Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5693
Revised: March 25, 2013
Accepted: March 28, 2013
Published online: September 14, 2013
Processing time: 282 Days and 20.9 Hours
AIM: To evaluate the contribution of the G-197A polymorphism in the interleukin-17 (IL-17) promoter region to gastric cancer risk in an Iranian population.
METHODS: We performed a case control study using samples from 161 individuals with gastric cancer and 171 healthy controls. For each individual, the G-197A genotype was determined by restriction fragment length polymorphism analysis of polymerase chain reaction-amplified fragments. Statistical analyses were performed to determine whether any demographic or behavioral factors, infection with Helicobacter pylori (H. pylori), or a particular G-197A genotype was associated with gastric cancer risk.
RESULTS: We found that the G-197A genotype was significantly associated with increased gastric cancer risk (P = 0.001). Patients who were homozygous (AA) at position -197 were 2.9 times more likely to develop disease (95%CI: 1.56-5.4; P = 0.001). Furthermore, logistic regression analysis revealed that the presence of a single A allele increased the risk of gastric cancer up to 1.7-fold (95%CI: 1.26-2.369; P = 0.001). This association was observed for early stage gastric adenocarcinomas only, and was not linked to H. pylori infection.
CONCLUSION: These results suggest that carrying one or more G-197A polymorphisms at position -197 in the IL-17 promoter region significantly increases gastric cancer risk in this patient population.
Core tip: There is currently a need for genetic markers to identify individuals at risk for developing gastric cancer. In this study, we describe one such marker, a G-197A polymorphism in the interleukin-17A (IL-17A) promoter. Within our study population, individuals who carry the G-197A polymorphism in the IL-17A promoter region may be at a significantly greater risk of developing gastric cancer. Importantly, the presence of this polymorphism alone was sufficient to increase risk of gastric cancer development.
- Citation: Rafiei A, Hosseini V, Janbabai G, Ghorbani A, Ajami A, Farzmandfar T, Azizi MD, Gilbreath JJ, Merrell DS. Polymorphism in the interleukin-17A promoter contributes to gastric cancer. World J Gastroenterol 2013; 19(34): 5693-5699
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5693
Gastric cancer is one of the most common causes of cancer-related deaths worldwide. Despite an overall decrease in gastric cancer incidence in recent years, this disease is still responsible for over 700000 deaths per year[1,2], and represents a significant medical burden in many countries. In Northern Iran, gastric cancer has a major impact on public health due to the high morbidity and mortality associated with this disease. Indeed, several Iranian provinces, including Manzadaran, Semnan, Golestan, and the greater Tehran area, report age-standardized incidence rates for gastric cancer ranging from 25.4-49.1[3]. While these high incidence rates may be partially explained by the fact that a significant proportion of this population is also colonized by the carcinogenic bacterium Helicobacter pylori (H. pylori)[3,4], the fact that this region maintains a high rate of gastric cancer despite an intensive H. pylori eradication program suggests that there are other host genetic and environmental factors involved in gastric cancer development.
Over the last several years, many studies have identified a variety of environmental, behavioral, and host genetic factors that play a role in gastric carcinogenesis across many patient populations. Among these behavioral factors are smoking and a high salt diet[5-8], which have been shown to be particularly important for disease development in Northern Iran[6]. However, there is currently a lack of information regarding which host genetic factors may play a role in carcinogenesis in the Iranian patient population. Previous reports have identified a group of host immune factors that, when aberrantly expressed, can influence the development of gastric disease. Among these factors are the interleukin-1 (IL-1), IL-8, IL-10, and tumor necrosis factor-α (TNF-α) genes, where specific polymorphisms have been associated with gastric cancer risk[9,10]. Additionally, in some instances, this effect can be compounded when the polymorphism exists in an H. pylori colonized individual. It is hypothesized that these polymorphisms result in a pro-inflammatory gastric environment, which may prime the tissue for cancer development.
Another, more recently described, pro-inflammatory cytokine is IL-17A (IL-17). This cytokine is one of a larger group of IL-17 family ligands and is primarily produced from a subset of CD4+ effector cells known as Th17 cells[11-13]. IL-17 is involved in both innate and adaptive immunity and can act on a variety of cell types[11,12]. Recently, reports have indicated that certain IL-17 polymorphisms are associated with autoimmune disease such as rheumatoid arthritis, graft vs host disease[14], and inflammatory diseases such as ulcerative colitis[15], suggesting that aberrant expression of this cytokine may polarize the body toward a variety of disease states. In addition, a recent study indicated that H. pylori-mediated induction of IL-17 may impact disease progression[16]; collectively, these studies highlight the importance of levels of IL-17 in a variety of diseases.
One particular IL-17 polymorphism (G-197A or rs22759133) has also been associated with certain types of gastric cancer in both Japanese and Chinese populations[17,18]. The guanine to adenine substitution at position -197 within the IL-17 promoter region is located in close proximity to 2 nuclear factors activated T cell binding motifs[19]. Because this region was shown to be required for IL-17 expression[19], it is believed that cells that harbor this mutation produce higher levels of IL-17, which in turn upregulates IL-17-mediated immune responses. This hypothesis is supported by the fact that various types of tumors express increased levels of IL-17[12], and patients with gastric cancer have a greater number of circulating IL-17-producing Th17 cells than healthy controls[20]. Taken together, these findings highlight the potential role of IL-17 in gastric cancer development.
Herein, we describe an epidemiologic study in which we investigate the role of the IL-17 G-197A promoter polymorphism in gastric cancer risk among individuals from Northern Iran, which is traditionally a poorly studied population. We found that within this patient population the G-197A polymorphism was significantly more frequent in gastric cancer patients compared with controls. This association was independent of H. pylori colonization status. These data indicate that the IL-17 G-197A polymorphism may be a good indicator for susceptibility to gastric cancer development in this patient population.
All aspects of the current study were approved by the Medical Research Ethics Committee at the Mandazaran University of Medical Sciences and conformed to the ethical guidelines set forth in the Declaration of Helsinki. Prior to enrollment, all patients were given an explanation of the nature of the study, and written informed consent was obtained from all individuals. Enrollees from the Mandazaran province of Iran were accepted after seeking treatment at Imam Teaching Hospital or Touba Polyclinic between April 2008 and November 2011. The diagnosis of gastric cancer cases were made based on gastric endoscopy, and cases were defined using the International Classification of Diseases for Oncology IX, Protocol 151 and Lauren criteria[21]. In order to simplify TNM staging[22], Stages IA and IB were grouped into “Stage I”, and Stages IIIA and IIIB were similarly combined into “Stage III”. We enrolled a total of 161 patients with gastric cancer (89 male, 72 female), with a mean age of 62.6 ± 12.4 years. One hundred seventy-one healthy controls (84 male and 87 female) were also enrolled, with a mean age of 60.8 ± 12.8 years. Subjects within the control group were matched to the case group with respect to age, sex, ethnic background, and geographic origin. Demographics and behavioral and epidemiological risk factors were self-reported by study participants using a written questionnaire. Cigarette smokers were defined as those participants who reported smoking at least one cigarette per day for 12 mo. Consumption of salted fish, pickles, and fast food was defined as eating these items at least once a week for 6 mo.
All patients were tested for H. pylori infection using H. pylori specific IgG by ELISA (Diagnostic Automation, CA, United States) and by the urea breath test. Individuals that tested positive by either of these methods were considered as positive for H. pylori.
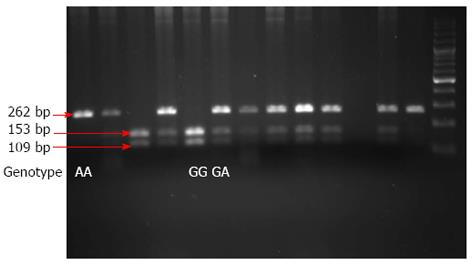
Venous blood collected from all study participants was used to isolate genomic DNA restriction fragment length polymorphism analysis of polymerase chain reaction-amplified fragments (PCR-RFLP) as previously described[18]. Briefly, each PCR amplification was performed using 1 μmol/L each of the forward (5’-AACAAGTAAGAATGAAAAGAGGACATGGT-3’) and reverse (5’-CCCCCAATGAGGTCATAGAAGAATC-3’) primers, 200 μmol/L of each dNTP, 2 mmol/L MgCl2, 0.4 U of Hot Start Taq polymerase (Takara), 1X Takara Hot Start Taq PCR buffer, and 100 ng of genomic DNA in a final volume of 25 μL. Each reaction was initially denatured at 95 °C for 4 min, followed by 35 cycles of denaturation at 95 °C for 40 s, primer annealing at 60 °C for 35 s, and extension at 72 °C for 30 s, followed by a final extension at 72 °C for 4 min. Amplified PCR products were subjected to enzymatic digestion with XagI (Fermentas) for 12 h at 60 °C and visualized after separation by 3% sodium dodecyl sulfate polyacrylamide gel electrophoresis and staining with ethidium bromide. This procedure allowed us to clearly differentiate between the homozygous GG, heterozygous GA, and homozygous AA genotypes: the resulting restriction digest products from individuals with a homozygous (GG) genotype were 153 bp and 109 bp; digestion products from individuals who were heterozygous (GA) were 262 bp, 153 bp, and 109 bp; a single 262 bp product was produced from individuals with a homozygous (AA) genotype (Figure 1).
After determining that all quantitative data were normally distributed (via Kolmograph-Smirnoph test), differences between patient populations were evaluated using the Student t-test. Qualitative differences between groups were assessed by the χ2 test as indicated. The association between IL-17 genotype and gastric cancer risk was determined using logistic regression analysis and an odds ratio (OR) with 95%CI. P-values ≤ 0.05 were considered significant for all tests.
The demographic data of gastric cancer patients and healthy controls are summarized in Table 1. Ages of study participants across the control group (n = 171) ranged from 24 to 87 years, and in the gastric cancer group (n = 161) from 28 to 86 years. The age difference between these 2 groups of participants was not statistically significant (P = 0.21). Similarly, the distribution of males and females in the study was also not significantly different between the gastric cancer group and the healthy controls (P = 0.16, χ2 test). We did note a statistically significant difference in the distribution of married individuals in the cancer group and the healthy controls, where individuals in the gastric cancer group were more likely to be single or divorced (P = 0.002, χ2 test). Similarly, we also noted that individuals within the gastric cancer group were more likely to be unemployed than those in the control group (P = 0.003, χ2 test). Finally, we also detected a difference in the level of education between patients in the 2 groups; a significantly higher number of the healthy controls had > 12 years of education compared with the gastric cancer patients (P = 0.003, χ2 test).
| Gastric cancer (n = 161) | Control (n = 171) | P-value1 | |
| Age (yr) | 62.56 ± 12.44 | 60.81 ± 12.76 | 0.21 |
| Sex (M/F) | 89/72 | 84/87 | 0.16 |
| Marital status | |||
| Single | 8 (5) | 3 (1.8) | |
| Married | 149 (92.5) | 166 (97.1) | 0.002 |
| Divorced | 4 (2.5) | 2 (1.2) | |
| Occupation | |||
| Unemployed | 4 (2.5) | 1 (0.6) | 0.003 |
| Employed | 24 (14.9) | 35 (20.5) | |
| Housewife | 48 (29.8) | 75 (43.9) | |
| Other | 85 (52.8) | 60 (35.1) | |
| Education 12 yr | 53 (32.9) | 85 (49.7) | 0.003 |
We next evaluated the distribution of the IL-17-197 alleles between the 2 study groups essentially as previously described[17,18]. The genotype frequencies of this polymorphism in controls were within the Hardy-Weinberg equilibrium (P = 0.49). As shown in Table 2, the predominant genotype found in gastric cancer patients was the heterozygous GA allele (38%), followed by the homozygous alleles GG (35%) and AA (27%). In contrast, within the healthy control group, the most common genotype was the wildtype GG allele (45.6%) followed by the heterozygous GA allele (42%) and the homozygous AA allele (12%). While the difference in the distribution of the GG and GA genotypes between the gastric cancer and control groups was not statistically significant, the finding that a larger number of cancer patients carried the AA allele was significant (P = 0.001, χ2 test). There was also a significant difference in the frequency of the A allele between the 2 groups; this allele was present in 46% of gastric cancer patients compared with only 33% of healthy controls (P = 0.001, χ2 test). We next performed a multivariate regression analysis to determine the predictive value of the G-197A polymorphism for gastric cancer development. After correcting for covariates such as age, sex, and H. pylori infection, this analysis indicated that the presence of the A allele increased gastric cancer risk by 1.7-fold (95%CI: 1.26-2.36; P = 0.001). The presence of the AA mutant genotype increased the odds of developing gastric cancer up to 2.9-fold (95%CI: 1.56-5.4; P = 0.001), indicating that the presence of the AA genotype at this locus is significantly associated with increased gastric cancer risk. Additionally, harboring the allele (AA + GA) enhanced the risk of gastric cancer up to 1.6-fold (95%CI: 1.01-2.45; P = 0.04).
| Genotype | Cancer (n = 161) | Controls (n = 171) | OR | CI | P-value1 |
| GG | 56 (34.8) | 78 (45.6) | 1.002 | ||
| GA | 61 (37.9) | 72 (42.1) | 1.2 | 0.73-1.91 | 0.53 |
| AA | 44 (27.3) | 21 (12.3) | 2.92 | 1.56-5.4 | 0.001 |
| G allele | 173 (53.7) | 228 (66.7) | 1.002 | ||
| A allele | 149 (46.3) | 114 (33.3) | 1.72 | 1.26-2.36 | 0.001 |
| A allele carriage (AA + GA vs GG) | 105 (64.2) | 93 (54.4) | 1.57 | 1.01-2.45 | 0.04 |
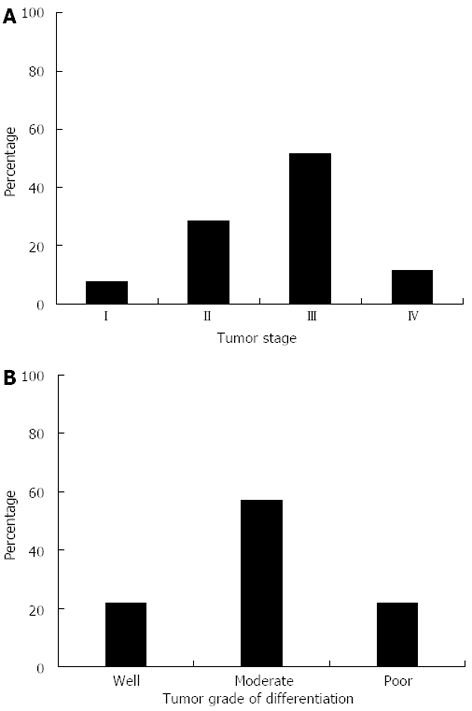
In order to determine whether the presence of the -197A allele was associated with disease progression within the gastric cancer group, we stratified a subset of the patients from this group based on TNM staging, extent of tumor cell differentiation, and the presence or absence of the mutant A allele (AA + GA vs GG); TNM information for 77 of the 161 patients enrolled in the gastric cancer group was available. A breakdown on tumor staging and degree of cellular differentiation are shown in Figure 2A and B, respectively. We placed individuals with lower grade malignancies (Stage I or II) in one group (n = 28), and those with Stage III or IV malignancies into a second group (n = 49) (Table 3). Within the group with Stage I or II malignancies, 22 patients had at least one A allele (GA or AA genotype), while only 6 patients had the GG genotype. This difference in Stage I/II patients was statistically significant (P = 0.001, χ2 test). Furthermore, the presence of the 197A allele increased the risk of gastric cancer development at the early stages of tumorigenesis by 6.3-fold (95%CI: 2.2-18.56; P = 0.001). In contrast, this association was not observed in patients with Stage III/Stage IV malignancies or when we grouped the cancer patients by age, sex, H. pylori status, or tumor cell differentiation (Table 3). These data suggest that while the presence of a mutant A allele at this locus increased the risk of developing a low grade (Stage I or II) malignancy, it was not a risk factor for progression to later stage cancer (Stage III or IV).
| GA + AA | GG | OR | 95%CI | P-value2 | |
| Age | |||||
| < 50 yr | 18 (17.1) | 4 (7.1) | 2.7 | 0.8-8.4 | 0.09 |
| ≥ 50 yr | 87 (82.9) | 52 (92.9) | |||
| Gender | |||||
| Male | 51 (48.6) | 21 (37.5) | 1.6 | 0.8-3.05 | 0.19 |
| Female | 54 (51.4) | 35 (62.5) | |||
| H. pylori+ | 65 (61.9) | 33 (58.9) | 0.88 | 0.45-1.71 | 0.74 |
| H. pylori- | 40 (38.1) | 23 (41.1) | |||
| TNM stage1 | |||||
| I-II | 22 (55) | 6 (16.2) | 6.3 | 2.2-18.5 | 0.001 |
| III-IV | 18 (45) | 31 (83.8) | |||
| Tumor differentiation | |||||
| Well | 25 (23.8) | 9 (16.1) | 1.003 | ||
| Moderate | 57 (54.3) | 35 (62.5) | 0.56 | 0.24-1.34 | 0.19 |
| Poor | 23 (21.9) | 12 (21.4) | 0.66 | 0.23-1.86 | 0.43 |
Gastric cancer remains a significant source of morbidity and mortality worldwide. As such, being able to identify which patients or patient populations are most at risk for developing this severe disease is of the upmost importance. This fact is particularly true for geographical regions such as Iran that have exceptionally high disease rates[23]. Indeed, despite the alarmingly high rates of gastric cancer in this region, few studies have focused on the identification of host factors or mutations in these factors that may predispose members of this population to gastric cancer development. Once identified, these factors or mutations could then be exploited to aid in the diagnosis of high-risk patients.
Numerous studies have attempted to unravel the complex nature of gastric cancer development. From these studies it has become clear that carcinogenesis is a multi-factorial process that involves a combination of environmental/behavioral, and genetic factors. For many populations/geographic areas, including the focus of the current study, major environmental/behavioral risk factors for gastric cancer development have been identified[2,5-8]. Additionally, there have been many studies that have identified potential genetic markers or polymorphisms that are associated with gastric cancer risk. Several of these factors play a role in maintaining proper immune homeostasis, including the pro-inflammatory cytokines IL-1β, inducible nitric oxide synthase, TNF-α, IL-8, IL-10[9,10,24], and more recently IL-17[17,18]. However, since many of these factors have only been studied in limited patient populations, it remains unclear whether or not the prognostic value of these markers applies equally to all groups. In fact, as more studies are performed across a variety of patient populations, it has become evident that the degree to which these factors impact on disease development is often dependent upon the group being studied[9,25-28]. As a result, there is a need to examine the role of these factors in additional populations.
Here, we described a case-control study that examined the association of the G-197A IL-17 promoter mutation with gastric cancer development in an Iranian population. This particular polymorphism has been previously associated with an increased risk of gastric mucosal atrophy and gastric cancer in a Japanese population[17], as well as gastric cancer risk in a Chinese population[18]. In accordance with those studies, we found that the G-197A polymorphism is significantly associated with an increase in gastric cancer risk (Table 2). Specifically, harboring 2 copies of the mutant allele (AA) at this locus increased a patient’s likelihood of developing cancer by a factor of 2.8 (Table 2). Furthermore, harboring only a single copy of this polymorphism (a heterozygous GA genotype) increased gastric cancer risk by 1.5 fold; this finding suggests that the effect of this polymorphism follows a dose-response. These data are consistent with the previous finding that the effect of the G-197A polymorphism on inflammation follows a similar dose-response pattern[17].
In healthy individuals, IL-17 is involved in both innate and adaptive arms of the immune system. Specifically, IL-17 is involved in induction of other pro-inflammatory cytokines as well as the recruitment and activation of inflammatory cells such as neutrophils and macrophages[11,29]. As the receptor for this cytokine is widely distributed on intestinal epithelial cells[12] and other tissue types[29], changes in the levels of IL-17 expression may have far reaching effects. This fact is illustrated by several studies, which have implicated increased IL-17 production with a variety of pathologic processes. Indeed, increased IL-17 transcript levels have been detected in patients with coronary artery disease[30], and inflammatory bowel disease[31], and specific IL-17 polymorphisms have been associated with ulcerative colitis[15], rheumatoid arthritis[32], and graft vs host disease[14]. While these conditions may present quite differently from gastric cancer, the underlying commonality among these diseases is their inflammatory origin.
While the precise mechanistic role of the G-197A polymorphisms in gastric cancer development remains unclear, a plausible hypothesis is that increased/constitutive expression of IL-17 may skew the gastric environment to become pro-inflammatory. As chronic inflammation is a known precursor for gastric cancer development[33], this IL-17-mediated inflammatory environment may result in an increase in carcinogenic cellular damage, which predisposes an individual to develop disease. Once these initial steps have begun, the cancer may progress in an IL-17 dependent or independent manner. In the current study, we found that the -197A allele was only significantly associated with the development of lower grade malignancies (Stage I or II) (Table 3). Similarly, a previous study linked the -197A polymorphism to an increased risk of poorly differentiated TNM Stage I/II cancer[18]. These data perhaps suggest that progression to more severe disease (Stages III or IV) occurs at least partially in an IL-17-independent manner. However, as 18 of the 49 TNM Stage III or IV cancer patients (Table 3) carried at least one mutant allele, we cannot completely rule out the possibility that this IL-17 polymorphism impacts on disease progression to some extent.
Gastric cancer remains a global health problem. As diagnosis of this disease often occurs only after the progression to more severe stages, there is a serious need for diagnostic markers that could help preemptively screen patients in high-risk populations and identify those who are most at risk of developing disease. Because the G-197A polymorphism in the IL-17 promoter region is consistently linked to gastric cancer development in multiple populations, it may be a good global candidate marker to identify gastric cancer risk.
The researchers would like to acknowledge the personnel of the endoscopic ward of Imam Hospital, the members of the Oncology wards of Amir Kola Hospital and Touba Polyclinic, and members of the Molecular and Cell Biology Research Center of Mazandaran University of Medical Sciences.
Individuals who carry specific genetic polymorphisms can be prone to cancer development. As a result, these polymorphisms may be used to identify these at risk individuals. However, before a particular polymorphism can be reliably used as a marker for cancer risk, the link between the polymorphism and disease propensity must be verified in a multiple populations of people with diverse genetic backgrounds.
Interleukin-17 (IL-17) is an important pro-inflammatory cytokine that is involved in both the innate and adaptive arms of the human immune system. One of the research hotspots in the field of IL-17 research is determining how increased or decreased levels of this cytokine effect human physiology and disease development.
Previous studies have identified the G-197A IL-17 polymorphism as a potential genetic marker for gastric cancer risk. However, those studies were performed on a limited population that had a similar genetic background. The current study verified the G-197A polymorphism as a potential genetic marker for gastric cancer risk in a genetically distinct population. Their results reinforce the possibility of using this IL-17 polymorphism as a marker for disease risk in many diverse populations.
The study highlights the possibility that the IL-17 G-197A polymorphism could be used as a marker for gastric cancer risk across diverse populations.
A polymorphism is where multiple forms of a DNA sequence may be present at a single genetic site.
The manuscript is quite well written. The results justify the conclusions drawn.
P- Reviewer D’Elios MM S- Editor Huang XZ L- Editor Cant MR E- Editor Zhang DN
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Pourfarzi F, Whelan A, Kaldor J, Malekzadeh R. The role of diet and other environmental factors in the causation of gastric cancer in Iran--a population based study. Int J Cancer. 2009;125:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, Yoonessi A, Tavangar M, Abedi BA, Sotoudehmanesh R. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37-42. [PubMed] |
| 4. | Sotoudeh M, Derakhshan MH, Abedi-Ardakani B, Nouraie M, Yazdanbod A, Tavangar SM, Mikaeli J, Merat S, Malekzadeh R. Critical role of Helicobacter pylori in the pattern of gastritis and carditis in residents of an area with high prevalence of gastric cardia cancer. Dig Dis Sci. 2008;53:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, Nichols R, Kesteloot H. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol. 1996;25:494-504. [PubMed] |
| 6. | Pakseresht M, Forman D, Malekzadeh R, Yazdanbod A, West RM, Greenwood DC, Crabtree JE, Cade JE. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 2011;22:725-736. [PubMed] |
| 7. | Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1-6. [PubMed] |
| 8. | Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 9. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1675] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 10. | Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631-636. [PubMed] |
| 11. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3754] [Cited by in RCA: 3789] [Article Influence: 236.8] [Reference Citation Analysis (0)] |
| 12. | Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 672] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 13. | Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483-5486. [PubMed] |
| 14. | Espinoza JL, Takami A, Onizuka M, Kawase T, Sao H, Akiyama H, Miyamura K, Okamoto S, Inoue M, Ohtake S. A single nucleotide polymorphism of IL-17 gene in the recipient is associated with acute GVHD after HLA-matched unrelated BMT. Bone Marrow Transplant. 2011;46:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Nakamura M, Yoshioka D, Arima Y. The influence of polymorphisms of interleukin-17A and interleukin-17F genes on the susceptibility to ulcerative colitis. J Clin Immunol. 2008;28:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Amedei A, Munari F, Bella CD, Niccolai E, Benagiano M, Bencini L, Cianchi F, Farsi M, Emmi G, Zanotti G. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Shibata T, Tahara T, Hirata I, Arisawa T. Genetic polymorphism of interleukin-17A and -17F genes in gastric carcinogenesis. Hum Immunol. 2009;70:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Wu X, Zeng Z, Chen B, Yu J, Xue L, Hao Y, Chen M, Sung JJ, Hu P. Association between polymorphisms in interleukin-17A and interleukin-17F genes and risks of gastric cancer. Int J Cancer. 2010;127:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Liu XK, Lin X, Gaffen SL. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J Biol Chem. 2004;279:52762-52771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 22. | Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 18 ed. New York: McGraw-Hill 2011; . |
| 23. | Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. [PubMed] |
| 24. | Goto T, Haruma K, Kitadai Y, Ito M, Yoshihara M, Sumii K, Hayakawa N, Kajiyama G. Enhanced expression of inducible nitric oxide synthase and nitrotyrosine in gastric mucosa of gastric cancer patients. Clin Cancer Res. 1999;5:1411-1415. [PubMed] |
| 25. | Atherton JC, Cao P, Peek RM, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771-17777. [PubMed] |
| 26. | Oleastro M, Cordeiro R, Ménard A, Yamaoka Y, Queiroz D, Mégraud F, Monteiro L. Allelic diversity and phylogeny of homB, a novel co-virulence marker of Helicobacter pylori. BMC Microbiol. 2009;9:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Oleastro M, Cordeiro R, Yamaoka Y, Queiroz D, Mégraud F, Monteiro L, Ménard A. Disease association with two Helicobacter pylori duplicate outer membrane protein genes, homB and homA. Gut Pathog. 2009;1:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Costa VS, Mattana TC, da Silva ME. Unregulated IL-23/IL-17 immune response in autoimmune diseases. Diabetes Res Clin Pract. 2010;88:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Zhang X, Pei F, Zhang M, Yan C, Huang M, Wang T, Han Y. Interleukin-17A gene variants and risk of coronary artery disease: a large angiography-based study. Clin Chim Acta. 2011;412:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. [PubMed] |
| 32. | Nordang GB, Viken MK, Hollis-Moffatt JE, Merriman TR, Førre ØT, Helgetveit K, Kvien TK, Lie BA. Association analysis of the interleukin 17A gene in Caucasian rheumatoid arthritis patients from Norway and New Zealand. Rheumatology (Oxford). 2009;48:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 612] [Article Influence: 34.0] [Reference Citation Analysis (0)] |