Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4897
Revised: April 12, 2013
Accepted: May 16, 2013
Published online: August 14, 2013
Processing time: 176 Days and 18.2 Hours
AIM: To evaluate 99mTc-ciprofloxacin scintigraphy compared with computed tomography (CT) for detecting secondary infections associated with severe acute pancreatitis (SAP) in swine.
METHODS: Six healthy swine were assigned to a normal control group (group A, n = 6). SAP was induced in group B (n = 9) and C (n = 18), followed by inoculation of the resulting pancreatic necroses with inactive Escherichia coli (E. coli) (group B) and active E. coli (group C), respectively. At 7 d after inoculation, a CT scan and a series of analyses using infecton imaging (at 0.5, 1, 2, 3, 4 and 6 h after the administration of 370 MBq of intravenous infecton) were performed. The scintigrams were visually evaluated and semi-quantitatively analyzed using region of interest assignments. The differences in infecton uptake and changes in the lesion-background radioactive count ratios (L/B) in the 3 groups were recorded and compared. After imaging detection, histopathology and bacterial examinations were performed, and infected SAP was regarded as positive. The imaging findings were compared with histopathological and bacteriological results.
RESULTS: In group A, 6 animals survived without infection in the pancreas. In group B, 7/9 swine survived and one suffered from infection. In group C, 15/18 animals survived with infection. Hence, the number of normal, non-infected and infected SAP swine was 6, 6 and 16, respectively. The sensitivity, specificity, accuracy, positive predictive value and negative predictive value of the infecton method were 93.8% (15/16), 91.7% (11/12), 92.9% (26/28), 93.8% (15/16) and 91.7% (11/12), whereas these values for CT were 12.5% (2/16), 100.0% (12/12), 50.0% (14/28), 100.0% (2/2) and 46.2% (12/26), respectively. The changes in L/B for the infected SAP were significantly different from those of the non-infected and normal swine (P < 0.001). The mean L/B of the infectious foci at 0.5, 1, 2, 3, 4 and 6 h was 1.17 ± 0.10, 1.71 ± 0.30, 2.46 ± 0.45, 3.36 ± 0.33, 2.04 ± 0.37 and 1.1988 ± 0.09, respectively. At 3 h, the radioactive counts (2350.25 ± 602.35 k) and the mean L/B of the infectious foci were significantly higher than that at 0.5 h (P = 0.000), 1 h (P = 0.000), 2 h (P = 0.04), 4 h (P = 0.000) and 6 h (P = 0.000).
CONCLUSION: 99mTc-ciprofloxacin scintigraphy may be an effective procedure for detecting SAP secondary infections with higher sensitivity and accuracy than CT.
Core tip: We successfully used a specific inflammatory agent, 99mTc-ciprofloxacin, which non-invasively detected secondary infections in an infective severe acute pancreatitis (SAP) model with higher sensitivity and accuracy than computed tomography. This method may be an effective tool for accurately diagnosing and assessing the severity of secondary infections in human SAP patients in the future. To our knowledge, there have been no previous studies that have compared the differential diagnosis of non-infectious and infectious SAP using 99mTc-ciprofloxacin imaging and histopathological and biological methods.
- Citation: Wang JH, Sun GF, Zhang J, Shao CW, Zuo CJ, Hao J, Zheng JM, Feng XY. Infective severe acute pancreatitis: A comparison of 99mTc-ciprofloxacin scintigraphy and computed tomography. World J Gastroenterol 2013; 19(30): 4897-4906
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4897.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4897
Infection of pancreatic or peripancreatic necroses occurs in 30%-70% of patients with severe acute pancreatitis (SAP)[1,2]. This disease is often accompanied by a late deterioration of organ function or generalized systemic illness[3], which is the leading cause of SAP-related deaths, with mortality rates of more than 30%[4-6]. Infected pancreatic necrosis in patients with clinical signs and symptoms of sepsis is an important indication for interventional therapy including surgery and drainage[7,8], whereas patients with sterile necrosis should be managed conservatively and undergo intervention only under certain circumstances[9].
However, until recently, the differential diagnosis of sterile and infectious SAP has remained a challenging issue. Effective biomarkers that will enable the localized diagnosis of infected pancreatic necrotic tissue are still under development and need to be confirmed in studies of larger patient cohorts[10,11]. Ultrasonography, computed tomography (CT) and magnetic resonance imaging have been used widely in the evaluation of pancreatitis, and CT is usually the first choice, however, these techniques have limitations in detecting secondary infections in cases of SAP i.e., unusual gas bubbles and typical manifestations of an abscess on the images. White blood cell (WBC) imaging is regarded as the principal nuclear medicine method for the imaging of infection and inflammation[12]. However, it is also difficult to distinguish between infective and sterile inflammatory conditions using this method[13]. Fine-needle aspiration has contributed to making a definitive diagnosis of infected pancreatic necrosis, but is an invasive procedure and there are difficulties in applying this technique to critically ill patients[10].
It is therefore essential to develop a sensitive and specific imaging methodology that will non-invasively detect secondary infections in SAP patients. Over the past few decades, a number of radiopharmaceuticals have been developed to investigate infective and non-infective inflammatory disorders[13-16]. In this regard, 99mTc-ciprofloxacin may be one of the most promising agents in the field of nuclear medicine[17,18]. This radiochemical combines the advantages of a 99mTc label and the broad-spectrum bacteria-localizing capability of ciprofloxacin, which has a higher sensitivity and specificity for bacterial infections than WBC scans[14,17-22].
We speculate that 99mTc-ciprofloxacin may have efficacy in the diagnosis of SAP secondary infections. In our study, a SAP secondary infection model was developed in swine as previously reported[23]. The features and effectiveness of 99mTc-ciprofloxacin scintigraphy in the diagnosis of secondary bacterial infection in this infective SAP animal model were then evaluated and compared with contrast-enhanced CT, and with histopathological and bacteriological testing.
This study was approved by the Animal Care Committee of Changhai Hospital. Healthy female Taihu swine (Experimental Animal Center of the Second Military Medical University, Shanghai, China), weighing 20-25 kg were acclimatized for one week before the start of the experiments. The animals had no access to food for 1 d and to water for 4 h prior to the start of the experiment.
Six healthy swine were assigned to group A as normal controls. SAP was induced in 27 animals as previously reported[23], and these animals were randomly assigned to group B (n = 9) and group C (n = 18). Two days after the onset of SAP, 4 mL of inactive Escherichia coli (E. coli) and active (108/mL) E. coli were inoculated into necrotic foci of the pancreas in group B and C swine by CT-guided puncture, respectively (Table 1). Imaging examinations were performed 7 d after inoculation. The swine received ketamine hydrochloride (0.1 mL/kg) before imaging examinations and received 2 mL pentobarbital (Bioszune Life Sciences, Beijing, China) solution (3% w/v) at 20-min intervals during the examinations.
| n | Inoculation | Survival number | Pathologic diagnosis and biological results | ||
| Infection (bacteria) | Non-infection | ||||
| Group A | 6 | No | 6 | 0 | 6 |
| Group B | 9 | Inactive E. coli | 7 | 1 (S. aureus/E.coli) | 6 |
| Group C | 18 | Active E. coli (108/mL) | 15 | 15 (14 E. coli and 1 S. aureus /E.coli) | 0 |
99mTc-ciprofloxacin was prepared by mixing 2 mg ciprofloxacin (Radiopharmaceuticals Laboratory of Beijing Normal University, China), 500 μg stannous tartrate, and 370 MBq freshly eluted sodium pertechnetate; then placed for 15 min at room temperature. Radiochemical purity was determined with a simple thin-layer chromatography technique, using 1-mm filters (Xinhua Group Co., Ltd., Hangzhou, China) in methyl ethyl ketone. 99mTc-ciprofloxacin remained at the base, and free pertechnetate moved with the solvent front. The Rf values of 99mTc-ciprofloxacin and 99mTcO4- were 1.0 and 0.0, respectively. The radiochemical purity and labeling rate of the radiopharmaceutical preparations were found to be greater than 90% at 6 h.
99mTc-ciprofloxacin was administered into the ear vein of the swine. Abdominal imaging was then performed using a dual-head single photon emission CT (SPECT) scanner (Philips, Forte, Netherlands). The energy peak was controlled at 140 KeV with a 15% window. Each animal underwent a 99mTc-ciprofloxacin scan at 0.5, 1, 2, 3, 4 and 6 h after the injection of radiolabeled ciprofloxacin. Multi-position graphic information with a total of 64 tomographic images was acquired continuously. The radioactivity counts for each frame were 300 k and the matrix size was 64 pixel × 64 pixel. Following acquisition, filter back projection reconstructions were performed.
The 99mTc-ciprofloxacin scintigrams were visually evaluated by three experienced nuclear medicine physicians in a blind fashion based on CT anatomic images. Sequential images captured from 0.5-6.0 h were mandatory for inclusion and interpretation. The scans were read independently, and any disagreements in interpretation were discussed and a consensus was reached on a majority basis. They were considered positive for infection when the pancreatic necrosis and peripancreatic tissue had a higher radionuclide uptake with a clear edge than the surrounding tissue, and negative for infection when the pancreatic necrosis and peripancreatic tissue had no significant radionuclide uptake. Diagnostic results were compared to bacterial culture and smear results. Semi-quantitative analysis was performed by determining the radioactivity counts of the pancreas, liver, spleen, renal, intestinal track, muscle and infectious foci in the pancreas using region of interest techniques, and the radioactivity of the muscle at the level of the pancreatic body was considered to be background. The measured values were averaged by three physicians. The lesion-to-background (L/B) ratios were then scored. The L/B curves changed with time in the groups and the optimal imaging time for diagnosis of infective SAP was thereby investigated.
CT was performed using a Sensation 64 scanner (Siemens Medical Solutions, Forchheim, Germany) 15 min after the SPECT scan. CT scanning (plain plus enhanced) was performed using the following parameters: a 3 mm slice thickness, 120 kV, 110 mAs, a 512 × 512 matrix, and 1.5 mL/kg of contrast material (Ultravist 300 mg I/mL; Schering AG, Germany) at a rate of 2 mL/s. The images were read by the same three nuclear medicine physicians and a consensus was reached on a majority basis according to the following criteria: visible gas bubbles scattered within the pancreatic necrosis or peripancreatic fluid on the CT images were considered positive[24].
After image examination, the animals were euthanized to remove the pancreas, and fluid was aspirated from the injection area or necrotic focus for bacterial culture or smear testing. Tissue samples were stained with hematoxylin and eosin (HE), and observed for evidence of pathologic changes to the pancreas. The criteria for diagnosing SAP secondary infection were as follows: (1) the appearance of the isolated pancreatic specimen was consistent with the pathologic diagnosis of SAP, whereby acute purulent inflammatory foci were present; and (2) the result of bacterial cultures from the necrotic area were positive or the presence of infection was confirmed using a smear. Diagnoses were made independently by a senior pathologist with no prior knowledge of the specimens.
Quantitative data were expressed as the mean ± SD. The sensitivity, specificity, accuracy, positive predictive value (PV+), and negative predictive value (PV-) of each imaging diagnosis were calculated. The effect of group and time on L/B was analyzed using two factor-repeated measure analysis of variance, the comparisons for the changes of L/B over time among groups were analyzed using one factor-repeated measure analysis of variance, the comparisons at the different time points in the same group and the comparisons among groups at the same time point were subjected to the Bonferroni test. Comparison of two rates was subjected to the χ2 test. SPSS 10.0 software (SPSS, Chicago, IL, United States) was used for analysis and P < 0.05 was considered statistically significant.
In group A, all six swine survived. In group B, 1 animal was excluded due to a main pancreatic duct intubation failure and another died of asphyxiation during anesthesia. In group C, one animal was also excluded from further analysis due to a main pancreatic duct intubation failure and two animals died of disease progression after the onset of SAP. Thus, in groups B and C, 7/9 and 15/18 were subjected to imaging analysis, respectively (Table 1).
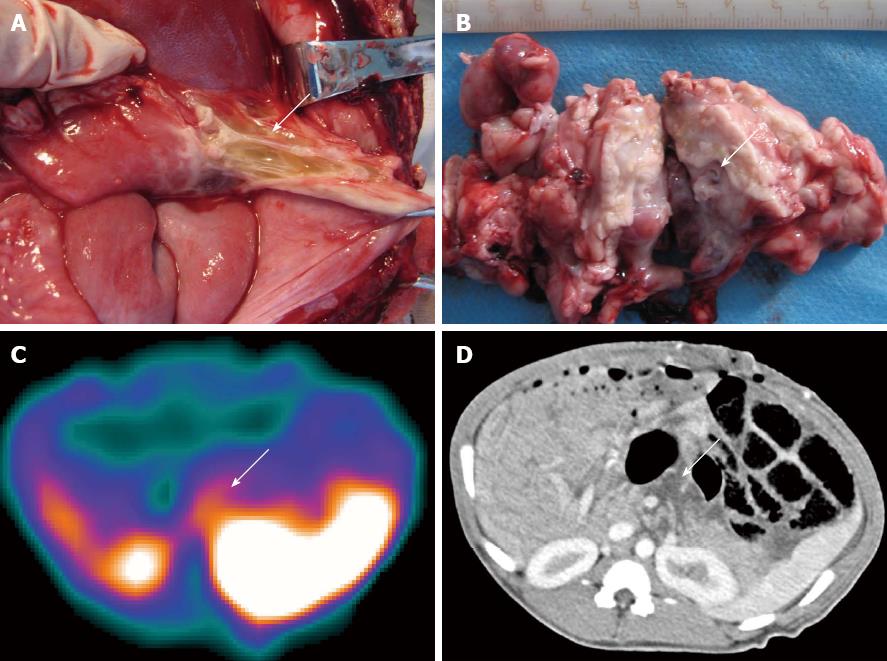
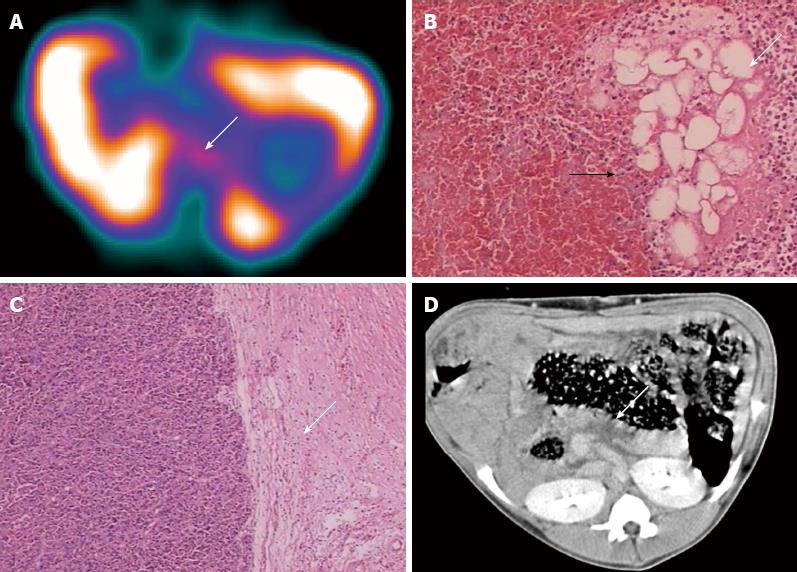
In group A, none of the swine showed infectious foci in the pancreas (Table 1). In group B, one of the seven animals (1/7) showed a focus with a Staphylococcus aureus/E. coli mixed infection. Light microscopy analysis of HE-stained sections revealed liquefactive necrosis in the center of this infectious focus. The remaining 6 animals in group B showed no bacterial infection (Table 1). In group C, 15 swine showed successful induction of a secondary infection. Bacterial culture and smear analysis of the necrotic foci in the pancreas showed that 14 SAP swine were infected with E. coli alone and 1 with a mixture of E. coli and Streptococcus, and showed intestinal perforation caused by this SAP secondary infection after paunching (Table 1). A total of 16 foci were found and yellow liquid flowed out of the cross-sections (Figure 1A and B). One animal had two cystic lesions in the pancreatic body and tail, with diameters of 19 and 5 mm, respectively. HE staining of infectious foci in group C showed liquefactive necrosis in most parts of the focal center, structureless substances in the fat cytoplasm, and coagulative necrosis in part of the foci.
In group A, 99mTc-ciprofloxacin scintigraphy revealed high radionuclide uptake in the kidneys, liver and spleen with excretion to the urinary bladder. No activity was observed in the area of the pancreas, normal bone marrow, muscle or gastrointestinal tract at any time point (Figure 2A). The CT images showed that the pancreatic parenchyma of all 6 animals were homogeneous and uniformly enhanced after contrast administration (Figure 2A).
In group B, no radionuclide uptake in the pancreatic areas was detected by SPECT in 5 of 7 animals at any time point (Figure 2B) and these swine were therefore diagnosed as negative for secondary infection (Table 2). Mild uptake in the pancreatic area was evident in one animal and the L/B was 2.15 at 3 h after administration, indicating a positive diagnosis of secondary infection (Table 2). However, pathology only displayed significant proliferation of granulated tissue at the edge of the necrotic area, no infectious focus was found in this animal by either pathologic or bacterial examinations (Figure 3A-C). Radionuclide uptake in the pancreatic area was detected in one animal, which was subsequently found to be infected with a mixture of S. aureus and E. coli in the pancreatic necrosis (Table 2). CT images revealed the pancreas had enlarged markedly and that the gastrointestinal tract was expanded and associated with effusion. Focal or pathy hypoattenuated areas within the pancreatic parenchyma were observed in all animals in group B and there were no signs of gas bubbles (Figures 2B and 3D).
| Pathologic diagnosis | SPECT | CT | |||
| Infection | Non-infection | Infection | Non-infection | ||
| Group A | Infection | 0 | 0 | 0 | 0 |
| Non-infection | 0 | 6 | 0 | 6 | |
| Group B | Infection | 1 | 0 | 0 | 1 |
| Non-infection | 1 | 5 | 0 | 6 | |
| Group C | Infection | 14 | 1 | 2 | 13 |
| Non-infection | 0 | 0 | 0 | 0 | |
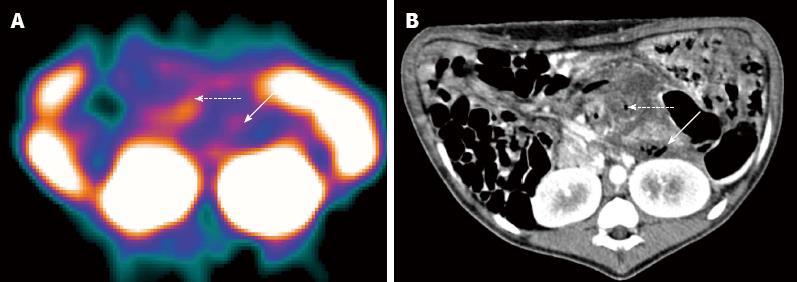
In group C, one animal was interpreted as negative by scintigraphy, which was proven to be a misdiagnosis by pathological examination, and by bacterial culture and smear testing. The remaining 14 animals in this group showed radioactive accumulations in the pancreatic area and were diagnosed as positive for secondary infection (Figures 1C and 2C), which was confirmed in each case by pathologic examination and bacterial culture (Table 2). One animal showed irregular patches of radioactivity accumulation around the pancreatic area due to intestinal perforations (Figure 4A). The animal with two lesions in the pancreatic body and tail was found to have a bigger focus in the pancreatic body, while smaller lesions were unclear.
CT images revealed that the pancreas was enlarged with irregular patchy and round-like cystic low-density necrotic areas, and effusion around the pancreas (Figures 1D and 2C). One animal had two round-like cystic lesions in the pancreatic body and tail, with diameters of 19 and 5 mm, respectively, without significant enhancement after contrast administration and were diagnosed as pseudocysts. Gas bubble signs were found in 2 swine, and one showed intestinal perforations and gas bubbles scattered throughout the necrotic area and in the peripancreatic fluid (Figure 4B), and the other showed gas bubbles in the pancreatic necrosis.
Based on our histopathological and biological results, the number of normal, non-infected and infected SAP swine was 6, 6 and 16, respectively (Table 1). It was calculated that 99mTc-ciprofloxacin scintigraphy had a sensitivity of 93.8% (15/16), a specificity of 91.7% (11/12), an accuracy of 92.9% (26/28), a PV+ of 93.8% (15/16), and a PV- of 91.7% (11/12) for detecting secondary bacterial infection associated with SAP (Table 3), and these values for CT were 12.5% (2/16), 100.0% (12/12), 50.0% (14/28), 100.0% and 46.2% (12/26), respectively. Of these parameters, sensitivity, accuracy and PV- were significantly lower than those of 99mTc-ciprofloxacin scintigraphy (P < 0.01) (Table 3).
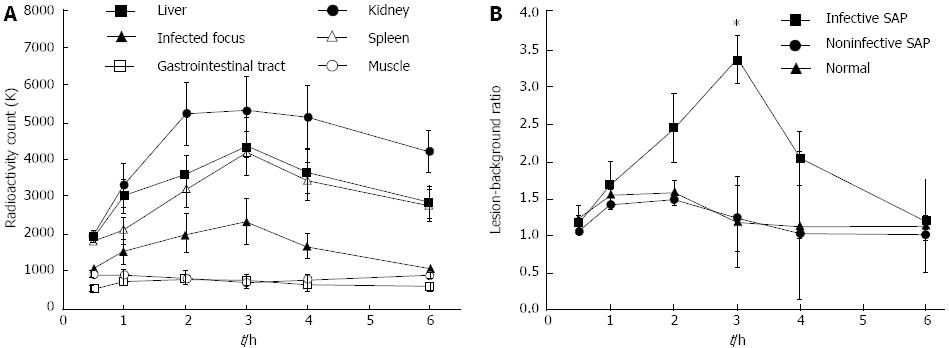
In infected SAP swine, the infectious foci in the pancreatic tissues showed no radionuclide uptake at 0.5 h, mild uptake at 1 and 2 h, and peak radioactivity counts at 3 h (2350.25 ± 602.35 k), and then gradually decayed from 4-6 h. The change was different in the kidney, liver, spleen, gastrointestinal tract and muscle (Figure 5A). The L/B in 6 normal swine, 6 non-infected SAP and 16 infected SAP animals at 0.5, 1, 2, 3, 4, 6 h after the administration of 99mTc-ciprofloxacin are presented in Figure 5B. There were significant differences in the L/B changes over time among the three study groups (F = 95.66, P < 0.001). These changes in the infected SAP animals differed significantly from those in the non-infected SAP (F = 88.63, P = 3.1e-16) and normal swine (F = 63.61, P = 8.2e-13). In contrast, no significant differences were found between the non-infected SAP and normal groups (t = 1.17, P = 0.251). The L/B ratio at 3 h after the administration of 99mTc-ciprofloxacin in the infected SAP swine reached 3.36 ± 0.33, which was significantly higher than at all other time points (P values were 1.1e-35, 3.5e-27, 3.9e-13, 4.2e-21 and 2.5e-35, respectively).
Secondary infection of pancreatic necrotic tissue is accepted as one of the most important prognostic indicators of disease severity and outcomes in SAP cases[4-6]. Early diagnosis is the key to improved treatment outcomes and reduced mortality. Although CT plays an important role in the diagnosis of SAP, it can not detect the sites of secondary infection with sufficient sensitivity as it can only do so if gas bubbles appear within the lesion, which occurs infrequently. Indeed, Bhansali et al[25] reported previously that bubbles were detectable in only 16% of SAP patients with secondary infections using CT imaging (21/131 cases). Our present experimental results indicate that the sensitivity of CT is too low (12.5%, 2/16) to accurately diagnose a SAP secondary infection.
The novel radiopharmaceutical used in our scintigraphy analysis is based on the 4-fluoroquinolone broad spectrum antibiotic ciprofloxacin. Following intravenous injection, ciprofloxacin is widely distributed in the body and is excreted via the kidneys. The mode of action of ciprofloxacin is mediated via inactivation of the bacterial DNA gyrase, which results in the retention of this agent at the sites of active bacterial infection[18]. Sierra et al[26] analyzed the mechanism of intracellular accumulation of 99mTc-ciprofloxacin in Staphylococcus aureus and Pseudomonas aeruginosa strains, and found that 99mTc-ciprofloxacin was equally accumulated intracellularly in all tested strains, while 99mTcO4- did not show accumulation in any of the strains. The absence of intracellular 99mTcO4- indicated that the entire radioactivity detected in the 99mTc-ciprofloxacin assay was due to the accumulation of the radiopharmaceutical compound, rather than free 99mTcO4-.
99mTc-ciprofloxacin has been widely tested in clinical studies and shown to be taken up by a wide range of live (but not dead) bacteria in vitro and in vivo [12,13,17,18]. It has also been reported that the specificity of 99mTc-ciprofloxacin scintigraphy reached 85%-96% in detecting both bone and joint bacterial infections[27-29] and showed a sensitivity and specificity of 91.7% and 75%, respectively, in the diagnosis of acute bacterial cholecystitis[19]. In our present study, the sensitivity, specificity and accuracy of 99mTc-ciprofloxacin were found to be 93.8%, 91.7% and 92.9%, respectively. The accuracy was higher than that of CT. We found that 99mTc-ciprofloxacin scintigraphy had some important advantages over WBC imaging which have also been reported by other studies. Firstly, the technique used for preparation is easy, and can be carried out without withdrawing blood, purifying leucocytes, labeling and reinjecting the radiolabeled cells, compared to WBC imaging. Secondly, no adverse effects were reported in response to intravenous administration of 99mTc-ciprofloxacin. Thirdly, both the radiochemical purity and labeling rate were found to be over 90% within 6 h at room temperature. Hence, 99mTc-ciprofloxacin is an ideal specific targeting agent for the detection of bacterial infection[17,27-29].
In infected SAP cases, the radioactivity levels in the bacterial foci were higher than in the surrounding SAP, muscle or soft tissues. Pronounced focal accumulation was evident at 3 h after administration, when both the radioactivity counts and lesion-background ratios were at peak levels. This suggests that 99mTc-ciprofloxacin scintigraphy is a suitable diagnostic test for SAP patients with a suspected secondary infection. However, both false-positive and false-negative results still arise in 99mTc-ciprofloxacin scintigraphy. In our present analyses, one false-negative result was found in a group C animal for which the pathology showed a pancreatic necrosis with a mild infection. This may suggest that the detectable uptake of 99mTc-ciprofloxacin has a severity of infection threshold. One false-positive result was also found in an animal showing marked hyperplasia of granulated and fibrous tissue at the edge of the pancreatic necrosis, which suggested that radioactive uptake may be related to granulated tissue repair at the edge of necrotic foci, as well as an increase in blood perfusion or capillary permeability[30].
We also found that SPECT visualization has disadvantages, including low resolution and poor display of anatomic structure. It can not display the shape and area of pancreatic necrotic foci, nor display the non-infected foci with peripancreatic fluid or pseudocysts. However, while the clinical use of SPECT-CT is widely accepted, the resolution and capability for displaying anatomical details of the SPECT-CT scanner are much improved[31]. Incorporating SPECT with CT in one scanner, which has the advantages of the two imaging techniques, makes it possible to evaluate and diagnose the infected foci with indefinite anatomical localization and low radioactive uptake.
In summary, 99mTc-ciprofloxacin scintigraphy has a higher sensitivity and accuracy in the detection of bacterial infections than CT. Moreover, this agent is not taken up in a normal pancreas and non-infected SAP, which could be highly useful in the detection of infectious SAP. This method may therefore become an effective tool in the future for accurately diagnosing and assessing the severity of secondary infections in human SAP patients. Undoubtedly, it is very important for clinicians to develop treatment programs and improve the efficacy of SAP. 99mTc-ciprofloxacin scintigraphy, 18F-FDG PET and diffusion-weighted imaging (DWI) have been applied widely for the diagnosis of infection, but they have advantages and limitations[32-36]. In the future, we will evaluate the efficacy of these commonly used techniques in the diagnosis of secondary infection of SAP.
The authors thank Dr. Feng Qian and Dr. Fang-Yuan Ren for technical support.
Secondary infection is one of the most challenging problems in the treatment of severe acute pancreatitis (SAP). Infected pancreatic necrosis in patients with the clinical signs and symptoms of sepsis is an important indication for interventional therapy including surgery and drainage, whereas patients with sterile necrosis should be managed conservatively and undergo intervention only under certain circumstances. Conventional scintigraphic and radiologic methods have limitations in the detection of secondary infection of SAP. It is therefore essential to develop a sensitive and specific imaging methodology that will non-invasively detect secondary infections in SAP patients. However, to their knowledge, only a few authors have applied traditional radionuclide imaging agents for the diagnosis of pancreatitis associated with infection. The utility of infecton in the detection of SAP secondary infection is still unclear.
In the area of SAP, one of the research hotspots is the detection of secondary infection of pancreatic necrosis. Over the past few decades, a number of radiopharmaceuticals have been developed to investigate infective and non-infective inflammatory disorders. In this regard, 99mTc-ciprofloxacin may be one of the most promising agents in the field of nuclear medicine. This radiochemical combines the advantages of a 99mTc label and the broad-spectrum bacteria-localizing capability of ciprofloxacin, which has a higher sensitivity and specificity for bacterial infections than white blood cell scans.
The authors successfully used a specific inflammatory agent, 99mTc-ciprofloxacin, which non-invasively detected secondary infections in an infective SAP model with higher sensitivity and accuracy than computed tomography (CT). To our knowledge, there have been no previous studies that have compared the differential diagnosis of non-infectious and infectious SAP using 99mTc-ciprofloxacin imaging and histopathological and biological methods.
This method may be an effective tool in the future for accurately diagnosing and assessing the severity of secondary infections in human SAP patients. Undoubtedly, it is very important for clinicians to develop treatment programs and improve the efficacy of SAP.
SAP is defined as necrosis involving at least 30% of the pancreas as visualized by contrast-enhanced CT, with greater involvement indicating greater severity of necrosis. It is a special type of acute pancreatitis with more complications and high mortality. The 4-fluoroquinolone broad spectrum antibiotic, ciprofloxacin, was labeled with 99mTcO4-. The mode of action of ciprofloxacin is mediated via the inactivation of bacterial DNA gyrase, which results in the retention of 99mTc-ciprofloxacin at the sites of active bacterial infection.
This is a well-presented manuscript describing a good research protocol on the use of 99mTc-ciprofloxacin in the detection of infection in severe acute pancreatitis in an animal model. The single photon emission CT (SPECT) images, with high background activity in the nearby organs, are indistinct, as is to be expected at this resolution. As the authors themselves have pointed out, SPECT-CT hybrid imaging would provide better images and localize the abnormal tracer activity more precisely to the focus of infection. The results are interesting and suggest that 99mTc-ciprofloxacin scintigraphy may therefore become an effective tool in the future for accurately diagnosing and assessing the severity instances of secondary infections in human SAP patients.
P- Reviewers Bradley EL, Bramhall SR, Kochhar R S- Editor Gou SX L- Editor Webster JR E- Editor Ma S
| 1. | Beger HG, Rau BM. Severe acute pancreatitis: Clinical course and management. World J Gastroenterol. 2007;13:5043-5051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Yousaf M, McCallion K, Diamond T. Management of severe acute pancreatitis. Br J Surg. 2003;90:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg. 1999;86:1020-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Sekimoto M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Hirota M, Kimura Y, Takeda K, Isaji S. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:10-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Le Mée J, Paye F, Sauvanet A, O’Toole D, Hammel P, Marty J, Ruszniewski P, Belghiti J. Incidence and reversibility of organ failure in the course of sterile or infected necrotizing pancreatitis. Arch Surg. 2001;136:1386-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Amano H, Takada T, Isaji S, Takeyama Y, Hirata K, Yoshida M, Mayumi T, Yamanouchi E, Gabata T, Kadoya M. Therapeutic intervention and surgery of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Uhl W, Warshaw A, Imrie C, Bassi C, McKay CJ, Lankisch PG, Carter R, Di Magno E, Banks PA, Whitcomb DC. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology. 2002;2:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, Imrie C, Tandon R. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17 Suppl:S15-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Schneider L, Büchler MW, Werner J. Acute pancreatitis with an emphasis on infection. Infect Dis Clin North Am. 2010;24:921-941, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Isenmann R, Beger HG. Natural history of acute pancreatitis and the role of infection. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Weiss G, Meyer F, Lippert H. Infectiological diagnostic problems in tertiary peritonitis. Langenbecks Arch Surg. 2006;391:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Hovi I, Taavitsainen M, Lantto T, Vorne M, Paul R, Remes K. Technetium-99m-HMPAO-labeled leukocytes and technetium-99m-labeled human polyclonal immunoglobulin G in diagnosis of focal purulent disease. J Nucl Med. 1993;34:1428-1434. [PubMed] |
| 13. | Vinjamuri S, Hall AV, Solanki KK, Bomanji J, Siraj Q, O’Shaughnessy E, Das SS, Britton KE. Comparison of 99mTc infecton imaging with radiolabelled white-cell imaging in the evaluation of bacterial infection. Lancet. 1996;347:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Halder KK, Nayak DK, Baishya R, Sarkar BR, Sinha S, Ganguly S, Debnath MC. (99m)Tc-labeling of ciprofloxacin and nitrofuryl thiosemicarbazone using fac-[(99m)Tc(CO)3(H2O)3] core: evaluation of their efficacy as infection imaging agents. Metallomics. 2011;3:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Sachin K, Kim EM, Cheong SJ, Jeong HJ, Lim ST, Sohn MH, Kim DW. Synthesis of N4’-[18F]fluoroalkylated ciprofloxacin as a potential bacterial infection imaging agent for PET study. Bioconjug Chem. 2010;21:2282-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Dahiya S, Chuttani K, Khar RK, Saluja D, Mishra AK, Chopra M. Synthesis and evaluation of Ciprofloxacin derivatives as diagnostic tools for bacterial infection by Staphylococcus aureus. Metallomics. 2009;1:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | De Winter F, Van de Wiele C, Dumont F, Van Durme J, Solanki K, Britton K, Slegers G, Dierckx RA, Thierens H. Biodistribution and dosimetry of 99mTc-ciprofloxacin, a promising agent for the diagnosis of bacterial infection. Eur J Nucl Med. 2001;28:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Choe YM, Choe W, Lee KY, Ahn SI, Kim K, Cho YU, Choi SK, Hur YS, Kim SJ, Hong KC. Tc-99m ciprofloxacin imaging in acute cholecystitis. World J Gastroenterol. 2007;13:3249-3252. [PubMed] |
| 20. | Malamitsi J, Papadopoulos A, Vezyrgianni A, Dalianis K, Boutsikou M, Giamarellou H. The value of successive Infecton scans in assessing the presence of chronic bone and joint infection and in predicting its evolution after treatment and after a prolonged follow-up. Nucl Med Commun. 2011;32:1060-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Hall AV, Solanki KK, Vinjamuri S, Britton KE, Das SS. Evaluation of the efficacy of 99mTc-Infecton, a novel agent for detecting sites of infection. J Clin Pathol. 1998;51:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | West JH, Vogel SB, Drane WE. Gallium uptake in complicated pancreatitis: a predictor of infection. AJR Am J Roentgenol. 2002;178:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Wang J, Shao C, Zuo C, Zheng J, Hao J, Shao C, Zhang F, Sun G, Liu Y. Establishment of a secondary infection model of severe acute pancreatitis in swine. Pancreas. 2011;40:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297-306. [PubMed] |
| 25. | Bhansali SK, Shah SC, Desai SB, Sunawala JD. Infected necrosis complicating acute pancreatitis: experience with 131 cases. Indian J Gastroenterol. 2003;22:7-10. [PubMed] |
| 26. | Sierra JM, Rodriguez-Puig D, Soriano A, Mensa J, Piera C, Vila J. Accumulation of 99mTc-ciprofloxacin in Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:2691-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Fuster D, Soriano A, Garcia S, Piera C, Suades J, Rodríguez D, Martinez JC, Mensa J, Campos F, Pons F. Usefulness of 99mTc-ciprofloxacin scintigraphy in the diagnosis of prosthetic joint infections. Nucl Med Commun. 2011;32:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Sonmezoglu K, Sonmezoglu M, Halac M, Akgün I, Türkmen C, Onsel C, Kanmaz B, Solanki K, Britton KE, Uslu I. Usefulness of 99mTc-ciprofloxacin (infecton) scan in diagnosis of chronic orthopedic infections: comparative study with 99mTc-HMPAO leukocyte scintigraphy. J Nucl Med. 2001;42:567-574. [PubMed] |
| 29. | Sharma R, Tewari KN, Bhatnagar A, Mondal A, Mishra AK, Singh AK, Chopra MK, Rawat H, Kashyap R, Tripathi RP. Tc-99m ciprofloxacin scans for detection of tubercular bone infection. Clin Nucl Med. 2007;32:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Britton KE, Vinjamuri S, Hall AV, Solanki K, Siraj QH, Bomanji J, Das S. Clinical evaluation of technetium-99m infecton for the localisation of bacterial infection. Eur J Nucl Med. 1997;24:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Filippi L, Uccioli L, Giurato L, Schillaci O. Diabetic foot infection: usefulness of SPECT/CT for 99mTc-HMPAO-labeled leukocyte imaging. J Nucl Med. 2009;50:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | van der Bruggen W, Bleeker-Rovers CP, Boerman OC, Gotthardt M, Oyen WJ. PET and SPECT in osteomyelitis and prosthetic bone and joint infections: a systematic review. Semin Nucl Med. 2010;40:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Brown TL, Spencer HJ, Beenken KE, Alpe TL, Bartel TB, Bellamy W, Gruenwald JM, Skinner RA, McLaren SG, Smeltzer MS. Evaluation of dynamic [18F]-FDG-PET imaging for the detection of acute post-surgical bone infection. PLoS One. 2012;7:e41863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Ioannou S, Chatziioannou S, Pneumaticos SG, Zormpala A, Sipsas NV. Fluorine-18 fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan contributes to the diagnosis and management of brucellar spondylodiskitis. BMC Infect Dis. 2013;13:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Israel O, Keidar Z. PET/CT imaging in infectious conditions. Ann N Y Acad Sci. 2011;1228:150-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Chiang IC, Hsieh TJ, Chiu ML, Liu GC, Kuo YT, Lin WC. Distinction between pyogenic brain abscess and necrotic brain tumour using 3-tesla MR spectroscopy, diffusion and perfusion imaging. Br J Radiol. 2009;82:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |