INTRODUCTION
Portal hypertension (PH) is a clinical syndrome that is usually secondary to obstruction of the intra- or extra-hepatic portal flow. It is considered the main complication of liver disease, being responsible for the development of other liver diseases, such as portal hypertensive gastropathy, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatopulmonary syndrome, portopulmonary hypertension, hyperkinetic syndrome and hepatic encephalopathy[1].
PH is characterized by an increase in pressure above 5 mmHg in the portal venous system. When the pressure reaches 8 to 10 mmHg, in the esophagus and stomach, gastroesophageal varices arise, which develop from a network of collateral circulation through the vessels that form the splanchnic circulation. Bleeding from gastroesophageal varices can occur when the portal pressure gradient reaches values above 12 mmHg[2-4].
Numerous veins dilate, including the hemorrhoidal plexus, abdominal wall and esophagogastric junction. The umbilical vein communicates with and dilates the superficial veins of the abdominal wall, and the presence of abdominal collateral circulation is an important clinical sign of portal hypertension, which is characterized by dilated and tortuous veins radiating from the navel to the upper abdomen and lower chest[5,6]. Collateral circulation of the left gastric vein to the azygos vein is responsible for esophagogastric varicose veins and increased circulation in the gastric mucosa, which characterize the complications of portal hypertension referred to as portal hypertension gastropathy (PHG)[5].
The vascular endothelium releases vasodilators, including nitric oxide (NO) and prostacyclins, and vasoconstrictors, including endothelin, angiotensin and thromboxane. The function of vascular tone is maintained by balancing these agents. The increased peripheral resistance is maintained by elevation of vasoconstrictors or vasodilators or by reducing the levels of both. The blood exerts a force against endothelial cells, which are the main agonist in the release of NO[7].
Increases in NO synthesis have also been reported in the liver of rats with PH. Moreover, NO production has been implicated in the pathogenesis of PHG, with increases in NO serum levels being detected in patients with PHG[5]. When present in high concentrations, NO acts as a free radical, forming two molecules of dinitrogen trioxide (N2O3) or peroxynitrite (ONOO), which are responsible for cytotoxic effects such as inflammation and septic shock. Recently, NO has been presented as an important signal of the maintenance of homeostasis, as well as a cytotoxic agent involved in numerous diseases[8-10].
Increased formation of blood vessels in the splanchnic region occurs through the process of angiogenesis, which is involved in the maintenance of hyperdynamic circulation in portal hypertension. This hypothesis is based on recent studies that demonstrate the presence of increased splanchnic angiogenesis and neovascularization, which are responsible for the formation of portosystemic collaterals in experimental models of PH[11]. The stimulus for the proliferation of new blood vessels occurs through a complex cascade of angiogenic events, and it is the main modulator of this mechanism, vascular endothelial growth factor, vascular endothelial growth factor (VEGF), that stimulates the proliferation and migration of endothelial cells. Overproduction of NO is stimulated by endothelial nitric oxide synthase (eNOS), which can be stimulated by both VEGF and phosphatidylinositol-3-kinase (PI3K). In turn, PI3K-Akt also receives stimulation through the VEGF pathway, and the shear stress that occurs in PH can be a factor in stimulation via PI3K-Akt as well[12-14]. The Akt protein directly stimulates eNOS by increasing the capacity of eNOS to generate NO. There are several ways to stimulate the Akt pathway, including through growth factors, cytokines and the mechanical force of shear stress in a blood vessel, which activates the NO release mechanism in a PI3K-dependent manner[15,16]. Overproduction of vascular NO plays a central role in both systemic and splanchnic vasodilations, which are characteristics of portal hypertension that cause it to be recognized as a major complication of liver cirrhosis. The increased expression and activity of eNOS are well-established events in chronic models of portal hypertension[17,18].
Glutamine, a nonessential amino acid, has received increasing attention because it becomes essential during stress and catabolic conditions[19]. Glutamine administration can result in an enhanced antioxidant capacity in various situations, such as critical illness or sepsis[20]. In the stomach, glutamine is able to protect against peptic ulceration and improves the healing of ulcers[21]. The present study was designed to investigate the potential beneficial effects of glutamine administration on gastric oxidative stress and to evaluate the role of the VEGF-PI3K-Akt-eNOS pathway in NO overproduction in an experimental model of PHG.
MATERIALS AND METHODS
Ethics
All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985).
Animals and experimental groups
Male Wistar rats with a mean weight of 250 g were used. The animals were obtained from the Center for Breeding of Laboratory Animals of the Federal University of Rio Grande do Sul. The rats were held in cages at 20-24 °C with a 12 h light/dark cycle and given free access to food and water. They were randomly divided into the following four groups of fourteen animals each: (1) [sham-operated (SO) rats receiving only NaCl as a vehicle]; (2) SO + G (SO rats receiving glutamine); (3) PPVL (PPVL rats receiving vehicle); and (4) PPVL + G (PPVL rats receiving glutamine).
Partial portal vein ligation and sham operation
During the procedure, rats were anesthetized with a ketamine (Ketalar, Parke Davis, 100 mg/kg) and xylazine 2% (Rompun, Bayer, 50 mg/kg) cocktail ip. PH was induced by partial portal vein ligation (PPVL) as described by Moreira et al[22]. Briefly, the portal vein was isolated, and a 3-0 silk ligature was tied around both the portal vein and an adjacent 20 gauge blunt-tipped needle. The needle was subsequently removed, and the vein was allowed to re-expand. The abdomen was then closed, and the animal was allowed to recover. Control rats underwent a similar operation but without ligature of the portal vein. Sham-operated animals received only vehicle (NaCl, 1 mL/kg, ig). Glutamine was administrated daily (14 mg/kg, daily, ig) for 7 d beginning on the eighth day after the surgical protocol. All rats were anesthetized and sacrificed on the fifteenth day of the protocol. Their stomachs were immediately removed and divided into four subsamples that were stored in a freezer at -80 °C for analysis of oxidative stress, total glutathione, immunohistochemistry and Western blotting.
Oxidative stress determinations
Gastric oxidative stress was determined by measuring the concentration of aldehydic products (TBARS). Briefly, the frozen tissue was homogenized in a solution containing 140 mmol KCl and 20 mmol phosphate buffer (pH 7.4) and centrifuged at 14000 g for 10 min. For TBARS analysis, the amount of aldehydic products generated by lipid peroxidation was measured via the thiobarbituric acid reaction using 3 mg of protein per sample. The samples were incubated at 90 °C for 30 min following the addition of 500 mL of 0.37% thiobarbituric acid in 15% trichloroacetic acid and then centrifuged at 2000 g for 15 min. The spectrophotometric absorbance of the supernatant was determined at 535 nm[23].
Nitric oxide quantification
Nitric oxide production in the gastric tissue was measured indirectly using a quantitative colorimetric assay based on the Griess reaction. This method is sensitive for both nitrite and nitrate ions[24]. Briefly, the samples were deproteinized and subsequently centrifuged for 20 min at 12000 g. After incubation of the supernatants with E. coli nitrate reductase (37 °C, for 30 min) to convert nitrates to nitrites, 1 mL of Griess reagent (0.5% naphthylethylenediamine dihydrochloride, 5% sulfonylamide, 25% phosphoric acid) was added. The reaction was allowed to proceed at room temperature for 20 min, after which the absorbance at 546 nm was measured using a sodium nitrate solution as a standard.
Antioxidant enzyme activities
Cytosolic superoxide dismutase (SOD; EC 1.15.1.1) was assayed spectrophotometrically based on the rate of epinephrine autooxidation, which is progressively inhibited by increasing amounts of SOD in the homogenate; the amount of enzyme that results in 50% of the maximum inhibition of autooxidation is defined as 1 unit of SOD activity[25]. For analysis of glutathione (GSH) and GSSG, the livers were homogenized with 5% (w/v) metaphosphoric acid. After centrifugation (16000 g for 2 min), the tissue homogenate was assessed spectrophotometrically (415 nm) in a microplate reader employing a modified version of the 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB, Sigma)/GSSG reductase (Sigma) recycling method using the 43 N-ethylmaleimide (NEM, Fluka) conjugating sample preparation technique for GSSG. Samples (10 L) for both GSH and GSSG determination were assayed in 96-well polystyrene plates (Corning) at 37 °C in the presence of 10 mmol DTNB, 0.17 mm β-NADPH (Sigma, dissolved in 0.5% (w/v) NaHCO3 as a Stabilizing agent) and 0.5 U/mL GSSG reductase[26].
Western blotting analysis
The technique used for this measurement was protein expression by Western blotting analysis employing the system described by Laemmli[27] for electrophoresis and the blotting technique described by Towbin et al[28]. Proteins (80 g) were separated in a 10%-15% polyacrylamide gel and transferred electrically to polvinylidene difluoro membranes (Millipore, Bedford, MA, United States). Subsequently, the membranes were placed in Tris/saline-tamponade/Tween-20 blocking solution (TBST-5% milk powder in Tris-buffered saline containing 0.05% Tween 20) for 60 min at 37 °C. The membrane was incubated overnight at 4 °C with polyclonal eNOS and VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, United States), P-Akt and PI3K (Cell Signaling Technology, Danvers, MA, United States). Thereafter, the membranes were washed with TBST and incubated for one hour at room temperature with an anti-rabbit immunoglobulin antibody coupled to HRP (SIGMA, Glostrup, Denmark). The proteins were detected by chemiluminescence using a commercial ECL kit (Amersham Pharmacia Biotech, Uppsala, Sweden), and the density of specific bands was quantified using a densitometer image (Image J, United States).
Immunohistochemistry
Tissues sections (4 m) soaked in a formalin fixative and embedded in paraffin were subjected to immunohistochemical analysis[29]. This technique consisted of the following steps: deparaffinization, rehydration, antigen retrieval, inactivation of endogenous peroxidase and blocking of nonspecific reactions. The samples were incubated with the primary antibody for 12 h at 4 °C using the specific dilution of each antibody indicated in the instructions. After we applied the streptavidin-biotin complex (LSAB, DAKO) using a diaminobenzidine revelation tetrahydrochloride Kit (DAB, DAKO) and the samples were counterstained with hematoxylin. The antibodies used in the gastric mucosa samples were eNOS and VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, United States), AKT (Cell Signaling Technology, Danvers, MA, United States) and NTT (Sigma, United States).
Statistical analysis
The data were calculated and analyzed using analysis of variance. A post hoc multiple comparisons test was performed using the Student Newman-Keuls test. Values were considered significant when P < 0.05. All calculations were performed using the statistical program Graphpad Prism, version 14.0 (SPSS Inc., Chicago, IL, United States).
DISCUSSION
PHG is recognized as a clinical condition in PHI, but the exact pathogenesis of PHG is still unclear. The PPVL model has been extensively studied and found to be a useful tool for understanding the pathophysiology of PHI and PHG. This model has been developed in different animal species, such as rats, mice and rabbits, and it is presently accepted to be suitable for investigating the pathogenesis of PHI and PHG because is highly reproducible and easy to perform, and portal hypertension develops very rapidly. The model used in our study is characterized by prehepatic portal hypertension with maintenance of hepatic structure, hyperdynamic circulation and portal-systemic shunting. Treatment of portal hypertension to prevent complications, particularly gastrointestinal bleeding, is of fundamental importance and occurs under three clinical scenarios: prevention of first bleeding (primary prophylaxis), treatment after an episode of bleeding and prevention of secondary bleeding (secondary prophylaxis). These treatments can be performed using vasoconstrictors, which reduce portal venous flow; vasodilators, which reduce intrahepatic resistance; or a combination of both treatments[30].
Treatment using chemicals or natural products that would prevent complications of PH would represent a significant advance in therapy and could be a way to decrease mortality. Development of experimental models exhibiting pathogenic characteristics similar to those of human disease may help in understanding the mechanisms of portal hypertension and allow testing of new therapeutic modalities. Thus, an experimental model of PH was employed in this study and has contributed to improving our understanding of the pathophysiological conditions presented in humans. PH may be triggered by various agents, leading to cirrhosis.
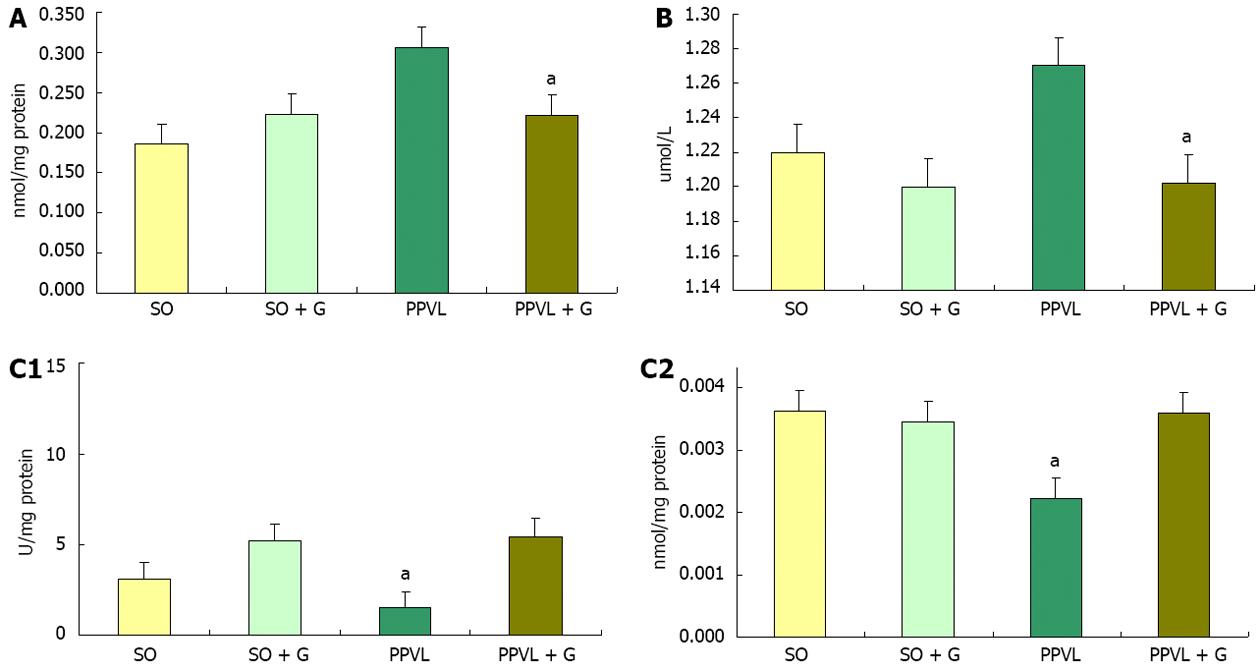
A study assessing the damage caused by oxidative stress in the gastric mucosa showed that there was an increase of LPO and decreases in the levels of the enzymes SOD, CAT and GPx in injured animals, suggesting that oxidative stress is involved in gastric tissue damage[31]. When we assessed lipid peroxidation in the stomachs of rats, an increase in the level of substances reacting with thiobarbituric acid (TBA-RS) was detected in the PPVL group compared with the SO group, which is suggested to be related to increased oxidative stress. This conclusion was supported by the amounts of TBA-RS observed in the PPVL + G group compared with the PPVL group. Similar results have been shown in other studies that used the flavonoid quercetin and N-acetylcysteine, suggesting that the amino acid glutamine exhibits antioxidant potential[32]. In the present study, glutamine reduced submucosal edema and vasodilation as well as reducing lipid peroxidation in homogenized stomachs from animals in the PPVL + G group. Glutamine, as a potential antioxidant, appears to protect the gastric mucosa and decrease oxidative damage to the gastrointestinal tract, which was observed both in this study and in previous studies of colitis employing glutamine[33].
The enzyme SOD, which is responsible for the dismutation of superoxide anion radicals into hydrogen peroxide, is the first line of defense against cellular oxidative stress. The SOD activity determined in the gastric mucosa in a study of patients with liver disease showed that this enzyme is found at reduced levels in these patients compared with control subjects with cirrhosis. However, individuals with chronic liver disease showed no significant changes in the levels of SOD. In liver diseases such as hepatitis, cirrhosis and hepatocellular carcinoma, there are high levels of LPO in the stomach, suggesting involvement of oxidative stress in gastric mucosal lesions in these liver diseases[34]. Studies relate the decrease in SOD enzyme activity to increased oxidative stress[35,36]. In the present study, we observed a decrease in SOD activity in the PPVL group compared with other groups, which could be related to the inactivation of superoxide anions, as this enzyme was acting in the dismutation of EAOS and the formation of H2O2. In contrast, animals in the PPVL + G group maintained values of SOD enzyme activity similar to controls. This increased activity of SOD in the PPVL group was due to the increased formation of EAOS, causing oxidative stress. Total GSH has a special physiological importance because glutamine serves as a substrate for glutathione formation. Studies have demonstrated the direct involvement of GSH in colitis[37]. A relationship was shown with glutathione colitis, in which rats with experimental colitis caused by TNBS showed a significant decrease in the level of glutathione compared with the control group[38].
In the evaluation of NO metabolites in stomach homogenates, we observed a reduction in the production of these metabolites in the PPVL + G group compared to the PPVL group. This increase in NO production in the PPVL group can be explained by the process of angiogenesis, which occurs in this model for the purpose of shunting of blood from the obstructed area to the systemic circulation. Moreover, these increased levels of nitric oxide could be reacting with superoxide anions to form peroxynitrite radicals, which are extremely harmful. This NO release becomes more pronounced due to the need for the formation of new blood vessels so that blood can reach the systemic circulation, triggering an increase in RL, thus stimulating lipid peroxidation and oxidative stress[39].
Oxidative stress plays an important role in the pathogenesis of PH because in association with the overproduction of superoxide anions and nitric oxide observed in the model of partial portal vein ligation, peroxynitrite formation occurs. Researchers have noted specific actions of ONOO as a modulator of intracellular signaling pathways regulating inflammatory responses, including induction of angiogenesis and VEGF.
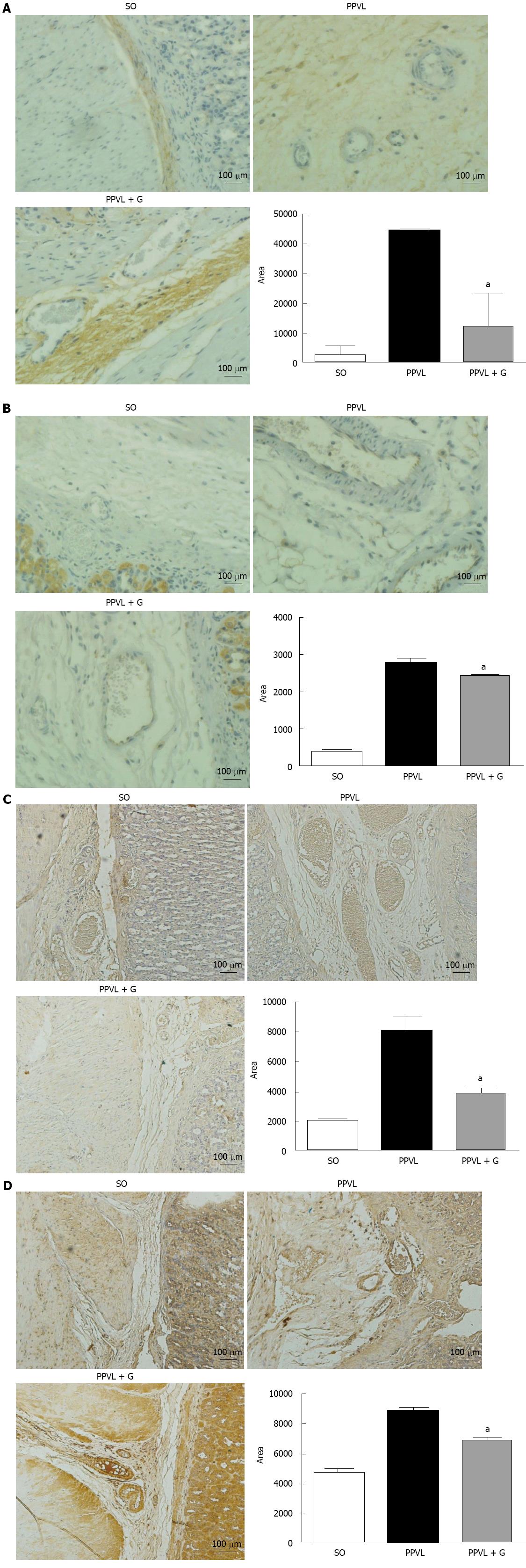
The significant reduction of lipid peroxidation observed in animals in the PPVL + G group demonstrates effective action of the glutamine in the process of lipid peroxidation. This result is consistent with the expected capacity of glutamine in the formation of inactivating EAOS, especially in reducing the formation of peroxynitrite. Peroxynitrite reacts with free tyrosine and tyrosine residues in protein molecules to produce nitrotyrosine. Alternatively, EAOS can activate tyrosine to form tyrosyl, a radical that in turn oxidizes NO to produce nitrotyrosine (NTT)[40,41]. The generation of peroxynitrite was assessed based on the level of expression and immunoreactivity of NTT, with significant reductions being observed for both parameters in animals in the PPVL + G group, demonstrating the effectiveness of glutamine with respect to the oxidative/nitrosative damage that occurs in the gastric mucosa.
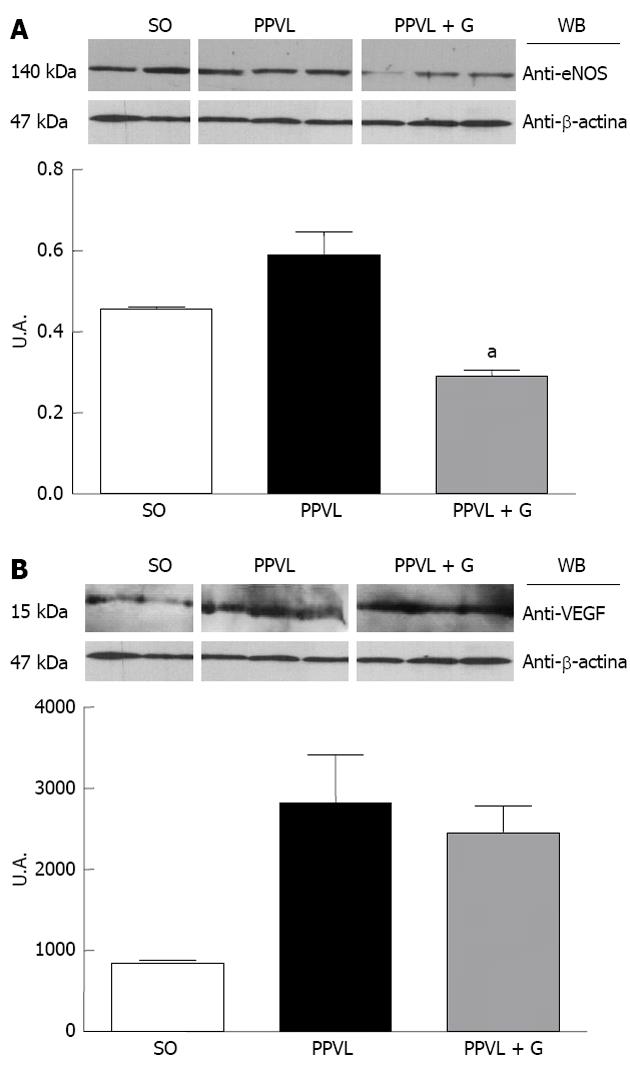
Upregulation of eNOS initiates the post-translational level mediated by Akt, which increases its activity at any concentration of cytosolic Ca2+[42]. During early cirrhosis, this pathway is stimulated by different types of stimuli, such as VEGF, inflammatory cytokines and mechanical shear forces[42]. In advanced stages of portal hypertension, bacterial translocation also activates eNOS via tumor necrosis factor, increasing tetrahydrobiopterin, which is an essential element that acts as a cofactor of the enzyme. According to several studies, other mechanisms, such as changes in the subcellular localization of eNOS[43], S-nitrosylation[44] or degradation of asymmetrical dimethylarginine, may be involved in regulating the activity of eNOS[45]. We observed in this study that glutamine reduced eNOS protein expression and the immunoreactivity of the enzyme in the gastric mucosa of animals in the PPVL + G group. One explanation for this finding is that glutamine, due to its involvement in NO synthesis, is involved in reduction of the cytosolic levels of Ca2+, which is related to the levels of Akt; in turn, Akt levels are stimulated by mechanical stress shear. Throughout this process, this hemodynamic mechanism would be inhibited due to reduced levels of NO triggered by the action of glutamine.
Angiogenesis is characterized by the formation of new vascular structures and a pathophysiological phenomenon that has been further investigated in recent years due to the critical role it plays in the pathogenesis of disease and its potential as a therapeutic target. Additionally, angiogenesis contributes to a number of physiological processes, such as wound healing and the reproductive cycle[46]. NO released by dilation and increased vascular permeability as well as the migration, proliferation and survival of endothelial cells plays a crucial role in angiogenesis. Many molecules have been implicated as modulators of the angiogenic process, such as tumor necrosis factor, interleukins, angiopoeitins and growth factors, including VEGF. Indeed, VEGF stimulates NO production by NOS, increasing vascular permeability and the proliferation and survival of endothelial cells[47].
Furthermore, overproduction of ONOO, which is formed by the reaction of superoxide anions and nitric oxide, is observed under inflammatory conditions[48], whereas cytotoxic actions of ONOO have been reported via the modulation of intracellular signaling pathways that regulate inflammatory responses, including induction of angiogenesis and increased levels of VEGF. Studies in the retina of rats with diabetes induced by streptozotocin demonstrated the formation of nitrotyrosine and increased effects regarding stimulation of VEGF. These conditions have been studied because they induce the generation of reactive oxygen species (ROS). EAOS are involved in triggering the overexpression of VEGF in various cell types[49,50], but the molecular mechanisms underlying this effect remain to be elucidated. Studies in patients, in animals and in vitro indicate that formation of nitrotyrosine, a marker of ONOO, is associated with increased VEGF expression during diabetic microvascular disease, atherosclerosis and tumor angiogenesis[51,52]. In addition, studies using a cultured cell line showed that formation of ONOO stimulates an increase in VEGF[53]. In the present study, we observed increased expression and immunoreactivity of VEGF in animals in the PPVL group, but there was no significant reduction of these parameters in animals in the PPVL + G group. We suggest that these results demonstrate direct action of glutamine on NO synthesis to reduce its levels, rather than only affect the action of growth factors and the associated cascade of events.
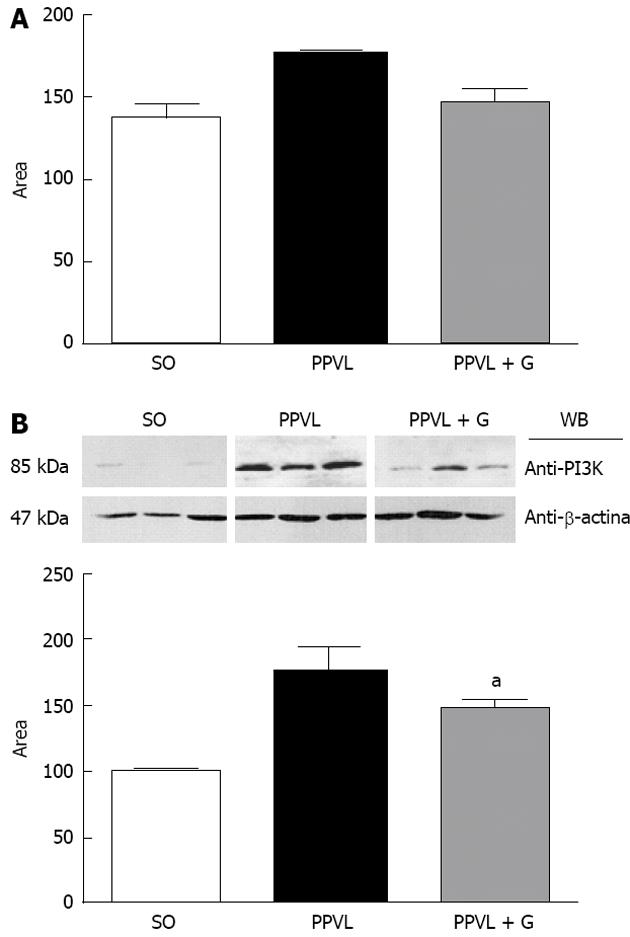
VEGF also acts by stimulating PI3K, which activates eNOS in an Akt-dependent manner, resulting in activation of eNOS and increased NO production. PI3K phosphorylates Akt, which can rapidly activate eNOS[54]. The Akt pathway has emerged as a signaling pathway in all cells of higher eukaryotes, and Akt has been found to be one of the most important and versatile kinases for understanding human physiology and disease. Recent studies have been performed to elucidate the molecular details of the regulation of Akt and its role in human disease. The NO released due to eNOS stimulation is involved in vasodilation, vascular remodeling and angiogenesis[55]. The Akt signaling pathway also leads to increased production of transcription factors induced by hypoxia (HIF1α and HIF2α), partly through the activation of an mTOR-dependent mechanism[56]. The activation of eNOS through Akt/B kinase leads to increased NO production, vasodilatation and increased splanchnic circulation[57]. Akt activation occurs due to an increase in shear stress-induced endothelial disruption, although other mechanisms may be involved[58].
In this study, we observed a significant increase in the expression and immunoreactivity of Akt in the PPVL group and a reduction in these parameters in the PPVL + G group. These results can be explained by the role that glutamine plays in the production of NO, which in turn, triggers a chain reaction, is overproduced and dilates the splanchnic vessels, gradually increasing shear stress, which is the mechanical stimulus for the activation of Akt. In addition, it was observed that VEGF levels are unchanged in the PPVL + G group compared to the PPVL group, and the VEGF RTK pathway acts as a means for the release of PIK3, eNOS and P-Akt, leading to the production of more NO, which is a feature present in the hyperdynamic circulation associated with PH. Therefore, glutamine acts to mitigate this situation not by reducing the levels of NO via VEGF, but by reducing the shear stress triggered by NO synthesis through inhibition of L-arginine, thereby reducing the levels of Akt, which is stimulated by mechanical stress in blood vessels. In conclusion, we describe the beneficial effects of glutamine treatment on oxidative stress, including reduced portal pressure, normalization of SOD and reduction of NO production by eNOS, mediated by the PI3K-Akt-eNOS pathway. VEGF levels tended to decrease, although this trend was not found to be statistically significant.