200; T-19), TβRII (1/200; C-16), p-Smad2/3 (1:50; Ser 433/435) (all obtained from Santa Cruz Biotechnology, Dallas, Texas, United States) and monoclonal α-smooth muscle actin antibody (α-SMA, 1:250; 1-4A; Sigma, St. Louis, MO, United States) were used to detect the expressions of TGF-β1 signaling proteins. Briefly, after deparaffinization, sections were incubated in a Decloaking Chamber (Biocare Medical, CA, United States) set point: SP1 123 °C for 2 min, SP2 85 °C for 10 s, SP limit 10 °C), soaked in 3% H2O2 methanol solution for 5 min, then 15 min in 1 × Universal blocking solution (Bio-Genex, CA, United States) and 20 min in 10% goat serum to prevent nonspecific staining. After that, sections were incubated with primary antibodies for 1 h at room temperature in a moist chamber. After three washes with 0.1% Tween 20 in PBS (PBST) for 5 min, EnvisionTM (DakoCytomation, Glostrup, Denmark) was applied according to the procedure and counterstained with Mayer’s hematoxylin (DakoCytomation) or DAPI (4’,6-diamidino-2-phenylindole 2HCI, D9542, Sigma).
Liver tissue was homogenized in an Ultra-Turrax homogenizer in RIPA buffer containing 1 mmol/L PMSF and protease inhibitors. After high-speed (12700 g) centrifugation at 4 °C, the protein in the supernatant was separated by 10% SDS-PAGE (20 μg per lane), and transferred onto a PVDF membrane. After blocking with 1.5% bovine serum albumin (BSA) in Tris-buffered saline, TGF-β1, TβRI, TβRII, p-Smad2/3, α-SMA and α1 (I) collagen were detected using rabbit polyclonal antibodies against TGF-β1 (1:1000), TβRI (1:1000), TβRII (1:1000), p-Smad2/3 (1:2000), α1 (I) collagen (1:2000), and α-SMA (1:400), respectively, and then incubated with anti-rabbit and mouse IgG HRP conjugated (Promega, Madison, WI, United States). Specific binding was detected using the Super Signal West Dura Extended Duration Substrate (PIERCE, Rockford, IL, United States) with a FluorTech 8800 gel doc system (Alpha Innotech, CA, United States) equipped with a chemiluminescent filter.
Total RNA was isolated using TRI-Reagent (Sigma). Real-time PCR was carried out as described[14]. DNase I-treated total RNA (1 μg) was used for synthesis of the first strand of cDNA. Reverse transcription conditions were as follows: 42 °C for 15 min, 95 °C for 5 min and 5 °C for 5 min (one cycle). Real-time PCR was carried out in 25 μL of reaction solution (2.5 μL of 10 × buffer, 5 mmol/L of each dNTP, 10 mmol/L MgCl2, 200 nmol/L primers and 0.75 unit of platinum® Taq polymerase; all from Invitrogen) plus 1 μL of SYBR Green (1:2000; BioWhittaker, Richland, ME, United States). No genomic DNA contamination or pseudogenes were detected by PCR in the absence of the reverse transcription step in the total RNA used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an invariant control. The reactions started at 95 °C for 7 min, followed by 40 cycles of 95 °C for 20 s, 54 °C for 30 s and 72 °C for 30 s. Melting peaks of PCR products were determined by heat denaturation from 60 to 95 °C at 0.2 °C/s. Fold changes in the mRNA levels of target genes relative to the endogenous GAPDH control were calculated as suggested by Schmittgen et al[15]. Table 1 lists the primers used in real-time PCR.
| Gene | Genbank no | Forward primer (5' to 3') | Reverse primer (5' to 3') |
| TGFβ1 | NM_011577 | TGCTAATGGTGGACCGCAA | CACTGCTTCCCGAATGTCTGA |
| TβRI | NM_009370 | ATGGTTCCGAGAGGCAGAGAT | CCATGTCCCATTGTCTTTGTTG |
| TβRII | NM_009371 | CCAGAAGTCCTGCATGAGCAA | TGGCAAACCGTCTCCAGAGTA |
| Smad2 | NM_010754 | TCTCCGGCTGAACTGTCTCCTA | GCGATTGAACACCAGAATGCA |
| Smad3 | NM_016769 | ATGGAGCTCTGTGAGTTTGCCT | TGGAGGTAGAACTGGCGTCTCT |
| α-SMA | NM_007392 | CTATTCAGGCTGTGCTGTCCCT | GCCCTCATAGATAGGCACGTTG |
| α1(I) collagen | NM_007742 | CCCAAGGAAAAGAAGCACGTC | AGGTCAGCTGGATAGCGACATC |
| GAPDH | NM_008084 | AGCCTCGTCCCGTAGACAAAA | TGGCAACAATCTCCACTTTGC |
Real-time PCR was performed for quantitative analyses according to standard protocol using the SYBR Green PCR Master Mix and ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Tokyo, Japan).
Livers were first perfused with PBS containing 0.2% BSA, passed through a nylon mesh, and resuspended in PBS/0.2% BSA (EMD chemicals, Gibbstown, NJ, United States). Hepatocytes were removed as pellets after centrifugation at 700 r/min for 60 s periods[16]. Lymphocytes from suspended liver cells were then isolated using Histopaque-1077 (Sigma Chemical Co. St. Louis, MO, United States). After centrifugation, cells were washed with PBS/0.2% BSA, and the viability of cells confirmed by trypan blue dye (Sigma Chemical Co. St. Louis, MO, United States) exclusion. Cell preparations were then pre-incubated with anti-mouse FcR blocking reagent and then incubated at 4 °C with a combination of fluorochrome-conjugated antibodies, including anti-CD4 FITC, anti-CD25 APC, anti-CD8 PECy5, anti-Foxp3 PE (all from eBioscience CA, United States). Multiple-color flow analyses were performed using a FACScan flow cytometer upregulated by Cytec Development (Fremont, CA, United States) to allow for 4-color analysis. Acquired data were analyzed with CELLQUEST Software (BD Biosciences CA, United States) and FlowJo Software (Tree star, Inc., Ashland, OR, United States).
Results are expressed as mean ± SD and were evaluated using Mann-Whitney U tests for comparison between samples from mouse model and littermates, with P < 0.05 considered significant.
The serum levels of ALT, ALP and total bilirubin in the mouse model were higher than in the control mice (105.5 ± 36.9 IU/L vs 28.2 ± 2.9 IU/L, P = 0.006; 138.2 ± 15.3 IU/L vs 74.8 ± l8.5 IU/L, P = 0.025; and 2.8 ± 0.4 mg/dL vs 0.95 ± 0.12 mg/dL, P = 0.043). Mouse model displayed an increase AMA titer over time. By week 24, serum samples of the six mouse models were all positive for AMA/M2. In the mouse model, the mean titer of anti-M2 was significantly higher at week 24 than at week 8 (P < 0.0005), while in the control mice AMA/M2 was not detected. The time table of AMA in the mouse model resembled that in human PBC, of which the disease is not observed in childhood and typically develops in the fourth or fifth decade of life.
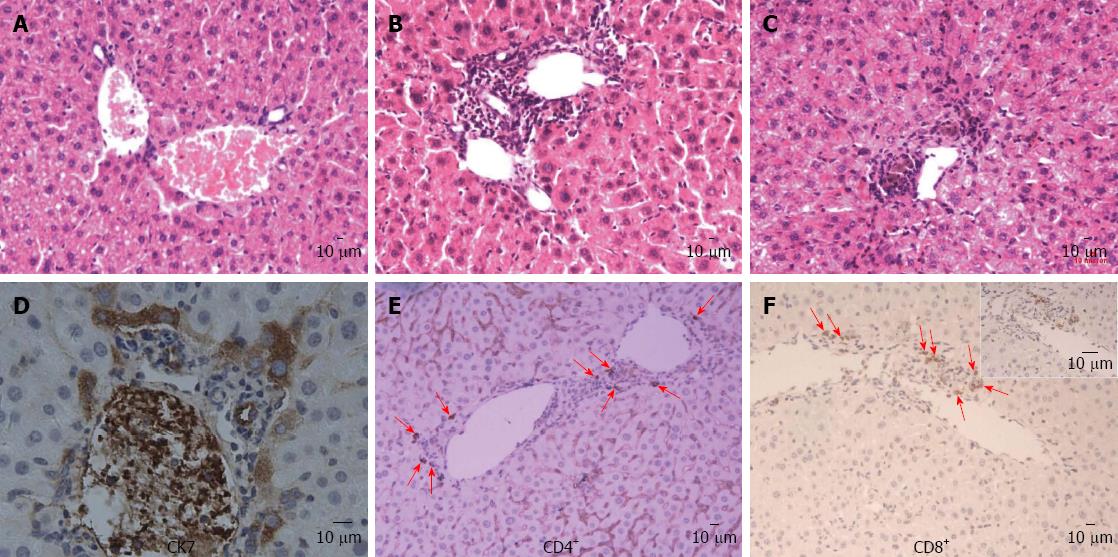
In the liver of the mouse model, moderate to severe infiltration of lymphoid cells was detected within the portal tracts in association with bile duct damage and a mild interface hepatitis (piecemeal necrosis) at week 8 (Figure 1B) and bile plugs were seen in canaliculi around portal tracts at week 24 (Figure 1C), which was absent in control mice (Figure 1A). Direct bile duct destruction was determined by the detection of scattered portal infiltration of CK-7 positive cells. Moreover, in liver tissues from some mice models, biliary cell destruction was so severe that identification of an intact bile duct structure was impossible and all biliary-type and hepatocytes were CK-7 positive, particularly in samples with cholestasis (Figure 1D). Immunohistochemical analysis demonstrated infiltration of CD4+ and CD8+ lymphocytes around small bile ducts that were absent in control mice (Figure 1E and F).
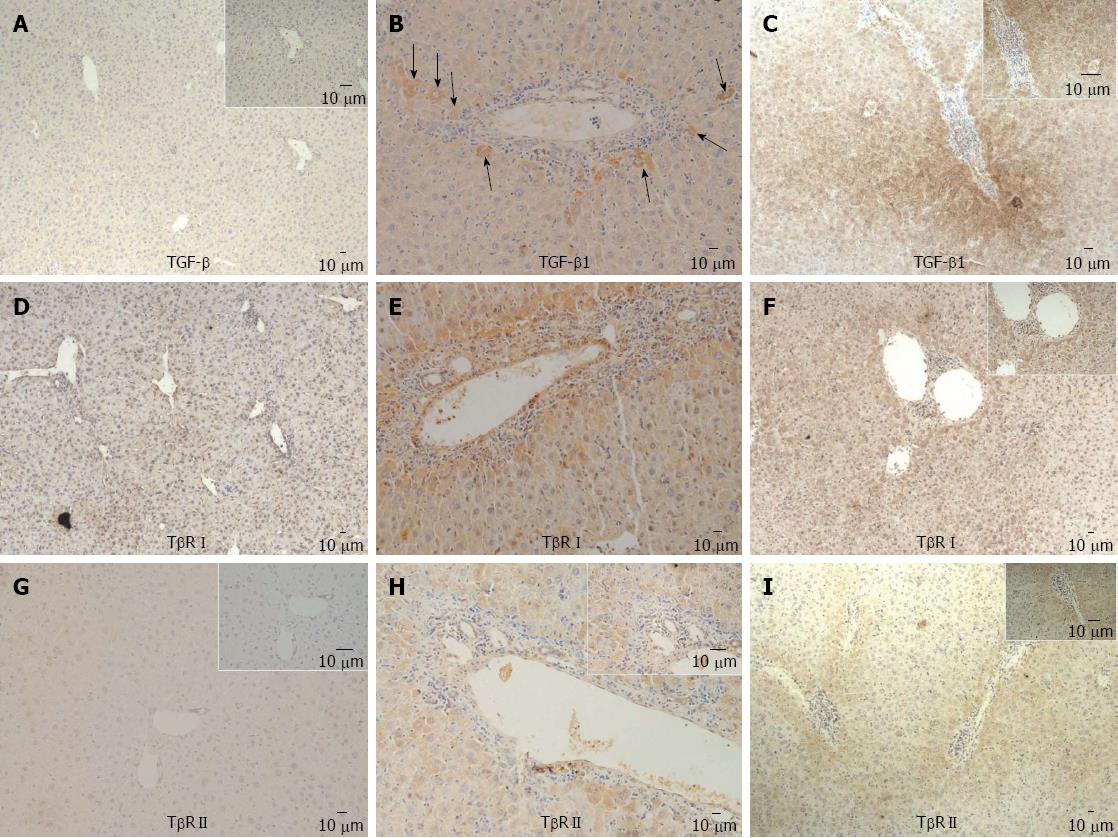
In mouse model, expression of TGF β1 in periportal and intralobular regions became more prominent over time (Figure 2A-D). At week 8, there were positive expressions of TβRI and TβRII in some periportal hepatocytes and biliary ductile endothelial cells (Figure 2E-H). At week 24, distribution of TβRI and TβRII became more extensive and prominent (Figure 2F and I).
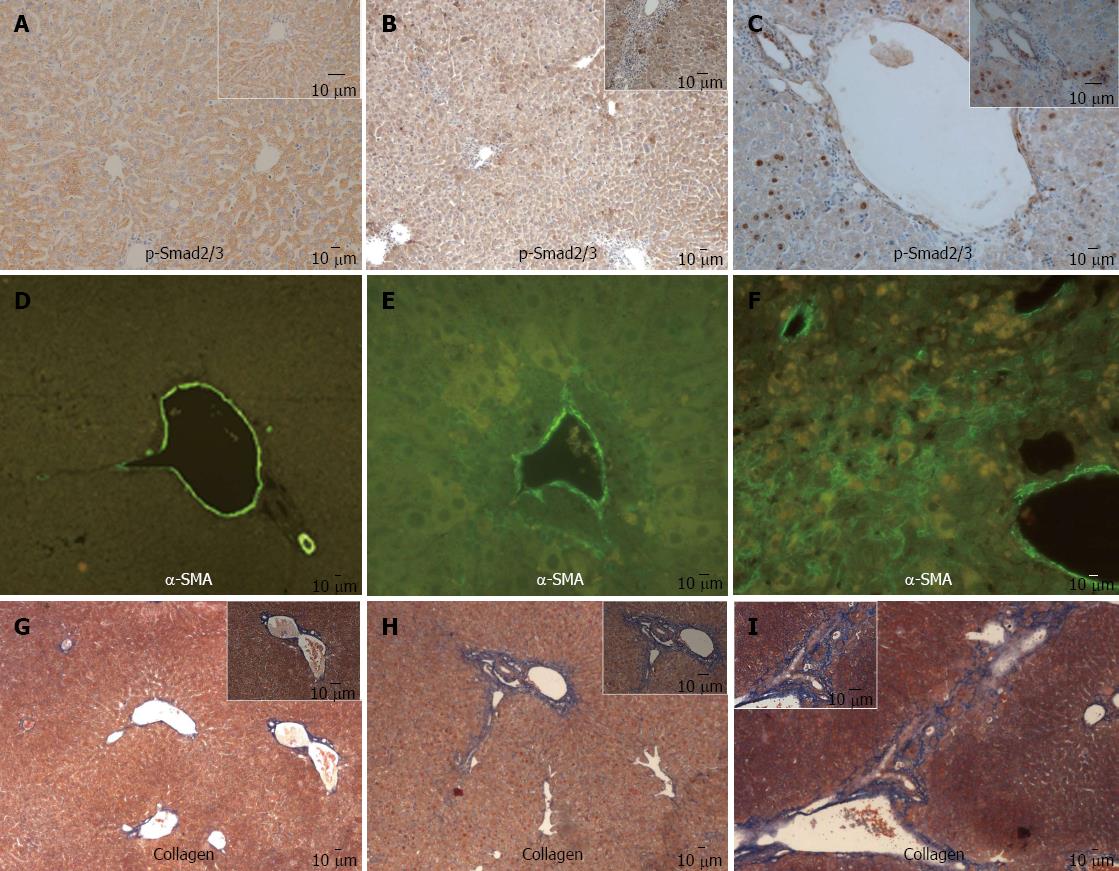
In mouse model, intranuclear staining of p-Smad2/3 was observed in some periportal and intralobular hepatocytes at week 8, and became more prominent at week 24 (Figure 3A-C), α-SMA positive staining and collagen deposition around portal areas were observed at week 8 (Figure 3E and H), and extension into surrounding parenchyma at week 24 (Figure 3F and I), which was absent in the liver of control mice (Figure 3D and G).
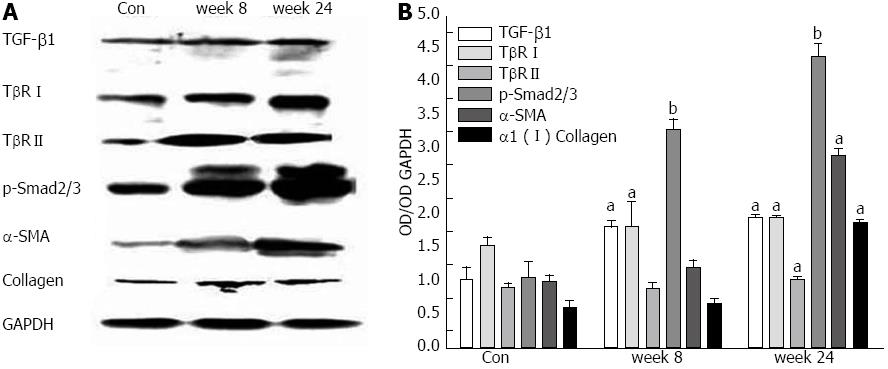
Immunoblot analysis of TGF-β1, TβRI, TβRII, p-Smad2/3, α-SMA and α1 (I) collagen of the liver homogenates from mouse model and control mice at week 8 and 24 is shown in Figure 4. Compared with that from control mice, there were increasing expressions of TGF-β1, TβRI, TβRII, p-Smad2/3, α-SMA and α1 (I) collagen of the liver homogenates from mouse model as time increased.
As shown in Table 2, the mRNA levels of TGF-β1, TβRI, TβRII, Smad2, Smad3, α-SMA and α1 (I) collagen of liver homogenates from mouse model at weeks 8 and 24 were higher than that from control mice.
After poly I: C injection, the total numbers of lymphocytes significantly increased in the liver of mouse model (Table 3). Although the total number of intrahepatic CD4+ lymphocytes increased, the percentage of CD4+ cells in the lymphocytes did not (Figure 5). In contrast, the CD8+ population in mouse model significantly increased in both total number and percentage compared with that in controls (Figure 5). In addition, the mouse model had a marked increase in the number as well as percentage of CD4+ CD25+ FOXP3+ lymphocytes compared with control mice (Table 3 and Figure 5). This finding is particularly interesting, as previous studies reported a decrease in precursors of CD4+ CD25+ regulatory T cells (Treg) in the peripheral blood of PBC patients[7,17,18], and several recent reports demonstrated increased infiltration of FOXP3+ Treg in damaged organ or target tissues in autoimmune diseases[19-21].
Our study demonstrated that this mouse model mimic several key phenotypic features of human PBC. It had elevated levels of ALP, AMA, portal bile ducts inflammation, and progressive collagen deposition. In human PBC, there is a ten-fold increase in frequency of CD8+ T cells specific for PDC-E2 in liver compared with that in peripheral blood, and it correlates with biliary ductular damage[18,22,23]. Interestingly, our mouse model also had increased CD8+ lymphocyte infiltration in liver, which is consistent with the chronic autoimmune nature of the disease. CK-7 is regarded as a histological marker for progression in PBC and indicates poor prognosis[24]. Hepatocytes do not normally express CK-7 except in the advanced stage of PBC, which was also observed in our study. Taken together, this animal model had several key phenotypic features and would allow us to analyze the early cellular events of PBC.
TGF-β1 is the key regulator in the pathogenesis of hepatic fibrosis, and appears to aggregate in the liver of PBC patients[25,26]. The selective abnormality of the TGF-β1 signaling pathway in T lymphocytes leads to impairment to peripheral tolerance and spontaneously development of features characteristic of PBC[7]. TGF-β1 is an essential modulator of Foxp3 expression in Tregs cells[20], conditioning their suppressive function. Recent studies have demonstrated reduction in the number of circulating Tregs in patients with PBC[21]. In addition, it is reported that the population of Tregs coexpressing Foxp3 and TGF-β1 decreases with age in female NOD mice[27]. Tregs produce elevated levels of TGF-β1, and the fact that TGF-β1 signaling receptors are up-regulated on the membrane of Tregs, underscores the potential for autocrine and/or paracrine receptor-ligand interaction in these cells. TGF-β1 is a positive regulator of Tregs expansion and inhibits autoimmune diseases via regulation of the size of Tregs pool in vivo[28]. Our study found elevated levels of TGF-β1 as well as the total number of CD4+ CD25+ FOXP3+ Treg in the liver of mouse model, which seems different from some studies[29-31]. However, there were also several reports demonstrating increased infiltration of FOXP3+ Tregs in damaged organ or target tissues in autoimmune diseases, suggesting that suppressor cells migrate to and/or multiply at the sites of inflammation as part of immune response to combat injurious inflammation[19-21], and in liver suppress hepatic immunity to autoantigens[32]. Taken together, our study illustrates that TGF-β1 regulation of FOXP3+ Tregs may be involved in the maintenance of chronic inflammation in PBC.
TGF-β1 down-regulates potentially harmful inflammatory responses in the liver, albeit at the expense of scar formation[33]. TGF-β1 signaling could induce phosphorylation of Smad2 and Smad3, which translocate into the nucleus to regulate expressions of specific target genes such as α1 (I) collagen and α-SMA[34]. Our study demonstrated that in the liver of mouse model, the levels of TGFβ1 as well as TβRI, TβRII, p-Smad2/3, α-SMA and α1 (I) collagen increased with age. These findings revealed that TGFβ1 may be involved in the fibrogenesis of the mice PBC model. Liver fibrosis occurs as a consequence of the differentiation of hepatic stellate cells (HSCs) into myofibroblasts, which is regulated by TGF β1[35]. Our study showed that the number of cells positive for α-SMA, which is a marker for myofibroblast-like cells[36], increased in aged mice in the animal model, which was coincident with increased expression of TGFβ1 and its signal molecules, supporting the finding that TGFβ1 signal pathway was involved in myofibroblast differentiation and subsequent liver fibrosis in the mouse PBC model.
In conclusion, although our data are derived from a murine model of PBC whose immunoregulation in PBC is likely to be far less complex than in human, the findings emphasize the role of TGFβ1 in development of PBC. TGFβ1 plays a dual role in development of PBC: it suppresses inflammatory response but operates to enhance fibrogenesis. The aberrant activity of TGF-β1 signaling contributes to the development of PBC.
We thank Dr. Wei-Xun Zhou for the histological studies.
Primary biliary cirrhosis (PBC) is an autoimmune liver disease. Recent studies suggest that transforming growth factor (TGF)-β1 signaling pathway might play an important role in the pathogenesis of PBC. However, whether TGF-β1 signaling pathway is involved in the development of PBC is still unknown.
TGF-β1 plays an important role in autoimmunity and liver fibrosis, and a TGF-β1 receptor knockout mouse has been recently proposed as a model for PBC. There is strong experimental evidence that TGF-β1 is implicated in the pathogenesis of PBC, probably through deregulation of T-reg.
An animal model of PBC was developed by polyinosinic polycytidylic acids (poly I:C) injection in genetically susceptible C57BL/6 female mice in this study. And the liver expressions of TGF-β1, TGF-β receptor I (TβRI), TβRII, p-Smad2/3, monoclonal α-smooth muscle actin antibody (α-SMA) and α1 (I) collagen in mouse model and control mice were evaluated. The relationship between TGF-β and Treg was also analyzed. The study found that TGFβ1 played a dual role in the development of PBC. The aberrant TGF-β1 signaling contributed to the development of PBC.
This study has provided new data of TGF-β1 signaling pathway involving the pathogenesis of PBC, which will pose significant impact on the understanding of the pathogeneses of PBC. Moreover, the data is the novel result of the role of TGF-β1 in the development of PBC. TGF-β1 signaling pathway is a potential target for PBC treatment.
This paper finds that aberrant TGF-β1 signaling contributes to the development in PBC. Until now we do not have a good answer for the role of TGF-β1 signaling in PBC. These findings may be related to the immunological abnormalities of PBC while the role of TGF-β1 signaling needs further investigation.
P- Reviewers Hardy T, Mason AL, Nakanuma Y S- Editor Song XX L- Editor Ma JY E- Editor Ma S
| 1. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 923] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 2. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3797] [Cited by in RCA: 4308] [Article Influence: 195.8] [Reference Citation Analysis (0)] |
| 3. | ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 932] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 4. | Lewindon PJ, Pereira TN, Hoskins AC, Bridle KR, Williamson RM, Shepherd RW, Ramm GA. The role of hepatic stellate cells and transforming growth factor-beta(1) in cystic fibrosis liver disease. Am J Pathol. 2002;160:1705-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Kikuchi K, Tanaka A, Matsushita M, Kitazawa E, Hosoya N, Kawashima Y, Selmi C, Gershwin ME, Miyakawa H. Genetic polymorphisms of transforming growth factor beta-1 promoter and primary biliary cirrhosis in Japanese patients. Ann N Y Acad Sci. 2007;1110:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 762] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 7. | Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, Ansari AA, Coppel RL, Li MO. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655-1660. [PubMed] |
| 8. | Mishra B, Tang Y, Katuri V, Fleury T, Said AH, Rashid A, Jogunoori W, Mishra L. Loss of cooperative function of transforming growth factor-beta signaling proteins, smad3 with embryonic liver fodrin, a beta-spectrin, in primary biliary cirrhosis. Liver Int. 2004;24:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Martinez OM, Villanueva JC, Gershwin ME, Krams SM. Cytokine patterns and cytotoxic mediators in primary biliary cirrhosis. Hepatology. 1995;21:113-119. [PubMed] |
| 10. | Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, Dickson ER, Haber J, Katz G, Lindor K. Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol. 2002;17:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Okada C, Akbar SM, Horiike N, Onji M. Early development of primary biliary cirrhosis in female C57BL/6 mice because of poly I: C administration. Liver Int. 2005;25:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Ambrosini YM, Yang GX, Zhang W, Tsuda M, Shu S, Tsuneyama K, Leung PS, Ansari AA, Coppel RL, Gershwin ME. The multi-hit hypothesis of primary biliary cirrhosis: polyinosinic-polycytidylic acid (poly I: C) and murine autoimmune cholangitis. Clin Exp Immunol. 2011;166:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Yoshioka K, Mori A, Taniguchi K, Mutoh K. Cell proliferation activity of proliferating bile duct after bile duct ligation in rats. Vet Pathol. 2005;42:382-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20-G30. [PubMed] |
| 15. | Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 790] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 16. | Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, Kikuchi K, Ikehara S, Gershwin ME. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323-2330. [PubMed] |
| 17. | Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, Ridgway W, Ueno Y, Ansari AA. IL-2 receptor alpha(-/-) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, Nakamura T, Saito S, Shimoda S. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Marazuela M, García-López MA, Figueroa-Vega N, de la Fuente H, Alvarado-Sánchez B, Monsiváis-Urenda A, Sánchez-Madrid F, González-Amaro R. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639-3646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Cao D, Börjesson O, Larsson P, Rudin A, Gunnarsson I, Klareskog L, Malmström V, Trollmo C. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol. 2006;63:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Sasaki M, Ikeda H, Sawada S, Sato Y, Nakanuma Y. Naturally-occurring regulatory T cells are increased in inflamed portal tracts with cholangiopathy in primary biliary cirrhosis. J Clin Pathol. 2007;60:1102-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Tsuda M, Ambrosini YM, Zhang W, Yang GX, Ando Y, Rong G, Tsuneyama K, Sumida K, Shimoda S, Bowlus CL. Fine phenotypic and functional characterization of effector cluster of differentiation 8 positive T cells in human patients with primary biliary cirrhosis. Hepatology. 2011;54:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Yang GX, Wu Y, Tsukamoto H, Leung PS, Lian ZX, Rainbow DB, Hunter KM, Morris GA, Lyons PA, Peterson LB. CD8 T cells mediate direct biliary ductule damage in nonobese diabetic autoimmune biliary disease. J Immunol. 2011;186:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Chatzipantelis P, Lazaris AC, Kafiri G, Papadimitriou K, Papathomas TG, Nonni A, Patsouris ES. Cytokeratin-7, cytokeratin-19, and c-Kit: Immunoreaction during the evolution stages of primary biliary cirrhosis. Hepatol Res. 2006;36:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Michel K, Roth S, Trautwein C, Gong W, Flemming P, Gressner AM. Analysis of the expression pattern of the latent transforming growth factor beta binding protein isoforms in normal and diseased human liver reveals a new splice variant missing the proteinase-sensitive hinge region. Hepatology. 1998;27:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Li Z, Dranoff JA, Chan EP, Uemura M, Sévigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 27. | Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, Peterson LB, Leung PS, Cheng C, Mackay IR. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 29. | Wang D, Zhang H, Liang J, Gu Z, Zhou Q, Fan X, Hou Y, Sun L. CD4+ CD25+ but not CD4+ Foxp3+ T cells as a regulatory subset in primary biliary cirrhosis. Cell Mol Immunol. 2010;7:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Zhang W, Sharma R, Ju ST, He XS, Tao Y, Tsuneyama K, Tian Z, Lian ZX, Fu SM, Gershwin ME. Deficiency in regulatory T cells results in development of antimitochondrial antibodies and autoimmune cholangitis. Hepatology. 2009;49:545-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, Oude Elferink RP, Prieto J, Medina JF. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 304] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Bayer EM, Herr W, Kanzler S, Waldmann C, Meyer Zum Büschenfelde KH, Dienes HP, Lohse AW. Transforming growth factor-beta1 in autoimmune hepatitis: correlation of liver tissue expression and serum levels with disease activity. J Hepatol. 1998;28:803-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 610] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 35. | Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int Suppl. 1996;54:S39-S45. [PubMed] |