Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5314
Revised: July 1, 2013
Accepted: July 12, 2013
Published online: August 28, 2013
Processing time: 92 Days and 7.2 Hours
AIM: To investigate the relaxant effect of chromane HEF-19 on colonic smooth muscles isolated from rabbits, and the underlying mechanisms.
METHODS: The relaxant effect and action mechanisms of HEF-19 were investigated using descending colon smooth muscle of the rabbits. Preparations 1 cm long were mounted in 15-mL tissue baths containing Tyrode’s solution, maintained at 37 ± 0.5 °C and aerated with a mixture of 5% CO2 in oxygen (Carbogen). The tension and amplitude of the smooth muscle strips were recorded after adding HEF-19 (10-6, 10-5 and 10-4 mol/L). After cumulative administration of four antispasmodic agents, including acetylcholine chloride (Ach) (10-4 mol/L), histamine (10-4 mol/L), high-K+ (60 mmol/L) and BaCl2 (8.2 mmol/L), HEF-19 (3 × 10-7-3 × 10-4 mol/L) was added to investigate the relaxant effect of HEF-19. CaCl2 (10-4-2.5 × 10-3 mol/L) was added cumulatively to the smooth muscle preparations pretreated with and without HEF-19 (1 × 10-6 or 3 × 10-6 mol/L) and verapamil (1 × 10-7 mol/L) to study the mechanisms involved. Finally, phasic contraction was induced with ACh (15 × 10-6 mol/L), and CaCl2 (4 × 10-3 mol/L) was added to the smooth muscle preparations pretreated with and without HEF-19 (3 × 10-6 mol/L or 1 × 10-5 mol/L) and verapamil (1 × 10-7 mol/L) in calcium-free medium to further study the underlying mechanisms.
RESULTS: HEF-19 (1 × 10-6, 1 × 10-5 and 1 × 10-4 mol/L) suppressed spontaneous contraction of rabbit colonic smooth muscles. HEF-19 (3 × 10-7-3 × 10-4 mol/L) relaxed in a concentration-dependent manner colonic smooth muscle preparations pre-contracted with BaCl2, high-K+ solution, Ach or histamine with respective EC50 values of 5.15 ± 0.05, 5.12 ± 0.08, 5.58 ± 0.16 and 5.25 ± 0.24, thus showing a spasmolytic activity. HEF-19 (1 × 10-6 mol/L and 3 × 10-6 mol/L) shifted the concentration-response curves of CaCl2 to the right and depressed the maximum response to CaCl2. The two components contracted by Ach were attenuated with HEF-19 (3 × 10-6 mol/L or 10-5 mol/L) in calcium-free medium.
CONCLUSION: HEF-19 inhibited rabbit colonic smooth muscle contraction, probably through inhibiting opening of voltage-dependent Ca2+ channels. HEF-19 reduced inflow and intracellular release of Ca2+ ions.
Core tip: This is a good descriptive study in which authors found a new L-calcium-antagonist relaxing rabbit colonic smooth muscles and analyzed its possible mechanism. It provides an opportunity to search for a new drug highly selective to the gastrointestinal tract, effectively relieving pain, diarrhea and intestinal discomfort, but without significant adverse effects on irritable bowel syndrome patients.
- Citation: Wei YY, Sun LL, Fu ST. HEF-19-induced relaxation of colonic smooth muscles and the underlying mechanisms. World J Gastroenterol 2013; 19(32): 5314-5319
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5314
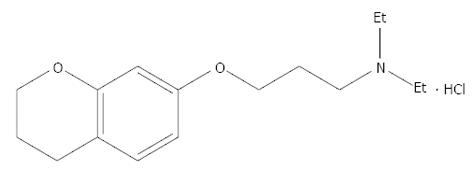
Irritable bowel syndrome (IBS) is a frequent gastrointestinal disease, characterized by a combination of several symptoms including abdominal pain or discomfort, flatulence, and problems related to bowel habits (constipation and/or diarrhea)[1]. Abnormal contraction of intestinal smooth muscle may be importantin producing the main IBS symptoms. thus, modifying the contractility is often the major aim in the treatment of IBS[2,3]. Calcium channel blockers have a good effect on IBS patients with abdominal pain and diarrhea[4].Calcium channel blockers have received increasing attention in the treatment of IBS. 3.4-Dihydro-7-[3-(diethylamino) propoxy] chroman hydrochloride (HEF-19) is a compound with a relaxant effect on colonic smooth muscles.
The present study investigated the relaxant effect of HEF-19 on isolated descending colon smooth muscle from rabbits, and the underlying mechanisms (Figure 1).
New Zealand rabbits of either sex (2.0-2.5 kg) were obtained from the Experimental Animal Center of Shenyang Pharmaceutical University (Certificate number: SCXK20030011). All care and handling of animals were approved by the Institutional Animal Ethical Committee.
Normal Tyrode’s solution contained: NaCl 136.86 mmol/L, KCl 2.68 mmol/L, NaHCO3 11.9 mmol/L, MgCl2 1.05 mmol/L, KH2PO4.H2O 0.41 mmol/L, CaCl2 1.8 mmol/L, and glucose 5.6 mmol/L. A high-K+ solution (KCl, 60 mmol/L) was obtained by equimolar replacement of NaCl by KCl in Tyrode’s solution[5]. Ca2+-free Tyrode solution was the solution in which CaCl2 was omitted and ethylenediaminetetra-acetic acid (EDTA, 0.1 mmol/L) was added[6]. Ca2+-free high-K+ solution was the Ca2+-free and high-K+ Tyrode solution. All chemicals were dissolved in distilled water. All solutions were stored at 4 °C and fresh dilutions were made daily.
HEF-19 (> 99.5% purity) was provided by Organic Chemistry Laboratory of Shenyang Pharmaceutical University and dissolved in distilled water. KCl was from Shenyang Chemical Reagent Factory, Shenyang, China, CaCl2 from Tianjin Bodi Chemical Co., Tianjin, China, BaCl2 from Shenyang Xingdong Reagent Factory, Shenyang, China, verapamil injection from Tianjin Heping Pharmaceutical Plant, Tianjin, China, and acetylcholine chloride (Ach) and histamine were from Sigma, United States.
The animals had free access to water but were fasted for 24 h before the experiments. The animals were killed by a blow to the head. The descending colon portion was isolated, washed, and freed from the mesentery. Preparations 1 cm long were mounted in 15-mL tissue baths containing Tyrode’s solution maintained at 37 ± 0.5 °C and aerated with a mixture of 5% CO2 in oxygen. A preload of 3 g was applied and the tissues were kept undisturbed for an equilibrium period of 60 min. During that time, the nutrient solution was changed every 20 min. Changes in isometric tension were measured with a force-displacement transducer (Chengdu Instrument Plant, Chengdu, China) and recorded by an RM6240B Multichannel Physiological Signal Collection and Handling System (Chengdu Instrument Plant)[7].
The normal tension and amplitude of the descending colonic smooth muscle strips were recorded after the contraction reached a stable plateau. HEF-19 (1 × 10-6, 1 × 10-5 and 1 × 10-4 mol/L) and vehicle were added to the tissue baths containing Tyrode’s solution.
The isolated colon smooth muscle preparations were contracted with Ach (1 × 10-4 mol/L), histamine (1 × 10-4 mol/L) High-K+ (60 mmol/L) or BaCl2 (8.2 mmol/L), after the contraction reached a stable plateau, and cumulative concentrations of HEF-19 (3 × 10-7 mol/L-3 × 10-4 mol/L) were added. The relaxant effect was expressed as a percentage of relaxation and the EC50 (concentration to produce a 50% maximal relaxation) was calculated using a multichannel physiological system.
The isolated preparations were allowed to stabilize in normal Tyrode’s solution and were replaced with Ca2+-free Tyrode’s solution for 30 min, and then K+-rich and Ca2+-free Tyrode’s solution. After 15 min incubation, Ca2+ was added in a cumulative fashion (1 × 10-4-2.5 × 10-3 mol/L) to obtain control concentration-response curves. The results were expressed as the percentage of the maximum contractile tension to CaCl2 before and after pretreatment with HEF-19 (1 × 10-6 or 3 × 10-6 mol/L) and verapamil (1 × 10-7 mol/L) respectively[8].
After the equilibration period, normal Tyrode’s solution was replaced with Ca2+-free Tyrode’s solution for 20 min. The phasic contraction caused by ACh (15 × 10-6 mol/L) was obtained, and tonic contraction was induced by further addition of CaCl2 (4 × 10-3 mol/L). After washing with normal Tyrode’s solution, the experiments were repeated with incubation for 10 min with HEF-19 (3 × 10-6 mol/L or 1 × 10-5 mol/L) and verapamil (1 × 10-7 mol/L) respectively[8,9].
Statistical evaluation of the data was performed using Student’s t test when appropriate. The data were expressed as mean ± SD or mean ± SEM and P < 0.05 was considered statistically significant.
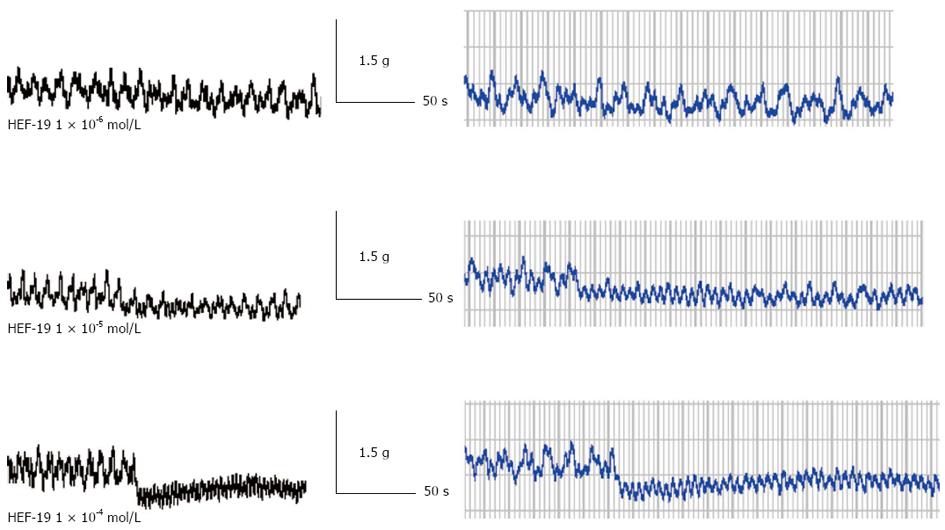
HEF-19 (1 × 10-6, 1 × 10-5 and 1 × 10-4 mol/L) significantly suppressed the tension and amplitude of spontaneous contraction, in a concentration-dependent manner. Figure 2 is print screen about tension and amplitude of spontaneous contraction of descending colonic smooth muscles. Tension is y-axis. Time is x-axis. Amplitude is difference between the peaks and troughs. The data of Figure 2 showed in Table 1.
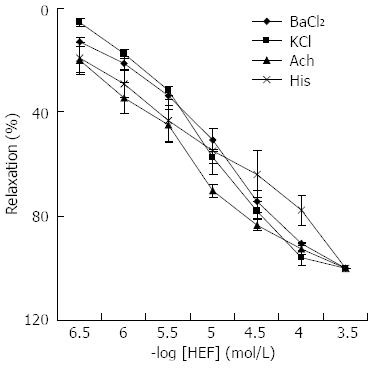
The maximum responses of the cumulative concentration-response curves to BaCl2, high-K+ solution, Ach or histamine were depressed by HEF-19 in a dose-dependent manner (3 × 10-7-3 × 10-4 mol/L). EC50 values were 5.15 ± 0.05, 5.12 ± 0.08, 5.58 ± 0.16 and 5.25 ± 0.24 (Figure 3).
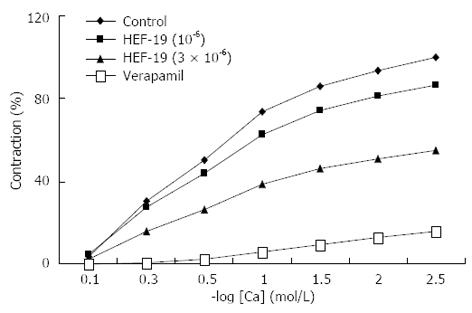
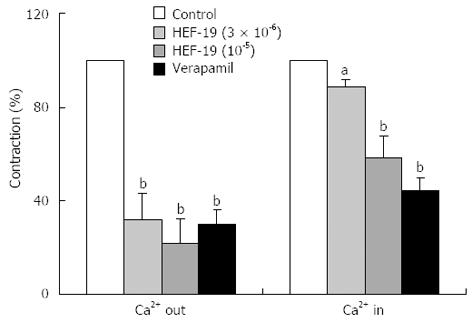
The maximum cumulative concentration-response curves for CaCl2-induced contraction were depressed by HEF-19 (1 × 10-6 and 3 × 10-6 mol/L) in a concentration-dependent manner. These results indicated that HEF-19 showed non-competitive antagonism (Figure 4).
The phasic and tonic contraction induced by ACh was decreased by HEF-19 (3 × 10-6 and 1 × 10-5 mol/L) in a concentration-dependent manner after pretreatment in calcium-free medium with EGTA (Figure 5).
Excitation-contraction coupling in smooth muscle occurs through two main mechanisms. Many smooth muscles are activated by Ca2+ signaling cascades. In addition, there is a Rho/Rho kinase signaling pathway that acts by altering the Ca2+ sensitivity of the contractile system[10,11]. The predominant source of activator and intracellular Ca2+ has little role to play in mediating excitation-contraction coupling by agonists. Both tonic and phasic (rhythmic) contraction are regulated by intracellular Ca2+ concentration. Ca2+ originates from the intracellular Ca2+ store, the sarcoplasmic reticulum, and influx from the extracellular space. Phasic contraction is influenced by neurotransmitters, hormones, and drugs. In circular muscle, these agents can also increase calcium by releasing it from intracellular stores, thus inducing tonic contraction[12-19].
Smooth muscle has the automatic rhythmicity. Spontaneous contraction shows the basic rhythmic depolarization wave. HEF-19 suppressed the spontaneous contractile amplitude and tension of rabbit colonic smooth muscle in a concentration-dependent manner. It has been reported that extracellular Ca2+ participates in spontaneous activity and enters the cytosol by L-type voltage-dependent Ca2+ channels[20].
The contraction induced by BaCl2, high-K+ solution, Ach or histamine was relaxed by HEF-19. High-K+ elicits an increase in intracellular Ca2+ and transient contractions[21,22]. ACh induces smooth muscle contraction via activating muscarinic receptors. Extracellular and intracellular Ca2+ participate in the Ach-induced contraction[23]. Histamine has a spasmogenic effect on the gastrointestinal tract through activating histaminergic receptors and increasing Ca2+ influx[24,25]. BaCl2 causes cell membrane depolarization and intracellular Ca2+ release, and it can cross the cell membrane through the Ca2+ channels to bind with troponin directly[26].
HEF-19 depressed the maximum cumulative concentration-response curve for CaCl2 in a non-competitive manner, similar to verapamil. The fact that HEF-19 inhibited CaCl2-induced smooth muscle contraction indicated that it inhibited the voltage-dependent Ca2+ channels, because CaCl2 can open these channels during high-K+ depolarization[27,28].
There are biphasic responses, including fast and slow components, in the contraction induced by ACh. The fast (phasic) phase is due to the release of intracellular Ca2+ induced by ACh in Ca2+-free medium[21], and the sustained (tonic) phase is largely dependent on the influx of external Ca2+ resulting from the reintroduction of CaCl2 into the medium. HEF-19 decreased the phasic and tonic contraction. The results showed that HEF-19 eventually inhibited the Ca2+ channels to reduced release of intracellular Ca2+ and influx of external Ca2+.
In conclusion, our results suggest that HEF-19 relaxed rabbit descending colonic smooth muscle by blocking voltage-dependent Ca2+ channels. HEF-19 inhibited the inflow of extracellular Ca2+ into cells, and intracellular release of Ca2+ ions. Ca2+ channels blocking effect of HEF-19 is fewer than verapamil on colonic smooth muscle. Calcium channel blockers are also reported to be effective in the treatment of IBS[3]. However, the adverse effects on the cardiovascular system of these blockers limit their further application on IBS patients. HEF-19, a L-type calcium channel blocker with selectivity for the gastrointestinal tract, is expected to be a safe and effective drug for treatment of abdominal pain and diarrhea symptoms associated with IBS.
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder in which abdominal pain is associated with changes in bowel habits and abdominal distension. Abnormal contraction of intestinal smooth muscle may be important in producing the main symptoms of IBS, thus, modifying contractility is often the major aim of treatment. Traditional cholinolytic and opioid drugs have been reported to have much adverse reactions. Some enteric spasmolytics agents have been found to treat IBS by selectively blocking voltage-dependent Ca2+ channels.
Current IBS pathophysiologic mechanisms are based on the abnormalities of brain-gut axis. With in-depth researches on various neurotransmitters, ion channel and receptors, designed as targets, new drugs are expected to appear against IBS. Since Pinaverium Bromide was developed and used clinically, there has been increasing concern to search for highly selective blockers of voltage-dependent Ca2+ channels to treat IBS patients with abdominal pain and diarrhea.
Chromane HEF-19 has a relaxant effect on colonic smooth muscles. It has previously been shown to have little activity on isolated vascular smooth muscle. The present study investigated the relaxant effect of HEF-19 on isolated descending colon smooth muscle of rabbits and the possible mechanisms. HEF-19 is expected to be a highly selective enteric spasmolytics agent through inhibition of opening of voltage-dependent Ca2+ channels in colonic smooth muscle. This is a potentially interesting study to find a drug for treatment of abdominal pain and diarrhea associated with IBS.
HEF-19 is expected to be a safe, effective and economic drug for treatment of abdominal pain and diarrhea symptoms associated with IBS.
HEF-19: HEF-19, 3.4-dihydro-7-[3-(diethylamino) propoxy] chroman hydrochloride, is highly selective enteric spasmolytics agent. IBS is a functional gastrointestinal disorder in which abdominal pain is associated with changes in bowel habits and abdominal distension. People with a functional gastrointestinal (GI) disorder have frequent symptoms, but the GI tract is not damaged. IBS is a group of symptoms that occur together. The most common symptoms of IBS are abdominal pain or discomfort, often reported as cramping, along with diarrhea, constipation, or both.
Very well written manuscript. In the manuscript entitled “HEF-19-induced relaxation of colonic smooth muscles and the underlying mechanisms”, the authors investigated the relaxant effect of chromane HEF-19 on colonic smooth muscles isolated from rabbits. This is a good descriptive study on a hot topic. The research is well done. The result is well discussed.
P- Reviewers Chorny M, Suzuki A, Wenzel SE S- Editor Wang JL L- Editor A E- Editor Zhang DN
| 1. | Forte E, Pizzoferrato M, Lopetuso L, Scaldaferri F. The use of anti-spasmodics in the treatment of irritable bowel syndrome: focus on otilonium bromide. Eur Rev Med Pharmacol Sci. 2012;16:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Dai Y, Liu JX, Li JX, Xu YF. Effect of pinaverium bromide on stress-induced colonic smooth muscle contractility disorder in rats. World J Gastroenterol. 2003;9:557-561. [PubMed] |
| 3. | Lu CL, Chen CY, Chang FY, Chang SS, Kang LJ, Lu RH, Lee SD. Effect of a calcium channel blocker and antispasmodic in diarrhoea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2000;15:925-930. [PubMed] |
| 4. | Krevsky B, Maurer AH, Niewiarowski T, Cohen S. Effect of verapamil on human intestinal transit. Dig Dis Sci. 1992;37:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Birk S, Edvinsson L, Olesen J, Kruuse C. Analysis of the effects of phosphodiesterase type 3 and 4 inhibitors in cerebral arteries. Eur J Pharmacol. 2004;489:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Wakabayashi I, Masui H, Groschner K. Intracellular alkalinization augments alpha(1)-adrenoceptor-mediated vasoconstriction by promotion of Ca(2+) entry through the non-L-type Ca(2+) channels. Eur J Pharmacol. 2001;428:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Gilani AH, Shah AJ, Ghayur MN, Majeed K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76:3089-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Small RC, Boyle JP, Elliott KR, Foster RW, Watt AJ. Inhibitory effects of AH 21-132 in guinea-pig isolated ileum and taenia caeci. Br J Pharmacol. 1989;97:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Zhang WW, Li Y, Wang XQ, Tian F, Cao H, Wang MW, Sun QS. Effects of magnolol and honokiol derived from traditional Chinese herbal remedies on gastrointestinal movement. World J Gastroenterol. 2005;11:4414-4418. [PubMed] |
| 10. | Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005;566:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Yamakage M, Namiki A. Calcium channels--basic aspects of their structure, function and gene encoding; anesthetic action on the channels--a review. Can J Anaesth. 2002;49:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Julou G, Freslon JL. Effects of calcium entry blockers on Ca2+-induced contraction of depolarized and noradrenaline-exposed rat resistance vessels. Eur J Pharmacol. 1986;129:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Fiorenza V, Yee YS, Zfass AM. Small intestinal motility: normal and abnormal function. Am J Gastroenterol. 1987;82:1111-1114. [PubMed] |
| 15. | Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047-5061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 16. | Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1443] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 17. | Abdel-Latif AA. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986;38:227-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Macara B, Rico JM. Effect of Ca2+ modulators on acetylcholine-induced phasic and tonic contractions and A23187-induced contractions in ileal longitudinal muscle and IP3 production. Eur J Pharmacol. 1992;218:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Zhou H, Kong DH, Pan QW, Wang HH. Sources of calcium in agonist-induced contraction of rat distal colon smooth muscle in vitro. World J Gastroenterol. 2008;14:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Grasa L, Rebollar E, Arruebo MP, Plaza MA, Murillo MD. The role of Ca2+ in the contractility of rabbit small intestine in vitro. J Physiol Pharmacol. 2004;55:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986;38:321-416. [PubMed] |
| 22. | Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157-230. [PubMed] |
| 23. | Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999;64:419-428. [PubMed] |
| 24. | Bolton TB, Clark JP. Effects of histamine, high potassium and carbachol on 42K efflux from longitudinal muscle of guinea-pig intestine. J Physiol. 1981;320:347-361. [PubMed] |
| 25. | Morel N, Hardy JP, Godfraind T. Histamine-operated calcium channels in intestinal smooth muscle of the guinea-pig. Eur J Pharmacol. 1987;135:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Clement JG. BaCl2-induced contractions in the guinea pig ileum longitudinal muscle: role of presynaptic release of neurotransmitters and Ca2+ translocation in the postsynaptic membrane. Can J Physiol Pharmacol. 1981;59:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Liu W, Yang XH, Zhou M, Li CD. Pharmacodynamical mechanisms of total flavonoids from chaenomeles Lagenaria koidz in the relaxation of gastrointestinal smooth muscles. Shijie Huaren Xiaohua Zazhi. 2007;15:165-167. |
| 28. | Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |