Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4887
Revised: June 6, 2013
Accepted: June 19, 2013
Published online: August 14, 2013
Processing time: 202 Days and 11.8 Hours
AIM: To evaluate the histopathological findings of type C liver disease to determine risk factors for development of hepatocellular carcinoma (HCC).
METHODS: We studied 232 patients, who underwent liver biopsy for type C chronic liver disease between 1992 and 2009, with sustained virological response (SVR) after interferon therapy. The patients were divided into two groups according to the F stage 0 + 1 + 2 group (n = 182) and F3 + 4 group (n = 50). We prospectively observed and compared the incidence of HCC of the patients with SVR in the F0 + 1 + 2 and F3 + 4 groups. Then, the background factors and liver histopathological findings, including the degree of fibrosis, F stage, inflammation, necrosis, bile duct obstruction, fat deposition, and degree of irregular regeneration (IR) of hepatocytes, were correlated with the risk of developing HCC.
RESULTS: HCC developed in three of 182 (1.6%) patients in the F0 + 1 + 2 group, and four of 50 (8.0%) in the F3 + 4 group. The cumulative incidence of HCC in the former group was found to be significantly lower than in the F3 + 4 group (log rank test P = 0.0224). The presence of atypical hepatocytes among IR of hepatocytes in the F3 + 4 group resulted in a higher cumulative incidence of HCC, and was significantly correlated with risk of HCC development (RR = 20.748, 95%CI: 1.335-322.5, P = 0.0303).
CONCLUSION: Atypical hepatocytes among the histopathological findings of type C liver disease may be an important risk factor for HCC development along with progression of liver fibrosis.
Core tip: To evaluate the histopathological findings of type C liver disease to determine risk factors for the development of hepatocellular carcinoma (HCC), we studied 232 patients, who underwent liver biopsy, with sustained virological response after interferon therapy. We investigated in detail the histopathological findings, and analyzed the findings to determine the risk factors. Consequently, atypical hepatocytes among irregular regeneration of hepatocytes may be an important risk factor for HCC development, along with progression of liver fibrosis. Clarification of atypical hepatocytes as a risk factor of carcinogenesis may aid in the early diagnosis of HCC.
- Citation: Matsumura H, Nirei K, Nakamura H, Higuchi T, Arakawa Y, Ogawa M, Tanaka N, Moriyama M. Histopathology of type C liver disease for determining hepatocellular carcinoma risk factors. World J Gastroenterol 2013; 19(30): 4887-4896
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4887.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4887
At our facility, we maintain a database of hepatitis patients who were recruited with informed consent from the 1990s. We previously reported the natural progression of patients with type C chronic hepatitis (CH) and liver cirrhosis (LC) - both attributable to hepatitis C virus (HCV) infection - over the course of about 30 years. The incidence of hepatocellular carcinoma (HCC) among those with LC was 7% per annum[1,2]. Many other studies have reported the progression of liver disease and the risk of development of HCC with HCV infection[3-8].
In this study, we collected liver biopsy samples from type C chronic liver disease patients before interferon (IFN) therapy and examined in detail the histopathological findings of the liver tissue. According to the progression of liver fibrosis (F stage), we examined the factors that may contribute to the development of HCC. We further studied the association of the condition of the hepatocytes, present during regeneration of the liver parenchyma destroyed by infection, with the incidence of HCC. The irregular regeneration (IR) of hepatocytes, which occurs as a result of repeated cycles of necrosis and regeneration of the liver parenchyma in CH, was found to be important for the prognosis of HCC[9-15]. A few studies have sought to determine the association between the histopathological findings from liver biopsies and the risk of developing HCC[4]. Therefore, patients with type C chronic liver disease who underwent liver biopsy were investigated in detail for findings on the sites and degree of fibrosis and inflammation, necrosis, IR of hepatocytes, and fat deposition in the liver. These findings were analyzed to determine the risk factors for development of HCC.
In this study, samples from 482 patients with type C liver disease who underwent liver biopsy between 1992 and 2009 were collected prior to the start of IFN therapy. Two hundred and thirty-two patients achieved a sustained virological response (SVR) to IFN therapy. We determined the F stage in the liver (stages 0-4, as described for histological scoring) by liver biopsy according to the method of Desmet et al[16], Knodell et al[17] and Ishak et al[18]. Then, 232 patients were divided into two groups according to the F stage: stages 0, 1 and 2 (F0 + 1 + 2 group, n = 182) and stages 3 and 4 (F3 + 4 group, n = 50). We prospectively observed and compared the incidence of HCC in the patients with SVR in the F0 + 1 + 2 and F3 + 4 groups. The observation period was from the time of diagnosis of SVR to the time of the final examination or of diagnosis of HCC. Then, the clinical background factors and the histopathological findings at the liver biopsy were correlated with the risk of developing HCC together with progressive liver fibrosis.
All of the patients were positive for serum HCV antibody (2nd generation ELISA; Dinabot, Tokyo, Japan) and HCV RNA in serum, and negative for serum hepatitis B surface antigen (HBsAg ELISA; Dinabot), anti-nuclear antibody (indirect immunofluorescence assay: IF, Special Reference Laboratory, Tokyo, Japan), anti-smooth muscle antibody (IF), and anti-mitochondrial antibody (IF). No heavy drinkers (more than 30 g of ethanol intake daily) were included.
We confirmed the positivity and measured the concentration of HCV RNA in the blood samples using the competitive reverse transcriptase-polymerase chain reaction and DNA probe methods (Special Reference Laboratory, Tokyo, Japan) or Amplicor monitoring method (Amplicor HCV Monitor, Roche Diagnostic K.K., Tokyo, Japan). Subjects without HCC were confirmed by abdominal computed tomography (CT) or ultrasonography within the 6 mo prior to initial liver biopsy. At the time of liver biopsy, we determined the patient’s body mass index, serum aspartate aminotransferase (AST) level (U/L), serum alanine aminotransferase (ALT) level (U/L), serum γ-glutamyltransferase (GT) level (U/L), serum platelet count (× 104/μL), and HCV genotype, and carried out a histopathological examination of the biopsy specimens. We recorded the patient’s sex and age, period of observation (years), and incidence of HCC. All patients gave their consent to be included in this study according to the Declaration of Helsinki.
Initial IFN therapy varied according to the time period when the patients were treated. From 1992 to 2001, IFN monotherapy was administered using natural (n) IFN (n-IFNα: Sumiferon, Sumitomo Pharma Co., Osaka, Japan) in 155 cases, recombinant (r)-α2a (r-IFNα2a: Canferon, Takeda Pharmaceutical Co., Osaka, Japan) in 44 cases, and r-α2b (r-IFNα2b:Intron, Schering-Plough Co., Osaka, Japan) in 121 cases. From 2001 to 2007, we treated 68 patients with combination therapy of IFN-α2b and ribavirin (Rebetol; Schering-Plough Co.). From 2007 to 2009, we administered combination therapy of Peg-IFN-α2b (Peg Intron; Schering-Plough Co.) and ribavirin in 93 cases. In accordance with the protocol based on the medical insurance system in Japan, considering the HCV genotype, we administered IFN therapy for 6-12 mo. The following classification of outcomes was determined according to the effects of IFN therapy: SVR was achieved in patients who became negative for serum HCV RNA for > 6 mo after termination of IFN therapy.
Liver tissues were taken from patients by percutaneous needle biopsy (MONOPTY, 14 or 16 gauge; C. R. Bard Inc., Tempe, AZ, United States) with the aid of an ultrasonic echo guide within the 6 mo preceding the start of IFN therapy. These tissue specimens were fixed in 2.5% formalin, embedded in paraffin, sectioned at 3-4 μm, and stained with hematoxylin and eosin (HE).
The tissues obtained by liver biopsy were evaluated for the sites and degree of fibrosis, inflammatory reaction, and necrosis, as well as the degree of IR of hepatocytes, bile duct obstruction, and fat deposition in the liver.
The histopathological findings were scored using 5 grades: score 0, none of the above features; score 1, minimal detection (observed in less than one-third of the field); score 2, mild (observed in one-third to less than two-thirds of the field); score 3, moderate (observed in two-thirds or more of the field), and score 4, severe (diffusely in all fields). The samples were scored blindly by two pathologists (Moriyama and the author), and the final value was calculated as the average of their scores (mean ± SD).
Only minimal fibrosis was observed in the portal areas of normal livers. Pericellular fibrosis was defined as fibrosis around the hepatocytes, perivenular fibrosis as fibrosis around the central vein, and portal sclerosis as fibrosing expansion of the portal area. The degree of pericellular fibrosis, perivenular fibrosis, and portal sclerosis were evaluated and assigned a score from 0 to 4 as described above for histopathological scoring. According to the method of Desmet et al[16] and Knodell et al[17], we determined the F stage in the liver (stages 0-4, as described above for histopathological scoring), including bridging fibrosis.
In evaluating hepatocellular necrosis, focal necrosis was defined as necrosis of several hepatocytes with infiltration of inflammatory cells such as macrophages and lymphocytes. Bridging necrosis refers to necrosis connecting areas, such as two portal tracts, leading to extensive hepatocellular necrosis and a state of acute hepatic failure. The patients were further defined as with (Y) or without bridging necrosis (N).
Infiltration of lymphocytes is a characteristic of viral hepatitis. The degree of inflammatory cell infiltration into the liver parenchyma (parenchymal infiltration) and portal area (periportal infiltration) were assessed, and assigned a score from 0 to 4 as described above for histopathological scoring. The degree of lymphocytic infiltration into the entire hepatic lobule (lymphoid reaction) and portal area (portal lymphocyte infiltration) was also evaluated and assigned a score from 0 to 4 as described above for histopathological scoring.
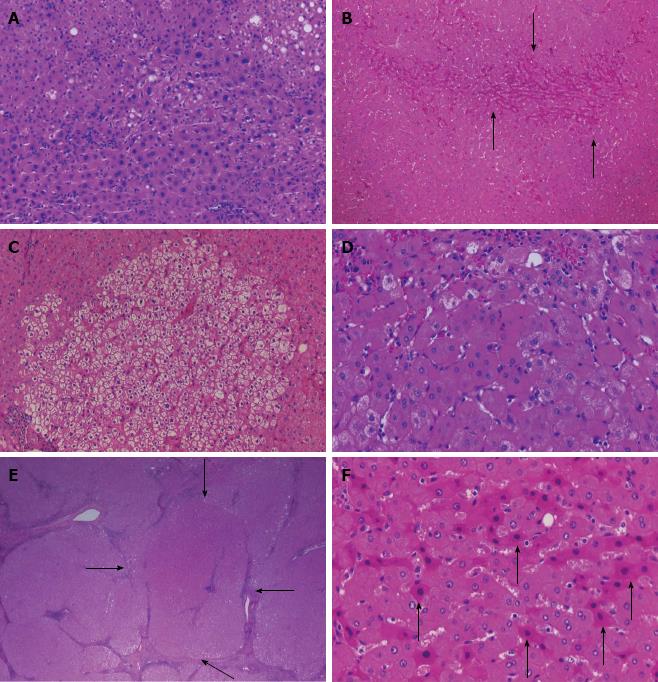
We also investigated the histopathological abnormalities characteristic of IR of hepatocytes (Figure 1), which were evaluated in each sample according to the criteria by Uchida[9], Shibata et al[10], Ueno et al[11] and Moriyama et al[12]. IR comprises the following six elements: dysplastic change, map-like distribution, bulging, oncocytes, nodularity, and atypical hepatocytes. Based on these, we examined the liver biopsy samples for: presence and degree of dysplastic change defined as anisocytosis characterized by variability of the cells; presence and degree of map-like distribution, which is defined as distinct populations of hepatocytes with a homogeneous appearance within each population and separated from each other by a sharp outline; presence and degree of bulging, which is defined as expansive proliferation of hepatocytes compressing the surrounding parenchyma; presence of oncocytes with nonuniformity of the cytoplasm; presence and degree of nodality, defined as nodular appearance of hepatocytes; and presence of atypical hepatocytes characterized by cellular degeneration. Each finding of IR was evaluated and assigned a score from 0 to 4 as described above for histopathological scoring.
Bile duct obstruction was investigated according to the degree of cholangitis by lymphocytes (bile duct damage) and assigned a score from 0 to 4 as described above for histopathological scoring. Finally, the degree of fat deposition in liver (steatosis) was assessed according to the method by Brunt et al[19] and Matteoni et al[20], and assigned a score from 0 to 4 as described above for histopathological scoring.
Patients with IFN therapy underwent abdominal ultrasonography every 3 or 6 mo and abdominal CT examination every 6-12 mo to check for the occurrence of HCC. When space occupying lesions (SOLs) were detected in the livers of the patients by dynamic CT, enhancement of SOLs was observed in the early phase of dynamic CT and the disappearance of SOL staining was observed in the late phase. A precise diagnosis was made by abdominal angiography. When SOLs in the liver were not enhanced in the early phase of dynamic CT, or if a precise diagnosis could not be made by abdominal angiography, tumor biopsy was carried out and a precise diagnosis was made on the basis of the pathological findings.
Sex, genotype, and bridging necrosis (Y/N) were compared using the χ2 test for independence. The remaining parameters including the clinical characteristics at the time of liver biopsy and the liver histopathological findings are shown as mean ± SD and were compared using the Mann-Whitney U test. Cumulative incidence curves were determined with the Kaplan-Meier method and the differences between groups were assessed using the log-rank test. Analysis of risk factors for HCC occurrence was made using the Cox proportional hazard model and these were compared by multivariate analysis. These were performed using SPSS 11.0 software (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered significant.
Comparison of clinical background factors at the time of liver biopsy and liver histopathological findings in SVR patients according to the F stage (F0 + 1 + 2, n = 182 and F3 + 4, n = 50) was performed (Tables 1 and 2). As demonstrated in Table 2, the number of patients for each score was compared, then the presence of bridging necrosis (Y/N) was compared, and the remaining parameters are presented as mean ± SD. The findings demonstrated that there was a higher level of deterioration of the liver in the F3 + 4 group as compared to the F0 + 1 + 2 group; liver fibrosis was more progressive, age, AST, ALT, and α-fetoprotein (AFP) were higher, platelet count was lower, and inflammatory reaction was stronger.
| Parameter | F0 + 1 + 2 | F3 + 4 | P value | |
| Number | 232 | 182 | 50 | |
| Gender (male) | 136 (58.6) | 104 (57.1) | 32 (64.0) | 0.3832 |
| Age (yr) | 47.9 ± 12.2 | 45.9 ± 12.4 | 55.2 ± 8.3 | 0.0001 |
| BMI | 22.6 ± 3.3 | 22.7 ± 3.3 | 22.2 ± 3.6 | 0.5466 |
| AST (U/L) | 55.2 ± 45.9 | 49.8 ± 46.6 | 74.5 ± 37.6 | 0.0007 |
| ALT (U/L) | 75.5 ± 62.7 | 70.5 ± 63.0 | 93.5 ± 58.9 | 0.0216 |
| γ-GT (U/L) | 62.3 ± 71.8 | 61.4 ± 76.7 | 65.8 ± 50.4 | 0.7048 |
| Platelet count (× 104/μL) | 18.8 ± 6.2 | 20.2 ± 6.0 | 13.9 ± 3.9 | 0.0001 |
| Genotype type 1/2 | 108 (46.5)/ 124 (53.5) | 86 (47.2)/ 96 (52.8) | 22 (44.0)/ 28 (56.0) | 0.6830 |
| AFP (ng/mL) | 7.7 ± 13.3 | 5.4 ± 8.6 | 15.5 ± 21.4 | 0.0008 |
| Observation period (yr) | 7.5 ± 4.9 | 7.4 ± 4.7 | 8.0 ± 5.5 | 0.4350 |
| Parameter | Score | F0+1+2 | F3+4 | P value | ||||
| 0 | 1 | 2 | 3 | 4 | ||||
| Irregular regeneration | ||||||||
| Dysplastic change | 109 | 50 | 49 | 21 | 3 | 0.769 ± 0.998 | 1.660 ± 1.081 | 0.0001 |
| Oncocytes | 211 | 16 | 4 | 1 | 0 | 0.066 ± 0.290 | 0.300 ± 0.647 | 0.0003 |
| Map-like distribution | 160 | 37 | 22 | 13 | 0 | 0.396 ± 0.756 | 0.960 ± 1.142 | 0.0001 |
| Nodular arrangement | 216 | 13 | 1 | 2 | 0 | 0.027 ± 0.164 | 0.320 ± 0.713 | 0.0001 |
| Bulging | 206 | 18 | 6 | 2 | 0 | 0.104 ± 0.400 | 0.340 ± 0.688 | 0.0022 |
| Atypical hepatocytes | 227 | 5 | 0 | 0 | 0 | 0.005 ± 0.074 | 0.080 ± 0.274 | 0.0012 |
| Inflammatory cell infiltration | ||||||||
| Peri-portal | 0 | 65 | 127 | 35 | 5 | 1.819 ± 0.652 | 2.260 ± 0.828 | 0.0001 |
| Parenchymal | 0 | 37 | 149 | 44 | 2 | 1.978 ± 0.585 | 2.300 ± 0.678 | 0.0010 |
| Portal lymphocyte | 0 | 5 | 103 | 121 | 3 | 2.456 ± 0.562 | 2.780 ± 0.507 | 0.0003 |
| Portal lymphoid reaction | 0 | 6 | 47 | 78 | 101 | 3.132 ± 0.850 | 3.360 ± 0.802 | 0.0904 |
| Fibrosis | ||||||||
| F stage | 5 | 128 | 49 | 34 | 16 | |||
| Portal sclerosis | 113 | 81 | 32 | 6 | 0 | 0.615 ± 0.783 | 1.020 ± 0.795 | 0.0014 |
| Pericellular fibrosis | 89 | 90 | 41 | 11 | 1 | 0.802 ± 0.870 | 1.260 ± 0.853 | 0.0011 |
| Perivenular fibrosis | 63 | 66 | 65 | 25 | 0 | 1.236 ± 1.016 | 1.243 ± 0.895 | 0.9691 |
| Bridging necrosis Y (presence) | 0/228 | 4/228 (1.7%) | 0.0020 | |||||
| Bile duct damage | 123 | 71 | 26 | 9 | 2 | 0.597 ± 0.861 | 1.000 ± 0.904 | 0.0041 |
| Steatosis | 96 | 46 | 68 | 18 | 1 | 0.944 ± 0.990 | 1.429 ± 1.118 | 0.0035 |
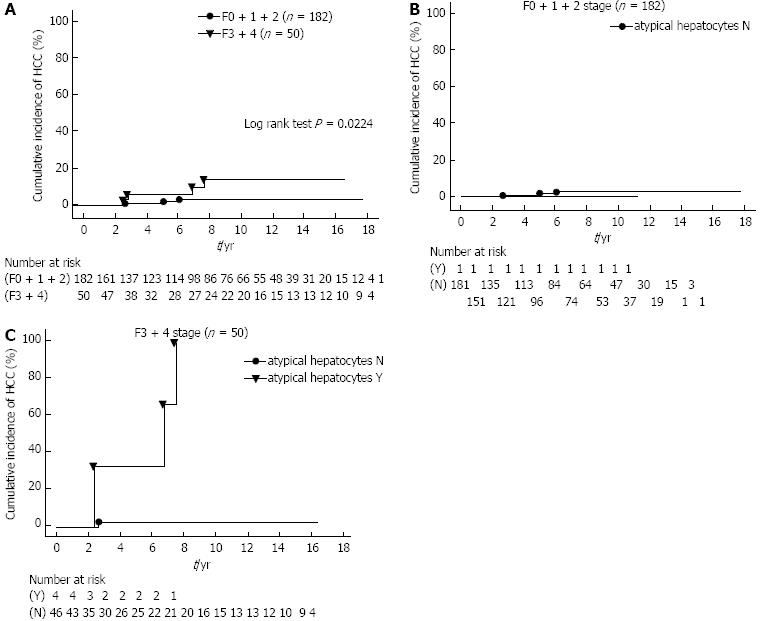
The cumulative incidence of HCC was compared between patients with SVR in the F0 + 1 + 2 and F3 + 4 groups by the Kaplan-Meier method (Figure 2A). HCC developed in seven of 232 (3.0%) patients, which included three of 182 (1.6%) patients in the F0 + 1 + 2 group, and four of 50 (8.0%) patients in the F3 + 4 group. The cumulative incidence of HCC in the former group was found to be significantly lower than in the F3 + 4 group (log rank test P = 0.0224).
Among the histopathological findings in the liver, the occurrence of the IR of hepatocytes is analyzed to determine the risk factors for development of HCC together with progressive liver fibrosis. In order to correct for the IR of hepatocytes, dysplastic change, map-like distribution, bulging, oncocytes, nodularlity, and atypical hepatocytes, the 232 patients were divided into two groups as follows: those with an absence (score 0) of IR of hepatocytes (N group) and those with presence (score 1-4) of IR of hepatocytes (Y group) according to the F0 + 1 + 2 and F3 + 4 groups. The cumulative incidence of HCC was compared with the IR of hepatocytes according to the F0 + 1 + 2 and F3 + 4 patients by the Kaplan-Meier method.
For example, to correct for the atypical hepatocytes, the 232 patients were divided into two groups as follows: those with an absence (score 0) of atypical hepatocytes (N group, n = 227) and those with presence (score 1-4) of atypical hepatocytes (Y group, n = 5). The Y group included all cases with score 1, but there were only cases with score 1. For the F3 + 4 patients, we found that there was a significantly lower cumulative incidence of HCC in the N group as compared to the Y group (log rank test, P = 0.0001, Figure 2B and C). However, there was no significant difference in the remaining IR of hepatocytes (data not shown). Thus, our findings demonstrate that with the progression of liver fibrosis, those patients with atypical hepatocytes among the IR of hepatocytes have a higher cumulative incidence of HCC.
In the F0 + 1 + 2 and F3 + 4 groups, the findings were analyzed to determine the risk factors for development of HCC using the Cox proportional hazard regression method. We compared the clinical background factors at liver biopsy of the patients with SVR in the F0 + 1 + 2 and F3 + 4 groups (Table 3). Univariate analysis using the Cox proportional hazard regression method was applied to these parameters to determine the clinical background factors that were associated with the development of HCC. None of the clinical background factors showed any significant associations with HCC. Blank values in the table signify that the bias was applied, but could not be statistically processed.
| Parameter | F0 + 1 + 2 | F3 + 4 | ||||
| RR | 95%CI | P value | RR | 95%CI | P value | |
| Clinical background factors1 | ||||||
| Gender (male) | 1.200 | 0.109-13.252 | 0.8815 | - | - | - |
| Age (yr) | 1.308 | 0.985-1.737 | 0.0635 | 1.123 | 0.959-1.314 | 0.1494 |
| BMI | 1.121 | 0.693-1.814 | 0.6415 | 0.987 | 0.549-1.772 | 0.9644 |
| AST (U/L) | 0.996 | 0.964-1.028 | 0.7903 | 1.003 | 0.971-1.037 | 0.8540 |
| ALT (U/L) | 0.992 | 0.965-1.020 | 0.5678 | 0.996 | 0.972-1.020 | 0.7494 |
| γ-GT (U/L) | 0.996 | 0.975-1.018 | 0.7169 | 1.001 | 0.977-1.025 | 0.9436 |
| Platelet count (× 104/μL) | 1.004 | 0.837-1.204 | 0.9634 | 1.088 | 0.855-1.384 | 0.4936 |
| Genotype type 1 | - | - | - | 0.285 | 0.030-2.752 | 0.2781 |
| AFP (ng/mL) | 0.989 | 0.867-1.129 | 0.8698 | 0.995 | 0.919-1.077 | 0.9046 |
| Histological findings | ||||||
| Irregular regeneration2 | ||||||
| Dysplastic change | 1.920 | 0.720-5.124 | 0.1926 | 1.330 | 0.543-3.256 | 0.5322 |
| Oncocytes | - | - | - | 1.788 | 0.510-6.265 | 0.3639 |
| Map-like distribution | - | - | - | 3.082 | 1.066-8.913 | 0.0378 |
| Nodularity | - | - | - | - | - | - |
| Bulging | - | - | - | - | - | - |
| Atypical hepatocytes | - | - | - | 42.055 | 4.303-411.029 | 0.0013 |
| Infiltration | ||||||
| Peri-portal | 3.248 | 0.537-19.655 | 0.1995 | 0.477 | 0.110-2.067 | 0.3225 |
| Parenchymal | 3.028 | 0.389-23.604 | 0.2902 | 0.420 | 0.092-1.921 | 0.2634 |
| Portal lymphocyte | 2.085 | 0.217-20.014 | 0.5241 | 1.213 | 0.177-8.333 | 0.8443 |
| Portal lymphoid reaction | 2.362 | 0.345-16.168 | 0.3812 | 3.364 | 0.484-23.407 | 0.2202 |
| Fibrosis | ||||||
| F stage | ||||||
| Portal sclerosis | 0.551 | 0.078-3.884 | 0.5500 | 1.293 | 0.444-3769 | 0.6373 |
| Pericellular fibrosis | 0.963 | 0.243-3.822 | 0.9576 | 0.534 | 0.117-2.440 | 0.4184 |
| Perivenular fibrosis | 0.627 | 0.168-2.344 | 0.4878 | 0.429 | 0.076-2.412 | 0.3370 |
| Bridging necrosis Y | - | - | - | - | - | - |
| Bile duct damage | 0.678 | 0.113-4.061 | 0.6707 | 1.305 | 0.374-4.555 | 0.6766 |
| Steatosis | 1.064 | 0.343-3.306 | 0.9142 | 1.660 | 0.623-4.421 | 0.3106 |
We compared the histopathological findings of liver biopsy in patients with SVR in the F0 + 1 + 2 and F3 + 4 groups (Table 3). Univariate analysis using the Cox proportional hazard regression method was applied to these parameters to determine the liver histopathological findings that were associated with the development of HCC. Significant correlations were found for map-like distribution (RR = 3.082, 95%CI: 1.066-8.913, P = 0.0378) and atypical hepatocytes (RR = 42.055, 95%CI: 4.303-411.0, P = 0.0013) in the F3 + 4 patients. In the F0 + 1 + 2 patients, none of the liver histopathological findings showed any significant associations with HCC. Blank values in the table signify that the bias was applied, but could not be statistically processed.
Multivariate analysis using the Cox proportional hazard regression method was applied to the parameters that could be of statistical significance in Table 3 in each of the F stage groups, and the findings are shown in Table 4. In the F0 + 1 + 2 group, none of the factors showed any significant correlation with the development of HCC. In the F3 + 4 group, map-like distribution (RR = 1.687, 95%CI: 0.404-7.053, P = 0.4734) was not found to have any correlation while the presence of atypical hepatocytes (RR = 20.748, 95%CI: 1.335-322.5, P = 0.0303) was shown to be significantly associated with HCC development.
| F3 + 4 parameter | RR | 95%CI | P value |
| Map-like distribution | 1.687 | 0.404-7.053 | 0.4734 |
| Atypical hepatocytes | 20.748 | 1.335-322.530 | 0.0303 |
In Japan, ≥ 90% of cases of LC associated with HCC are attributable to hepatitis B or C virus infection. Many studies of hepatitis C have reported risk factors for HCC other than the virus, such as the progression of fibrosis, male sex, increasing age, consumption of a large amount of alcohol, and excess iron in the liver[7,21-23]. It has been reported that the occurrence of HCC may be suppressed by reducing the degree of inflammation, regardless of the presence or absence of HCV[24-26]. Furthermore, among the liver histopathological findings, it has been reported that the progression of liver fibrosis (F stage) constitutes a risk factor for carcinogenesis[4]. Therefore, we sought to identify the liver histopathological findings, other than the F stage, that are correlated with the risk of carcinogenesis. Previously, we have reported that the degree of IR is related to factors other than liver fibrosis that contribute to liver carcinogenesis. It has been reported that the cumulative incidence of HCC was significantly lower in cases in which the degree of IR had improved after IFN therapy than in cases without IFN therapy[11,12]. If the degree of inflammation and necrosis in the liver were also improved by IFN therapy, this would be reflected by an improvement in the histopathological findings, particularly the degree of IR and liver fibrosis, and by a reduction in the incidence of HCC. Moreover, it is generally accepted that HCV may be a direct cause of HCC[27]. Thus, by considering the differences in liver histopathological findings in patients with SVR following viral clearance, it was possible to eliminate the direct effects of the virus on HCC progression. If the degree of inflammation and necrosis in the liver was improved following elimination of the virus by IFN therapy, the development of HCC would be attributable, at least in part, to nonviral factors. Therefore, we investigated the risk factors in detail, including the degree of liver IR and the histopathological findings of liver biopsy specimens, and prospectively examined the findings separately according to the degree of liver fibrosis in SVR patients.
In type C chronic disease, it is said that active inflammation persists in the liver, with the formation of lymphoid follicles, varying degrees of liver parenchyma failure, and IR of hepatocytes, ultimately leading to the formation of small regenerative nodules. In other words, IR of hepatocytes, which occurs in the liver as a result of repeated cycles of necrosis and regeneration, is recognized as a population of hepatocytes that differ from normal ones in size, appearance, and arrangement of the nucleus. As a result, nodular lesions often are detectable in the livers of patients with type C chronic disease. These nodular lesions may be classified into two types: dysplastic nodules and early HCC. In addition, dysplastic nodules are divided into low-grade and high-grade dysplastic nodules or borderline lesions[28]. Fatty changes may be seen in 40% of high-grade dysplastic nodules, but are not observed in the low-grade ones, and regenerative large nodules are observed at high frequency in early HCC[29,30].
We compared the cumulative incidence of HCC between patients with low (F0 + 1 + 2) and high (F3 + 4) degree of liver fibrosis, and it was found that the former group had a significantly lower rate than the F3 + 4 group, suggesting that as fibrosis progresses in the liver, HCC is more likely to occur. It is clear that liver fibrosis progresses and also contributes to an increased risk of the development of HCC. In this study, factors associated with the development of HCC were identified by comparing patients with SVR by multivariate analysis using the Cox proportional hazard regression method, which was applied to the clinical and histopathological parameters. We found that among all the investigated factors, the presence or absence of atypical hepatocytes among IR of hepatocytes may be an important risk factor for HCC development along with progression of liver fibrosis.
Moreover, patients with progressive liver fibrosis and atypical hepatocytes among the IR of hepatocytes also had a higher cumulative incidence of HCC. Therefore, our results clearly demonstrate the occurrence of atypical hepatocytes in progressive liver fibrosis as a risk factor of HCC. This finding is also considered important for determination of a patient’s therapeutic options.
HCC, stemming from LC induced by HCV infection, and other nonviral liver diseases, such as nonalcoholic steatohepatitis (NASH)[31-33] and autoimmune hepatitis[34], may develop through carcinogenic mechanisms based on inflammation[35,36]. Accordingly, we consider that the atypical hepatocytes may also contribute to the development of HCC in these nonviral diseases. Thus, during the assessment of the histopathological findings from liver biopsies, attention should be paid to the liver fibrosis, and additionally the presence or absence of atypical hepatocytes according to the type of liver disease.
In conclusion, among the histopathological findings in the liver of type C chronic disease, the occurrence of atypical hepatocytes in the IR of hepatocytes is significantly correlated with the risk of developing HCC together with progressive liver fibrosis. We believe that clarification of this finding as a risk factor of carcinogenesis may aid in the early diagnosis of HCC, and it would be meaningful to perform liver biopsy in patients with progression of liver fibrosis. Thus, by treating patients for both hepatitis C infection and atypical hepatocytes, we may attain an increase in the survival rate and a lower incidence of HCC.
The authors thank all the members of the Liver Group in Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine.
A few studies have sought to determine the association between thehistopathological findings from liver biopsies and the risk of developing hepatocellular carcinoma (HCC). The authors studied 232 patients who underwent liver biopsy, with a sustained virological response (SVR) after interferon (IFN) therapy. They investigated in detail the histopathological findings and analyzed the findings to determine the risk factors.
It has been reported that progression of liver fibrosis (F stage) constitutes a risk factor for carcinogenesis. Therefore, the authors sought to identify the liver histopathological findings, other than F stage, that are correlated with the risk of carcinogenesis. If the degree of inflammation and necrosis in the liver were improved following elimination of the virus by IFN therapy, the development of HCC would be attributable, at least in part, to nonviral factors. Then, they investigated the risk factors in detail, including the degree of liver irregular regeneration (IR) and the histopathological findings of liver biopsy specimens, and prospectively examined the findings separately according to the degree of liver fibrosis in SVR patients.
Atypical hepatocytes among IR of hepatocytes may be an important risk factor for HCC development along with progression of liver fibrosis.
The authors believe that clarification of this finding as a risk factor of carcinogenesis may aid in the early diagnosis of HCC, and it would be meaningful to perform liver biopsy for patients with progression of liver fibrosis. Then, by treating patients for both hepatitis C infection and atypical hepatocytes, they may attain an increase in the survival rate and a lower incidence of HCC.
IR of hepatocytes, which occurs as a result of repeated cycles of necrosis and regeneration of the liver parenchyma in chronic hepatitis, is divided into six elements: dysplastic change; anisocytosis characterized by variability of cell size with focal dysplastic change; map-like distribution; distinct populations of hepatocytes with a homogeneous appearance within each population, which are separated from each other by a sharp outline; bulging; expansive proliferation of hepatocytes compressing the surrounding parenchyma. Oncocytes: oncocytic change of hepatocytes. Nodularlity: nodular arrangement of the parenchyma. Atypical hepatocytes: degeneration of hepatocytes.
In this study, the authors analyzed the risk factors for HCC associated with type C chronic liver disease. They found that the presence of atypical hepatocytes was significantly correlated with risk of HCC development, and therefore concluded that atypical hepatocytes among the histopathological findings of type C liver disease may be an important risk factor for HCC development along with progression of liver fibrosis.
P- Reviewer Luo GH S- Editor Huang XZ L- Editor Kerr C E- Editor Ma S
| 1. | Matsumura H, Moriyama M, Goto I, Tanaka N, Okubo H, Arakawa Y. Natural course of progression of liver fibrosis in Japanese patients with chronic liver disease type C--a study of 527 patients at one establishment. J Viral Hepat. 2000;7:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Leone N, Rizzetto M. Natural history of hepatitis C virus infection: from chronic hepatitis to cirrhosis, to hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2005;51:31-46. [PubMed] |
| 3. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 4. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 315] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [PubMed] |
| 6. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [PubMed] |
| 7. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1792] [Article Influence: 85.3] [Reference Citation Analysis (2)] |
| 8. | Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, Tanaka E. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Uchida T. Small hepatocellular carcinoma: its relationship to multistep hepatocarcinogenesis. Pathol Int. 1995;45:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Shibata M, Morizane T, Uchida T, Yamagami T, Onozuka Y, Nakano M, Mitamura K, Ueno Y. Irregular regeneration of hepatocytes and risk of hepatocellular carcinoma in chronic hepatitis and cirrhosis with hepatitis-C-virus infection. Lancet. 1998;351:1773-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Ueno Y, Moriyama M, Uchida T, Arakawa Y. Irregular regeneration of hepatocytes is an important factor in the hepatocarcinogenesis of liver disease. Hepatology. 2001;33:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Moriyama M, Matsumura H, Oshiro S, Nakamura H, Arakawa Y, Nirei K, Aoki H, Yamagami H, Kaneko M, Tanaka N. Interferon therapy improves the irregular regeneration of hepatocytes in liver in patients with C-viral chronic hepatitis and liver cirrhosis. Intervirology. 2007;50:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Taguchi K, Aishima S, Matsuura S, Terashi T, Nishiyama K, Shirabe K, Shimada M, Maehara Y, Tsuneyoshi M. Significance of the relationship between irregular regeneration and two hepatocarcinogenic pathways: “de novo” and so-called “dysplastic nodule-hepatocellular carcinoma” sequence. J Surg Oncol. 2005;92:100-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Miyakawa H, Fujikawa H, Kikuchi K, Kitazawa E, Kawashima Y. Irregular regeneration of hepatocytes and development of hepatocellular carcinoma in primary biliary cirrhosis. Am J Gastroenterol. 2002;97:488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Moriyama M, Mikuni M, Matsumura H, Nakamura H, Oshiro S, Aoki H, Shimizu T, Yamagami H, Shioda A, Kaneko M. SEN virus infection influences the pathological findings in liver but does not affect the incidence of hepatocellular carcinoma in patients with chronic hepatitis C and liver cirrhosis. Liver Int. 2005;25:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [PubMed] |
| 17. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2507] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 18. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] |
| 19. | Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 617] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 20. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] |
| 21. | Yamasaki T, Terai S, Sakaida I. Deferoxamine for advanced hepatocellular carcinoma. N Engl J Med. 2011;365:576-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Choi J. Oxidative stress, endogenous antioxidants, alcohol, and hepatitis C: pathogenic interactions and therapeutic considerations. Free Radic Biol Med. 2012;52:1135-1150. [PubMed] |
| 23. | Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 607] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 25. | Cammà C, Giunta M, Andreone P, Craxì A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol. 2001;34:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Kumar M, Kumar R, Hissar SS, Saraswat MK, Sharma BC, Sakhuja P, Sarin SK. Risk factors analysis for hepatocellular carcinoma in patients with and without cirrhosis: a case-control study of 213 hepatocellular carcinoma patients from India. J Gastroenterol Hepatol. 2007;22:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 912] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 28. | International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 589] [Article Influence: 36.8] [Reference Citation Analysis (2)] |
| 29. | Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol. 2000;33:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 175] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Miyaaki H, Fujimoto M, Kurogi M, Nakashima O, Eguchi K, Kojiro M. Pathomorphological study on small nodular lesions in hepatitis C virus-related cirrhosis. Oncol Rep. 2005;14:1469-1474. [PubMed] |
| 31. | Tokushige K, Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Shiratori K. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol. 2010;45:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1013] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 33. | Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46 Suppl 1:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 34. | Migita K, Watanabe Y, Jiuchi Y, Nakamura Y, Saito A, Yagura M, Ohta H, Shimada M, Mita E, Hijioka T. Hepatocellular carcinoma and survival in patients with autoimmune hepatitis (Japanese National Hospital Organization-autoimmune hepatitis prospective study). Liver Int. 2012;32:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Jiang Z, Jhunjhunwala S, Liu J, Haverty PM, Kennemer MI, Guan Y, Lee W, Carnevali P, Stinson J, Johnson S. The effects of hepatitis B virus integration into the genomes of hepatocellular carcinoma patients. Genome Res. 2012;22:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 36. | Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol. 2011;17:4853-4857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |