Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4374
Revised: April 1, 2013
Accepted: May 18, 2013
Published online: July 21, 2013
Processing time: 150 Days and 20.6 Hours
AIM: To validate the usefulness of screening endoscopy findings for predicting Helicobacter pylori (H. pylori) infection status.
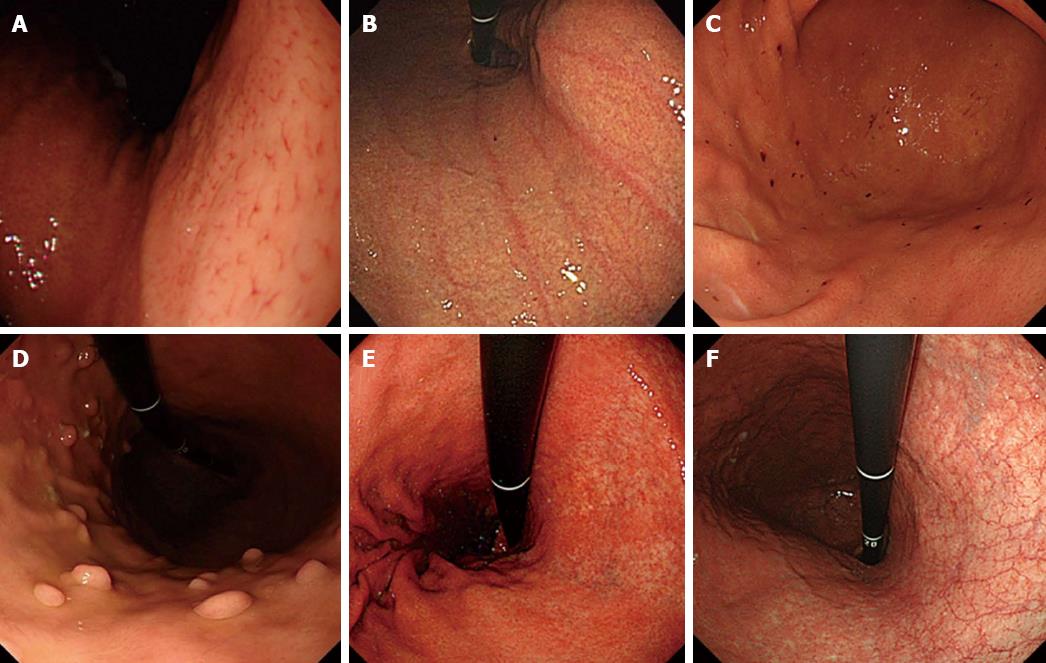
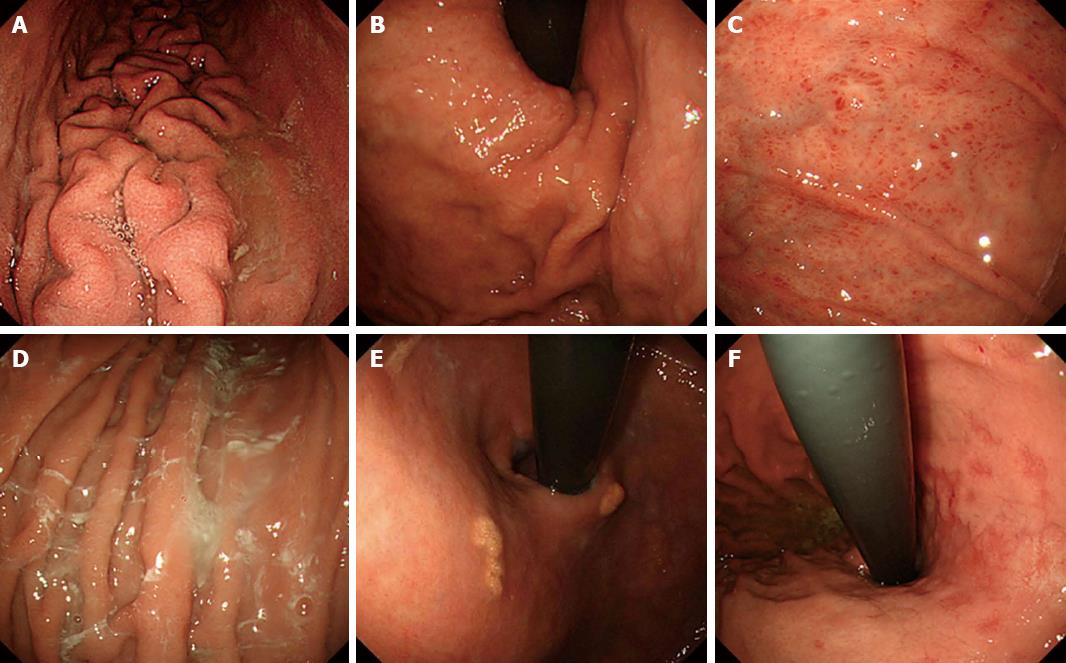
METHODS: H. pylori infection status was determined by histology, serology, and the urea breast test in 77 consecutive patients who underwent upper endoscopy. Based on the findings, patients were categorized as H. pylori-uninfected, -infected, or -eradicated cases. Using six photos of certain sites in the stomach per case, we determined the presence or absence of the following endoscopic findings: regular arrangement of collecting venules (RAC), linear erythema, hemorrhage, fundic gland polyp (FGP), atrophic change, rugal hyperplasia, edema, spotty erythema, exudate, xanthoma, and mottled patchy erythema (MPE). The diagnostic odds ratio (DOR) and inter-observer agreement (Kappa value) for these 11 endoscopic findings used in the determination of H. pylori infection status were calculated.
RESULTS: Of the 77 patients [32 men and 45 women; mean age (SD), 39.7 (13.4) years] assessed, 28 were H. pylori uninfected, 28 were infected, and 21 were eradicated. DOR values were significantly high (< 0.05) for the following H. pylori cases: uninfected cases with RAC (11.5), linear erythema (24.5), hemorrhage (4.1), and FGP (34.5); for infected cases with atrophic change (8.67), rugal hyperplasia (15.8), edema (14.2), spotty erythema (11.5), and exudate (3.52); and for eradicated cases with atrophic change (32.4) and MPE (103.0). Kappa values were excellent for FGP (0.93), good for RAC (0.63), hemorrhage (0.79), atrophic change (0.74), and MPE (0.75), moderate for linear erythema (0.51), rugal hyperplasia (0.49), edema (0.58), spotty erythema (0.47), and exudate (0.46), and poor for xanthoma (0.19).
CONCLUSION: The endoscopic findings of RAC, hemorrhage, FGP, atrophic change, and MPE will be useful for predicting H. pylori infection status.
Core tip: To determine useful findings for predicting Helicobacter pylori (H. pylori)-uninfected, -infected, or -eradicated cases, we evaluated following 11 endoscopic findings, regular arrangement of collecting venules (RAC), linear erythema, hemorrhage, fundic gland polyp (FGP), atrophic change, rugal hyperplasia, edema, spotty erythema, exudate, xanthoma, and mottled patchy erythema (MPE). Among these, RAC, hemorrhage, FGP, atrophic change, and MPE were found to be predictive findings for H. pylori infection status on screening endoscopy. The knowledge of these findings may contribute to the early detection of gastric cancer.
-
Citation: Watanabe K, Nagata N, Nakashima R, Furuhata E, Shimbo T, Kobayakawa M, Sakurai T, Imbe K, Niikura R, Yokoi C, Akiyama J, Uemura N. Predictive findings for
Helicobacter pylori -uninfected, -infected and -eradicated gastric mucosa: Validation study. World J Gastroenterol 2013; 19(27): 4374-4379 - URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4374
Gastric cancer remains the second leading cause of cancer death, accounting for 600000 deaths annually worldwide[1]. The incidence of gastric cancer is particularly high in Asia, especially in China, Japan and Korea where Helicobacter pylori (H. pylori) infection is highly prevalent. The risk of gastric cancer can differ depending on whether individuals are uninfected or infected with H. pylori or whether the infection has been eradicated[2-4]. It is therefore extremely important in the early detection of gastric cancer that H. pylori infection status is determined for each of these groups of individuals.
Endoscopy is an essential diagnostic tool for gastric cancer, enabling various findings induced by histological inflammation of the gastric mucosa to be detected. The development of histological gastritis is regarded as rare in H. pylori-uninfected cases, but it is usually noted in H. pylori-infected cases and improved by eradication therapy. This difference is reflected in the appearance of the gastric mucosa, which has been reported in the form of endoscopic findings in several studies[5-12]. However, the predictive value of the findings has not yet been validated.
In this study, we assessed, in a systematic manner, 11 endoscopic findings in dyspeptic patients and evaluated which of the findings were associated with H. pylori-uninfected, -infected, and -eradicated cases.
A total of 148 consecutive patients with dyspepsia who had undergone upper gastrointestinal endoscopy and had been strictly diagnosed with H. pylori infection between December 2008 and April 2009 at the National Center for Global Health and Medicine (NCGM) were identified from an endoscopic electronic database. The exclusion criteria applied were the use of non-steroidal anti-inflammatory drugs or anti-thrombogenic drugs and a history of gastric surgery, hemorrhagic disease, liver cirrhosis, renal failure, heart failure, or early or advanced gastric cancer, because these conditions could affect the mucosal appearance of the stomach[13-16]. After exclusion, 77 cases remained for analysis.
Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki and its subsequent revision. The study protocol was approved by the Ethics Committee of NCGM (approval No. 811).
H. pylori infection status was evaluated by the presence of serum immunoglobulin G antibody against H. pylori (HM-CAP enzyme immunoassay, Enteric Products, Westbury, NY, United States), the [13C]-labeled urea breath test (13C-UBT, with a cut-off value of 2.5‰; Ubit, Otsuka Pharmaceuticals, Tokyo, Japan), and histological examination with toluidine blue staining of 3 endoscopic biopsy specimens taken from the greater curvature of the upper gastric body, angulus, and antrum, respectively. When all three methods yielded negative results, H. pylori infection status was considered “uninfected”. When one or more of these methods yielded a positive result and there was no history of previous eradication therapy, H. pylori infection status was considered “infected”. When histological examination and 13C-UBT yielded negative results and a history of eradication therapy was recorded, H. pylori infection status was considered “eradicated”.
All endoscopies were performed by well-trained endoscopists using a high resolution videoendoscope (GIF-260H, Olympus Co., Tokyo, Japan) with a pre-endoscopic oral solution containing dimethylpolysiloxane (Balgin Antifoaming Oral Solution 2%, Kaigen Co., Ltd., Osaka, Japan). In all cases, around 50-60 endoscopic images had been routinely recorded at fixed sites in the esophagus, stomach, and duodenum and saved to the electronic endoscopic database (Solemio ENDO, Olympus Co.). Six of the recorded images - specifically of the antrum, angulus, lesser and greater curvature of the lower body, greater curvature of the upper body, and cardia of the stomach - were used for analysis in each case.
The presence or absence of the following 11 distinctive endoscopic findings were evaluated (Figures 1 and 2): regular arrangement of collecting venules (RAC)[5], linear erythema[9], hemorrhage[9] , fundic gland polyp (FGP)[10], atrophic change[6,7], severity of atrophy (open/closed)[6,7], rugal hyperplasia[8], edema[9] (which is visible as a thickened mucosal layer especially at the angulus and cardia), spotty erythema[9], exudate[9], xanthoma[11], and mottled patchy erythema (MPE)[12]. A well-experienced endoscopist (Kobayakawa M) assessed the findings, and to determine inter-observer agreement, another experienced endoscopist (Sakurai T) also assessed these findings. Both were blinded to clinical information in the cases examined.
To identify the predictive endoscopic findings for H. pylori infection status from among the 462 endoscopic images for the 77 patients, diagnostic odds ratios (DOR) for the 11 endoscopic findings in H. pylori-uninfected, -infected, and -eradicated cases were calculated. In addition, 95%CI were also estimated. DOR is defined as the positive likelihood ratio divided by the negative likelihood ratio. Positive likelihood ratio was calculated by sensitivity/1-specificity, and negative likelihood ratio was calculated by specificity/1-sensitivity[17].
The inter-observer agreement for each endoscopic finding among the two endoscopists was measured using kappa statistics. Kappa values (k) > 0.80 denoted excellent agreement, > 0.60-0.80 good, > 0.40-0.60 moderate, > 0.20-0.40 fair, and ≤ 0.20 poor[18]. Values of P < 0.05 were considered significant. All statistical analysis was performed using Stata version 10 software (StataCorp, Lakeway Drive College Station, TX, United States).
Of the 77 patients [32 men and 45 women; mean age (SD), 39.7 (13.4) years] assessed, 28 were H. pylori uninfected, 28 were infected, and 21 were eradicated.
The DOR for each endoscopic finding in the diagnosis of the three groups of H. pylori infection status are shown in Table 1. In cases diagnosed as H. pylori uninfected, RAC (11.5), linear erythema (24.5), hemorrhage (4.1), and FGP (34.5) had high DORs and were significantly associated (P < 0.05). In infected cases, atrophic change (8.67), rugal hyperplasia (15.8), edema (14.2), spotty erythema (11.5), and exudate (3.52) had high DORs and were significantly associated (P < 0.05). Lastly, in eradicated diagnosis, atrophic change (32.4) and MPE (103.0) had high DORs and were significantly associated (P < 0.05).
| Endoscopic finding | H. pylori | ||
| Uninfected | Infected | Eradicated | |
| RAC | 11.5 (3.73-35.1) | 0.03 (0.00-0.14)a | 1.65 (0.61-4.47)a |
| Linear erythema | 24.51 (4.12-146) | 0.051 (0.00-0.83)a | 0.18 (0.00-1.20)a |
| Hemorrhage | 4.11 (1.54-11.0) | 0.03 (0.00-0.19)a | 2.52 (0.92-6.95)a |
| Fundic gland polyp | 34.51 (1.89-632) | 0.101 (0.01-1.81)a | 0.151 (0.01-2.81)a |
| Atrophic change | 0.01 (0.00-0.06) | 8.671 (2.11-35.6)a | 32.41 (1.87-562)a |
| Rugal hyperplasia | 0.021 (0.00-0.34) | 15.8 (4.86-51.4)a | 0.66 (0.22-2.03) |
| Edema | 0.10 (0.03-0.31) | 14.2 (4.52-44.1)a | 0.66 (0.24-1.81) |
| Spotty erythema | 0.09 (0.00-0.60) | 11.5 (3.03-42.7)a | 0.35 (0.00-1.54) |
| Exudate | 0.27 (0.00-1.18) | 3.52 (1.16-11.6)a | 0.77 (0.21-2.93) |
| Xanthoma | 0.86 (0.22-3.46) | 2.45 (0.64-9.29) | 0.30 (0.00-2.02) |
| Mottled patchy erythema | 0.071 (0.00-1.17) | 0.071 (0.00-1.17) | 1031 (5.64-1888)a |
Open type atrophy was significantly (P < 0.05) more frequent on endoscopy in H. pylori infected patients (20/28, 71.4%) than in H. pylori eradicated patients (8/21, 38.1%).
The kappa value indicating agreement between the two endoscopists for each endoscopic finding is shown in Table 2.
| Endoscopic finding | Kappa value |
| RAC | 0.63 |
| Linear erythema | 0.51 |
| Hemorrhage | 0.79 |
| Fundic gland polyp | 0.93 |
| Atrophic change | 0.74 |
| Rugal hyperplasia | 0.49 |
| Edema | 0.58 |
| Spotty erythema | 0.47 |
| Exudate | 0.46 |
| Xanthoma | 0.19 |
| Mottled patchy erythema | 0.75 |
This study identified several endoscopic findings that are clearly associated with uninfected, infected, and eradicated H. pylori infection. Some endoscopic findings have been previously reported to be correlated with H. pylori infection. Atrophic change was found to be associated with the infection in an aged group (OR = 9.8)[19] as well as in general[7,20]. Rugal hyperplasia was reported to be correlated with H. pylori infection[21]. Edema, with or without exudate, and spotty erythema were considered to be a result of mucosal inflammation, but these positive findings were not definitive[9]. Lastly, xanthoma, which refers to yellowish-white small nodules or plaques in the gastric mucosa, is considered to be related with H. pylori infection[11]. Among these findings, we clarified that all of them - atrophic change, rugal hyperplasia, edema, spotty erythema, and exudate - are valuable endoscopic findings of H. pylori infection.
In regard to the predictive findings related with H. pylori uninfected mucosa, RAC has been well studied and showed a positive association[5,22]. Fundic gland polyp is also considered to be a finding associated only with uninfected cases[10]. In the present study these endoscopic findings showed high odds ratios and thus support the results of earlier studies. While hemorrhage and linear erythema were found to be associated with well-preserved gastric acid secretion, they were not clearly associated with H. pylori uninfected mucosa[9]. In the present study, however, these two findings had high odds ratios, suggesting they are valuable for predicting H. pylori-uninfected cases.
This study also investigated H. pylori-eradicated cases, because the preventive effect of H. pylori eradication therapy for gastric cancer has been reported[3,4] and therefore an increasing number of patients will likely receive H. pylori eradication therapy into the future[23,24]. Gastric cancer can, however, still occur in eradicated cases, but it is generally difficult to diagnose and few predictive endoscopic findings have been reported. Of those that have been suggested are the disappearance of rugal hyperplasia[8] and hyperplastic polyp[25], but as these findings need to be compared before and after eradication therapy, on their own it seems difficult to apply them to clinical use. Atrophic change, occurring as a result of H. pylori infection, is thought to remain after eradication therapy[26]. Moreover, in our previous study, MPE, which is recognized as a flat or slightly depressed reddish lesion that is distinguishable from the congested mucosa, emerged after H. pylori eradication therapy[12]. In the study too, atrophic change and MPE was highly predictive of H. pylori eradicated mucosa, suggesting that a combination of these findings is highly valuable in clinical practice.
In regard to the reproducibility of endoscopic image evaluation for these 11 findings, among the positive endoscopic findings found, RAC, hemorrhage, fundic gland polyp, atrophic change, and MPE all showed good inter-observer agreement, suggesting that these findings can be easily identified and will be generally useful. The other positive findings of edema, rugal hyperplasia, and spotty erythema showed moderate agreement, suggesting that they could also be suitable for general use.
There are several important strengths of this study, including that the diagnosis of H. pylori infection status was accurate, made on the basis of a combination of three different diagnostic tests to overcome any shortcomings of a single test. Moreover, DOR was used to estimate the diagnostic value: DOR is not influenced by prevalence rate, so the results are applicable in other populations with a different prevalence of H. pylori infection.
Nonetheless, this study has several limitations. First, the sample size of 77 patients is relatively small, and did not permit multivariate analysis to exclude confounders. Second, the population consists of relatively young patients, therefore cases in whom H. pylori is naturally eradicated without eradication therapy as a result of long-term course of severe atrophic gastritis, may not be included. Furthermore, photographic not video images were used for analysis and therefore the whole stomach was not observed, so some findings that may be present were not assessed.
In conclusion, the endoscopic findings associated with H. pylori infection status that are common and have good inter-observer agreement were clarified to be RAC, hemorrhage, fundic gland polyp, atrophic change, and MPE. These findings should be generally useful in clinical practice and contribute to the early detection of gastric cancer.
We wish to thank Hisae Kawashiro, Clinical Research Coordinator, for assistance with data collection.
The risk of gastric cancer can differ among Helicobacter pylori (H. pylori) uninfected, infected, and eradicated patients, therefore it is important to determine H. pylori infection status. Endoscopic prediction of H. pylori infection status can be extremely useful for early detection of gastric cancer, but the diagnostic value of H. pylori related endoscopic findings has not yet been validated.
Some endoscopic findings were reported to be related with H. pylori uninfected, infected case, and little has been reported on eradicated case. The accuracy of these findings varies in each studies, and have not been well examined. In this study, the authors demonstrate the accuracy and reproducibility of endoscopic findings which have been previously reported to be correlated with H. pylori infection.
This is the first study which evaluate the value of predictive endoscopic findings separately for 3 groups of H. pylori infection status; uninfected, infected, and eradicated. Moreover, the authors used diagnostic odds ratio (DOR) to estimate the diagnostic value of endoscopic findings. DOR is not influenced by prevalence rate, so the results are applicable in other populations with a different prevalence of H. pylori infection.
The endoscopic findings associated with H. pylori infection status that are common and have good reproducibility were clarified to be regular arrangement of collecting venules, hemorrhage, fundic gland polyp, atrophic change, and mottled patchy erythema. These findings should be generally useful in clinical practice and contribute to the early detection of gastric cancer.
DOR is defined as the positive likelihood ratio divided by the negative likelihood ratio. DOR is single indicator of diagnostic test, and higher value is indicative of better test performance, irrespective of prevalence rate.
The authors showed endoscopic features of gastric mucosa according to H. pylori infection status. Although most endoscopists are usually aware of the correlation between endoscopic findings and H. pylori status, the simplification and clarification of the correlation by showing typical endoscopic findings and their diagnostic odds ratios may be worth publication.
P- Reviewer Kato J S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Pisani P, Parkin DM, Bray F, Ferlay J. Erratum: Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer, 83, 18-29 (1999). Int J Cancer. 1999;83:870-873. [PubMed] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3182] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 3. | Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-642. [PubMed] |
| 4. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 935] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 5. | Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39-45. [PubMed] |
| 6. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. |
| 7. | Kawaguchi H, Haruma K, Komoto K, Yoshihara M, Sumii K, Kajiyama G. Helicobacter pylori infection is the major risk factor for atrophic gastritis. Am J Gastroenterol. 1996;91:959-962. [PubMed] |
| 8. | Yasunaga Y, Shinomura Y, Kanayama S, Yabu M, Nakanishi T, Miyazaki Y, Murayama Y, Bonilla-Palacios JJ, Matsuzawa Y. Improved fold width and increased acid secretion after eradication of the organism in Helicobacter pylori associated enlarged fold gastritis. Gut. 1994;35:1571-1574. [PubMed] |
| 9. | Kaminishi M, Yamaguchi H, Nomura S, Oohara T, Sakai S, Fukutomi H, Nakahara A, Kashimura H, Oda M, Kitahora T. Endoscopic classification of chronic gastritis based on a pilot study by the research society for gastritis. Digestive Endoscopy. 2002;14:138-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Sakai N, Tatsuta M, Hirasawa R, Iishi H, Baba M, Yokota Y, Ikeda F. Low prevalence of Helicobacter pylori infection in patients with hamartomatous fundic polyps. Dig Dis Sci. 1998;43:766-772. [PubMed] |
| 11. | Isomoto H, Mizuta Y, Inoue K, Matsuo T, Hayakawa T, Miyazaki M, Onita K, Takeshima F, Murase K, Shimokawa I. A close relationship between Helicobacter pylori infection and gastric xanthoma. Scand J Gastroenterol. 1999;34:346-352. [PubMed] |
| 12. | Nagata N, Shimbo T, Akiyama J, Nakashima R, Kim HH, Yoshida T, Hoshimoto K, Uemura N. Predictability of gastric intestinal metaplasia by mottled patchy erythema seen on endoscopy. Gastroenterology Research. 2011;4:203-209. |
| 13. | Primignani M, Carpinelli L, Preatoni P, Battaglia G, Carta A, Prada A, Cestari R, Angeli P, Gatta A, Rossi A. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC). Gastroenterology. 2000;119:181-187. [PubMed] |
| 14. | Laine L. Review article: the effect of Helicobacter pylori infection on nonsteroidal anti-inflammatory drug-induced upper gastrointestinal tract injury. Aliment Pharmacol Ther. 2002;16 Suppl 1:34-39. [PubMed] |
| 15. | Sotoudehmanesh R, Ali Asgari A, Ansari R, Nouraie M. Endoscopic findings in end-stage renal disease. Endoscopy. 2003;35:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kawai T, Watanabe M, Yamashina A. Impact of upper gastrointestinal lesions in patients on low-dose aspirin therapy: preliminary study. J Gastroenterol Hepatol. 2010;25 Suppl 1:S23-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129-1135. [PubMed] |
| 18. | Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257-268. [PubMed] |
| 19. | Ohkuma K, Okada M, Murayama H, Seo M, Maeda K, Kanda M, Okabe N. Association of Helicobacter pylori infection with atrophic gastritis and intestinal metaplasia. J Gastroenterol Hepatol. 2000;15:1105-1112. [PubMed] |
| 20. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [PubMed] |
| 21. | Mond DJ, Pochaczevsky R, Vernace F, Bank S, Chow KW. Can the radiologist recognize Helicobacter pylori gastritis. J Clin Gastroenterol. 1995;20:199-202. [PubMed] |
| 22. | Machado RS, Viriato A, Kawakami E, Patrício FR. The regular arrangement of collecting venules pattern evaluated by standard endoscope and the absence of antrum nodularity are highly indicative of Helicobacter pylori uninfected gastric mucosa. Dig Liver Dis. 2008;40:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Graham DY, Shiotani A. The time to eradicate gastric cancer is now. Gut. 2005;54:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1350] [Article Influence: 75.0] [Reference Citation Analysis (1)] |
| 25. | Ohkusa T, Miwa H, Hojo M, Kumagai J, Tanizawa T, Asaoka D, Terai T, Ohkura R, Sato N. Endoscopic, histological and serologic findings of gastric hyperplastic polyps after eradication of Helicobacter pylori: comparison between responder and non-responder cases. Digestion. 2003;68:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Satoh K, Kimura K, Takimoto T, Kihira K. A follow-up study of atrophic gastritis and intestinal metaplasia after eradication of Helicobacter pylori. Helicobacter. 1998;3:236-240. [PubMed] |