Published online Jun 28, 2013. doi: 10.3748/wjg.v19.i24.3872
Revised: March 12, 2013
Accepted: April 3, 2013
Published online: June 28, 2013
Processing time: 198 Days and 9.7 Hours
AIM: To compare radiofrequency ablation (RFA) and transcatheter arterial chemoembolization (TACE) with RFA monotherapy in hepatocellular carcinoma (HCC).
METHODS: We searched PubMed, Medline, Embase and Chinese databases (CBMdisc and Wanfang data) for randomized controlled trails comparing RFA plus TACE and RFA alone for treatment of HCC from January 2000 to December 2012. The overall survival rate, recurrence-free survival rate, tumor progression rate, and safety were analyzed and compared. The analysis was conducted on dichotomous outcomes and the standard meta-analytical techniques were used. Pooled odds ratios (ORs) with 95%CIs were calculated using either the fixed-effects or random-effects model. For each meta-analysis, the χ2 and I2 tests were first calculated to assess the heterogeneity of the included trials. For P < 0.05 and I2 > 50%, the assumption of homogeneity was deemed invalid, and the random-effects model was used; otherwise, data were assessed using the fixed-effects model. All statistical analysis was conducted using Review manager (version 4.2.2.) from the Cochrane collaboration.
RESULTS: Eight randomized controlled trials were identified as eligible for inclusion in this analysis and included 598 patients with 306 treated with RFA plus TACE and 292 with RFA alone. Our data analysis indicated that RFA plus TACE was associated a significantly higher overall survival rate (OR1-year = 2.96, 95%CI: 1.84-7.74, P < 0.001; OR2-year = 3.72, 95%CI: 1.24-11.16, P = 0.02; OR3-year = 2.65, 95%CI: 1.81-3.86, P < 0.001) and recurrence-free survival rate (OR3-year = 3.00, 95%CI: 1.75-5.13, P < 0.001; OR5-year = 2.26, 95%CI: 1.43-3.57, P = 0.0004) vs that of RFA alone. The tumor progression rate in patients treated with RFA alone was higher than that of RFA plus TACE (OR = 0.60, 95%CI: 0.42-0.88, P = 0.008) and there was no significant difference on major complications between two different kinds of treatment (OR = 1.20, 95%CI: 0.31-4.62, P = 0.79). Additionally, the meta-analysis data of subgroups revealed that the survival rate was significantly higher in patients with intermediate- and large-size HCC underwent RFA plus TACE than in those underwent RFA monotherapy; however, there was no significant difference between RFA plus TACE and RFA on survival rate for small HCC.
CONCLUSION: The combination of RFA with TACE has advantages in improving overall survival rate, and provides better prognosis for patients with intermediate- and large-size HCC.
Core tip: This study aimed to compare the effectiveness and prognosis of combination of transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) with that of RFA alone in hepatocellular carcinoma (HCC). To the best of our knowledge, there has been no comprehensive comparison on these two treatments in terms of small-, intermediate- and large-size HCC. Our analysis demonstrated that effectiveness of TACE combined with RFA was better than that of RFA for treatment of intermediate- and large-size HCC. We provide important evidence that TACE-RFA for intermediate- and large-size HCC may be performed more widely in clinical practice.
- Citation: Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2013; 19(24): 3872-3882
- URL: https://www.wjgnet.com/1007-9327/full/v19/i24/3872.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i24.3872
Hepatocellular carcinoma (HCC) is one of the most common malignancies. HCC ranks fifth for men and eighth for women and accounts for > 660000 new cases annually worldwide[1-3]. Due to poor baseline liver function, over tumor burden, or hepatic vessel invasion of HCC patients, it is barely possible to perform surgical resection. Transcatheter arterial chemoembolization (TACE) as a palliative therapy has become one of the most widely performed treatments for unresectable HCC[4,5]. However, the complete necrosis rate of tumors after TACE have only reached 10%-20%, and the 1-, 3- and 5-year overall survival rates range from 49%-71.9% to 23%-62.5% and 9%-17% in most studies[6-11]. Radiofrequency ablation (RFA) as a thermal in situ destruction technique has been proved to be a safe and effective treatment. RFA has been accepted as one of the best treatment options for small HCC[12,13]. However, it is difficult for RFA to achieve complete ablation in the treatment of relatively large HCC. Therefore, novel approaches to treating HCC patients have been extensively pursued and may offer opportunities for longer survival of patients with HCC. In recent years, the combination of interventional therapies has been widely performed for treatment of HCC. One such combined strategy is the combination of RFA and TACE.
Previous studies have reported that combination of RFA and TACE is more effective for induction of a significantly higher complete tumor necrosis rate than RFA monotherapy is, and improves overall survival rate in patients with HCC[14-16]. However, other studies assessing the clinical efficacy of RFA plus TACE and RFA alone for treatment of HCC have reported conflicting outcomes[17-19]. Hence, whether RFA combined with TACE or RFA monotherapy is the better treatment choice for HCC has long been debated. Meta-analysis is a suitable method to resolve this conflict. Several randomized controlled trials have been published in an attempt to answer the above question. A meta-analysis of these trials to analyze and compare comprehensively the clinical efficacy and safety of RFA combined with TACE and RFA monotherapy will provide clinicians with an unbiased opinion and valuable information about the efficacy of these treatment options. Comparison of these two treatments could help stratify the benefits of treatment choices for patients with HCC. Hence, this meta-analysis was designed to compare comprehensively the efficacy and safety of combination of RFA and TACE with RFA monotherapy for treatment of patients with HCC.
A search of the literature was conducted in PubMed, Medline, Embase and Chinese databases (CBMdisc and Wanfang data) from January 2000 to December 2012, using the following MeSH search headings: “hepatocellular carcinoma”, “radiofrequency ablation” and “transcatheter arterial chemoembolization”. A limit was set on the randomized controlled trials, which was conducted to identify studies comparing the effectiveness and safety of the combination of RFA and TACE with that of RFA monotherapy for HCC. No language restriction was imposed in this search.
To be eligible for the present meta-analysis, studies were required to have an integrated baseline of patients and outcomes: (1) study design: randomized controlled trials on RFA plus TACE vs RFA monotherapy in the treatment of HCC; (2) baseline of population: randomization of no fewer than 30 formally diagnosed HCC patients with average age, percentage male, Child-Pugh class, tumor size, and tumor stage; and (3) results: studies were required to have good descriptions of the results for overall survival rate, recurrence-free survival rate, tumor progression rate, and major complications. Abstracts, letters, reviews without original data, expert opinions, editorials, case reports and studies lacking control groups were excluded from the analysis.
All analysis was conducted on dichotomous outcomes and the standard meta-analytical techniques were used. Pooled odds ratios (ORs) with 95%CIs were calculated using either the fixed-effects or random-effects model. For each meta-analysis, the χ2 and I2 tests were first calculated to assess the heterogeneity of the included trials. P < 0.05 and I2 > 50% was considered significant. For P < 0.05 and I2 > 50%, the assumption of homogeneity was deemed invalid, and the random-effects model was used; otherwise, data were assessed using the fixed-effects model. The risk of the publication bias of the included trials was assessed using the symmetry of the funnel plot. Statistical analysis was performed using the software programs Review manager (version 4.2.2.) from the Cochrane collaboration. P < 0.05 was considered significant.
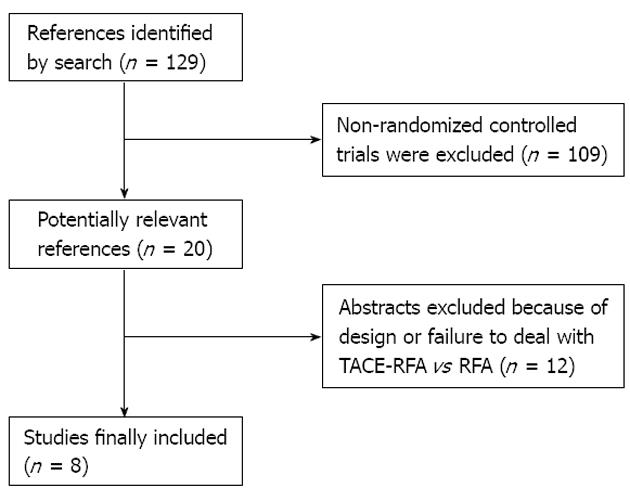
This meta-analysis yielded a total of 129 studies. Based on the inclusion and exclusion criteria, we included eight randomized controlled trials[14,15,18,20-24] (Figure 1). There was a total of 598 patients with 306 treated with RFA plus TACE and 292 treated with RFA alone (Table 1). Among these studies, there were eight, three, six and two studies that reported comparative data for overall survival rate at 1, 2, 3 and 5 years, respectively; five, three and two studies reported comparative data for recurrence-free survival rate at 1, 3 and 5 years, respectively (Tables 2 and 3). In the small HCC (tumor size ≤ 3 cm) subgroup, there were two studies with comparative data on survival rate at 1 and 3 years, respectively. In the intermediate-size HCC (3 cm < tumor size ≤ 5 cm) subgroup, there were four, three and two studies with comparative data on survival rate at 1, 3 and 5 years, respectively. In the large-size HCC (tumor size > 5 cm) subgroup, there were four and three studies with comparative data on survival rate at 1 and 3 years, respectively (Tables 2 and 3). There were six and three studies with comparative data on tumor progression rate and major complications, respectively (Tables 2 and 3). The quality of the included studies was detected using Review manager (version 4.2.2.) programs, and was judged to be high quality (Figure 2A-D).
| Ref. | Country | Design | Treatment | No. of patients | Age (yr) | Sex (male/female) | Tumor size (cm) | Child-Pugh class (A/B/C) |
| Peng et al[14] | China | RCT | TACE + RFA | 69 | 57.5 ± 10.0 | 60/9 | ≤ 5.01 | 60/9/0 |
| RFA | 70 | 55.1 ± 9.5 | 55/15 | - | 59/11/0 | |||
| Cheng et al[15] | China | RCT | TACE + RFA | 96 | ≤ 751 | NA | 3 < TS ≤ 7.51 | NA |
| RFA | 100 | - | - | - | NA | |||
| Yang et al[16] | China | RCT | TACE + RFA | 24 | 59.1 ± 11.4 | 18/6 | 6.6 ± 0.6 | NA |
| RFA | 12 | 61.0 ± 10.4 | 8/4 | 5.2 ± 0.4 | NA | |||
| Shibata et al[18] | Japan | RCT | TACE + RFA | 46 | 67.2 ± 8.9 | 31/15 | 1.7 ± 0.6 | 32/14/0 |
| RFA | 43 | 69.8 ± 8.0 | 33/10 | 1.6 ± 0.5 | 33/10/0 | |||
| Morimoto et al[20] | Japan | RCT | TACE + RFA | 19 | 70 (57-78) | 15/4 | 3.6 ± 0.7 | 12/7/0 |
| RFA | 18 | 73 (48-84) | 12/6 | 3.7 ± 0.6 | 16/2/0 | |||
| Kang et al[22] | China | RCT | TACE + RFA | 19 | 52.2 | 14/5 | 6.7 ± 1.1 | 12/7/0 |
| RFA | 18 | 50.7 | 14/4 | 6.2 ± 1.2 | 12/6/0 | |||
| Shen et al[23] | China | RCT | TACE + RFA | 18 | 52.7 (20-72) | 5/13 | 5.6 (2.2-15.8) | 4/14/0 |
| RFA | 16 | 56.1 (36-75) | 3/13 | 5.0 (2.3-12.3) | 6/10/0 | |||
| Zhang et al[24] | China | RCT | TACE + RFA | 15 | 57.8 (39-72) | 12/3 | 4.6 (2.3-7.1) | NA |
| RFA | 15 | 61.8 (38-78) | 13/2 | 4.1 (2.4-6.0) | NA |
| Ref. | Treatment | No. of patients | Recurrence- free survival rate | Overall survival rate | |||||
| 1 yr | 3 yr | 5 yr | 1 yr | 2 yr | 3 yr | 5 yr | |||
| Peng et al[14] | TACE+RFA | 69 | 80.00% | 45.00% | 40.00% | 94.00% | NA | 69.00% | 46.00% |
| RFA | 70 | 64.00% | 18.00% | 18.00% | 82.00% | 47.00% | 36.00% | ||
| Cheng et al[15] | TACE+RFA | 96 | NA | NA | 58.00% | 83.00% | NA | 55.00% | 31.00% |
| RFA | 100 | 42.00% | 67.00% | 32.00% | 8.00% | ||||
| Yang et al[16] | TACE+RFA | 24 | 29.00% | NA | NA | 68.00% | NA | NA | NA |
| RFA | 12 | 34.70% | 57.00% | ||||||
| Shibata et al[18] | TACE+RFA | 46 | 71.30% | 48.80% | NA | 100.00% | 100.00% | 84.80% | NA |
| RFA | 43 | 74.30% | 29.70% | 100.00% | 88.80% | 84.50% | |||
| Morimoto et al[20] | TACE+RFA | 19 | 67.00% | NA | 100.00% | 93.00% | 93.00% | NA | |
| RFA | 18 | 56.00% | 28.00% | 89.00% | 89.00% | 80.00% | |||
| Kang et al[22] | TACE+RFA | 19 | NA | NA | NA | 84.20% | 42.10% | 36.80% | NA |
| RFA | 18 | 66.10% | 22.20% | 16.70% | |||||
| Shen et al[23] | TACE+RFA | 18 | 63.90% | 50.00% | NA | 87.50% | NA | 73.30% | NA |
| RFA | 16 | 30.00% | 18.70% | 52.20% | 20.40% | ||||
| Zhang et al[24] | TACE+RFA | 15 | NA | NA | NA | 100.00% | NA | NA | NA |
| RFA | 15 | 80.00% | |||||||
| Variables | No. of studies furnishing data | Results | OR (95%CI) | P value | I2 | |
| RFA-TACE | RFA | |||||
| Efficacy overall survival rate | ||||||
| 1 yr | 8 | 89.20% | 76.00% | 2.96 (1.84- 7.47) | < 0.001 | 0.00% |
| 2 yr | 3 | 85.70% | 73.40% | 3.72 (1.24-11.16) | 0.02 | 0.00% |
| 3 yr | 6 | 66.70% | 45.70% | 2.65 (1.81- 3.86) | < 0.001 | 13.10% |
| 5 yr | 2 | 37.50% | 19.40% | 2.78 (0.85-9.12) | 0.09 | 79.30% |
| Recurrence-free survival rate | ||||||
| 1 yr | 5 | 67.60% | 60.30% | 1.59 (0.99-2.55) | 0.05 | 3.50% |
| 3 yr | 3 | 46.60% | 22.40% | 3.00 (1.75-5.13) | < 0.001 | 0.00% |
| 5 yr | 2 | 50.90% | 32.30% | 2.26 (1.43-3.57) | 0.0004 | 0.00% |
| Survival rate (TS ≤ 3 cm) | ||||||
| 1 yr | 2 | 98.90% | 91.00% | 8.42 (1.01- 70.56) | 0.05 | NA |
| 3 yr | 2 | 78.10% | 71.90% | 1.35 (0.67-2.74) | 0.4 | 0.00% |
| Survival rate (3 cm < TS ≤ 5 cm) | ||||||
| 1 yr | 4 | 95.30% | 84.60% | 3.46 (1.29-9.28) | 0.01 | 0.00% |
| 3 yr | 3 | 78.20% | 53.90% | 3.58 (1.79-7.15) | 0.0003 | 28.60% |
| 5 yr | 2 | 49.30% | 15.40% | 5.34 (2.42-11.75) | < 0.001 | 0.00% |
| Survival rate (TS < 5 cm) | ||||||
| 1 yr | 4 | 75.90% | 52.50% | 2.91 (1.60-5.29) | 0.0004 | 0.00% |
| 3 yr | 3 | 43.10% | 10.30% | 6.96 (3.01-16.07) | 0.00001 | 0.00% |
| Tumor progression rate | 6 | 31.60% | 42.80% | 0.60 (0.42-0.88) | 0.008 | 34.80% |
| Major complications | 3 | 3.70% | 3.10% | 1.20 (0.31-4.62) | 0.79 | 0.00% |
Heterogeneity assessment: In the analysis of the effects of 1-, 2- and 3-year overall survival rates (P1-year = 0.77, I2 = 0%; P2-year = 0.56, I2 = 0%; P3-year = 0.33, I2 = 13.1%); 1-, 3- and 5-year recurrence-free survival rates (P1-year = 0.39, I2 = 3.5%; P3-year = 0.60, I2 = 0%; P5-year = 0.37, I2 = 0%); tumor progression rates (P = 0.18, I2 = 34.8%); major complications (P = 0.91, I2 = 0%), the meta-analysis data indicated that there was no significant heterogeneity among the studies, thus the fixed-effects model was used to pool the results. However, in the analysis of the effect of 5-year overall survival rates, there was significant heterogeneity among the studies (P5-year = 0.03, I2= 79.3%), thus the random-effects model was used to pool the results (Figures 2 and 3).
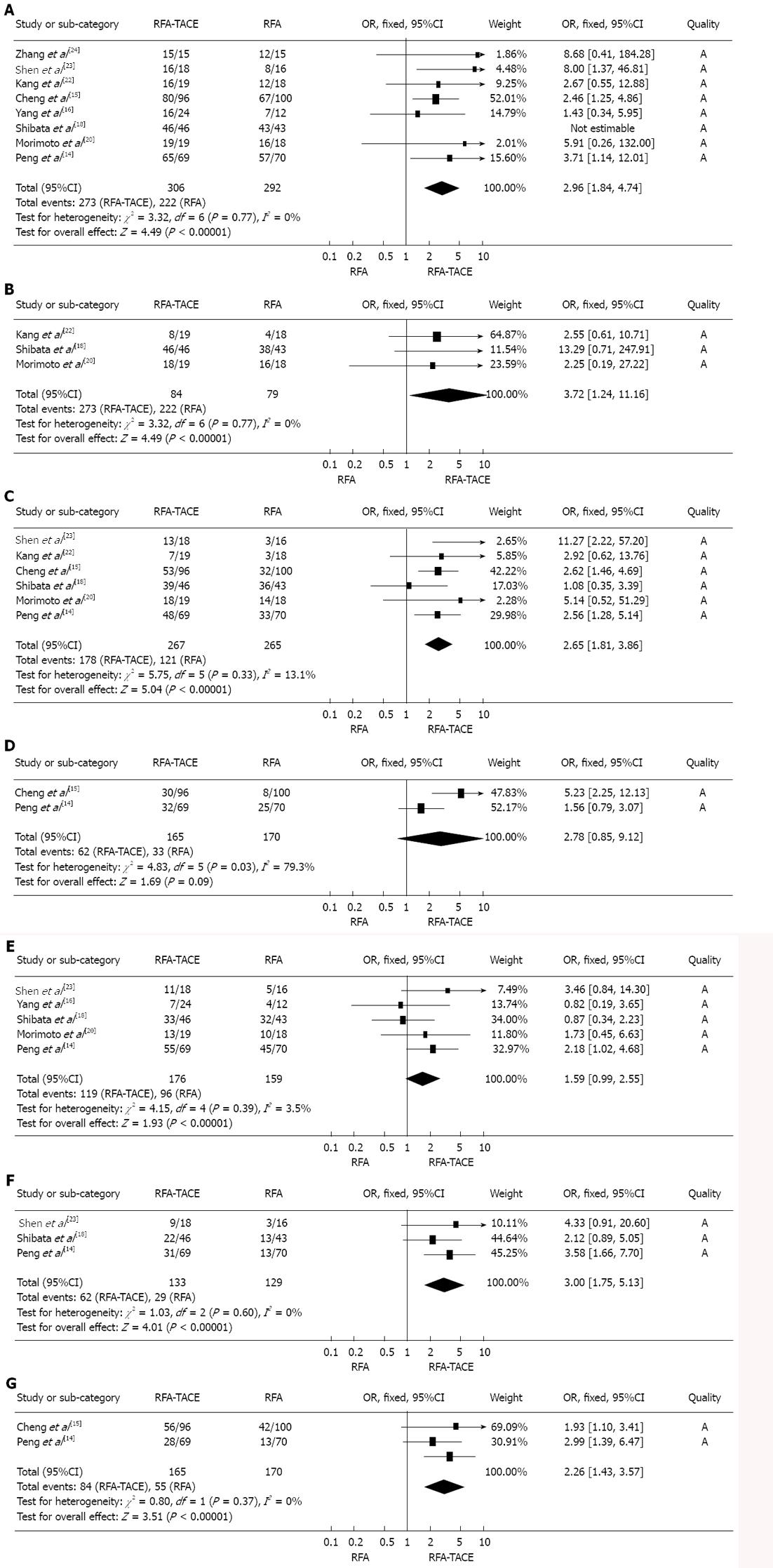
Overall survival rate: There was a significant difference in 1-, 2- and 3-year overall survival rate between treatment with RFA plus TACE and RFA alone, and the meta-analysis data suggested that RFA plus TACE was associated with a significantly higher 1-, 2- and 3-year overall survival rate than RFA monotherapy was (OR1-year = 2.96, 95%CI: 1.84-7.74, P < 0.001; OR2-year = 3.72, 95%CI: 1.24-11.16, P = 0.02; OR3-year = 2.65, 95%CI: 1.81-3.86, P < 0.001). However, there was no significant difference in overall survival rate between these two treatments for 5-year overall survival rate (OR5-year = 2.78, 95%CI: 0.85-9.12, P = 0.09) (Figure 2A-D).
Recurrence-free survival rate: There was a significant difference in 3- and 5-year recurrence-free survival rate between treatment with RFA plus TACE and RFA alone, and the meta-analysis data suggested that RFA plus TACE was associated with a significantly higher 3-and 5-year recurrence-free survival rate than RFA monotherapy was (OR3-year = 3.00, 95%CI: 1.75-5.13, P < 0.001; OR5-year = 2.26, 95%CI: 1.43-3.57, P = 0.0004). However, there was no significant difference in recurrence-free survival rate between the two treatments at 1 year (OR1-year = 1.59, 95%CI: 0.99-2.55, P = 0.05) (Figure 2E-G).
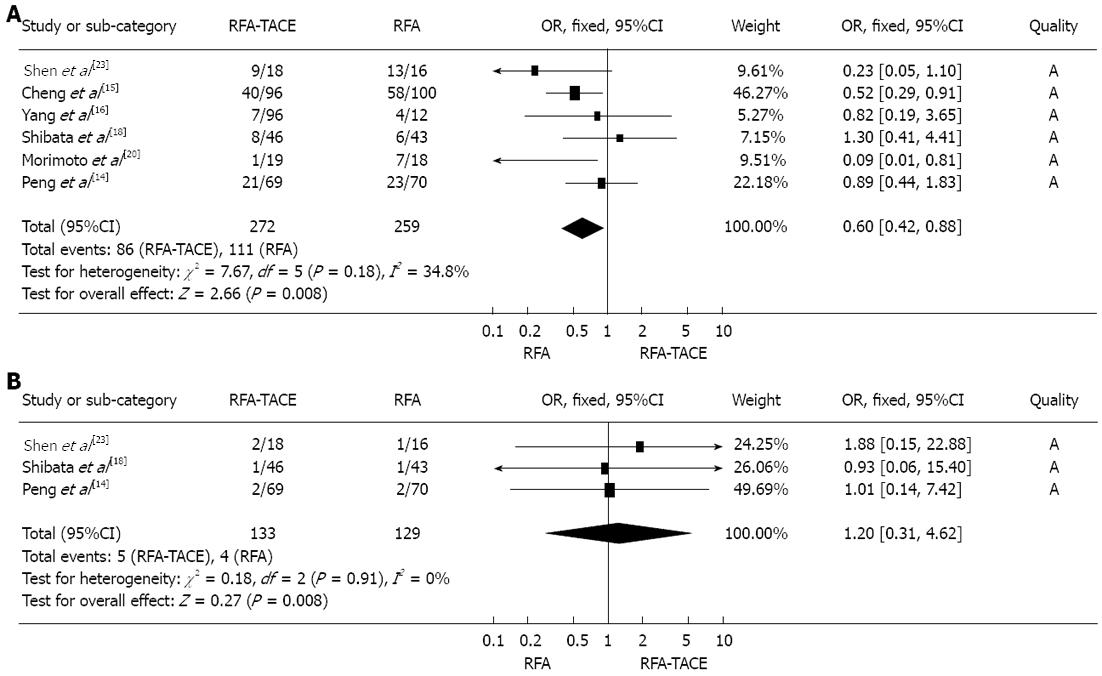
Tumor progression rate: There was a significant difference in tumor progression rate between treatment with RFA plus TACE and RFA alone, and the meta-analysis data suggested that RFA monotherapy was associated with a significantly higher tumor progression rate than RFA plus TACE treatment was (OR = 0.60, 95%CI: 0.42-0.88, P = 0.008) (Figure 3A).
Meta-analysis data showed that there was no significant difference in major complications between treatment with RFA plus TACE and RFA monotherapy (OR = 1.20, 95%CI: 0.31-4.62, P = 0.79) (Figure 3B).
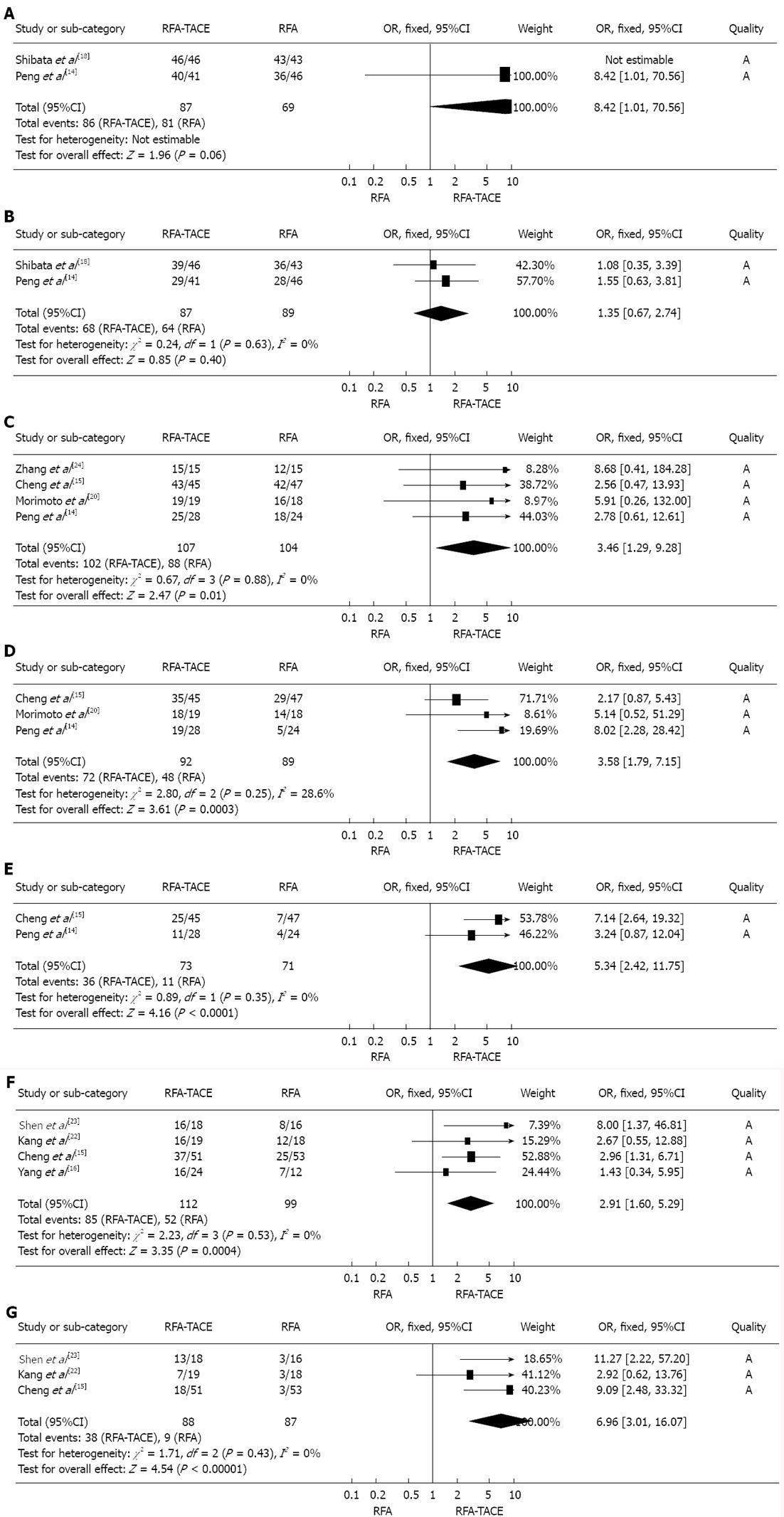
The meta-analysis data revealed that there was no significant difference in survival rate between the two treatments at 1 year (OR: 8.42, 95%CI: 1.01-70.56, P = 0.05) and 3 years (OR: 1.35, 95%CI: 0.67-2.74, P = 0.40) (Figure 4A and B).
There was a significant difference in survival rate between the two treatments at 1, 3 and 5 years, and the meta-analysis data indicated that RFA plus TACE was associated with a significantly higher 1-, 3- and 5-year survival rate than RFA monotherapy was (OR1-year = 3.46, 95%CI: 1.29-9.28, P = 0.01; OR3-year = 3.58, 95%CI: 1.79-7.15, P = 0.0003; OR5-year = 5.34, 95%CI: 2.42-11.75, P < 0.001) (Figure 4C-E).
There was a significant difference in survival rate between the two treatments at 1 and 3 years, and the meta-analysis data revealed that RFA plus TACE was associated with a significantly higher 1- and 3-year survival rate than RFA monotherapy was (OR1-year = 2.91, 95%CI: 1.60-5.29, P = 0.0004; OR3-year = 6.69, 95%CI: 3.01-16.07, P < 0.001) (Figure 4F and G).
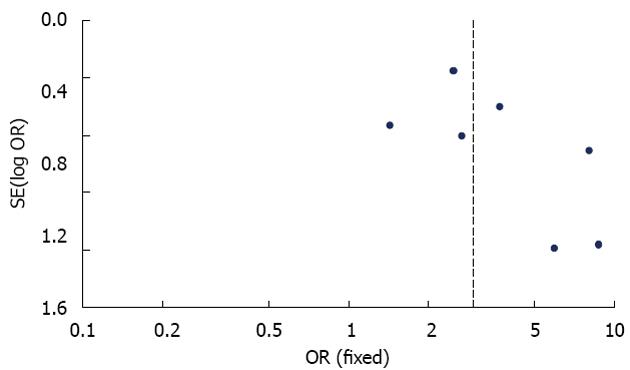
The publication bias in this study was detected using the funnel plot of the meta-analysis results. All of the included studies reported comparative data on 1-year overall survival rate. In the analysis of the effect of 1-year overall survival rate, the symmetry of the funnel plot shape suggested that there was no obvious publication bias in this meta-analysis (Figure 5).
According to our data analysis, we found that the combination of TACE and RFA was associated with higher overall and recurrence-free survival rates than RFA monotherapy was in the treatment of HCC. Additionally, the subgroups analysis indicated that although there were no significant differences between TACE plus RFA and RFA alone in the treatment of small-size HCC, combination of TACE and RFA was associated with a higher survival rate than was RFA monotherapy for patients with intermediate- and large-size HCC. Thus, our analysis suggested that TACE combined with RFA was more effective than RFA monotherapy for treatment for intermediate- and large-size HCC.
Previous studies have reported that RFA combined with TACE is more effective than RFA monotherapy for treatment of HCC[14-16]. However, some other studies have reported conflicting results[17-19]. Meta-analysis is a method that combines data from all eligible studies, and has the advantage of reducing random error, thus obtaining more precise estimates and defining the effect of clinical interventions more precisely[25,26].
Tumor recurrence and progression are the major risk factors that affect the prognosis of HCC patients. A high rate of intrahepatic recurrence and progression after RFA plus TACE and/or RFA is the main cause of late death of patients with HCC. In the current analysis, the recurrence-free survival and progression rates were compared and analyzed. Our meta-analysis indicated that the 1-, 3- and 5-year recurrence-free survival rate was higher after RFA plus TACE than after RFA alone. However, RFA monotherapy was found to be associated with a higher tumor progression rate than RFA plus TACE was. The residual tumor tissue after RFA was the main cause of tumor recurrence and progression, which may be attributable to insufficient ablation of the primary tumor after RFA monotherapy. RFA combined with TACE has advantages in improving tumor necrosis rate. TACE is expected to reduce the cooling effect of hepatic blood flow in RFA by decreasing hepatic arterial flow, and plays a primary role in inducing tumor destruction[14,20,21]. Additionally, RFA cannot be a suitable treatment for tumors with multiple nodules, and TACE after RFA can effectively induce necrosis of multiple nodules and improve the tumor necrosis rate. This may explain the better results following RFA plus TACE in the treatment of HCC.
As regards the subgroups in our analysis, we found that there was no significant difference in survival rate between the two treatments for small HCC. However, there was a significant difference in survival rate between RFA plus TACE and RFA alone for treatment of intermediate- and large-size HCC, and our meta-analysis data showed that RFA plus TACE was associated with a significantly higher survival rate than RFA monotherapy was. RFA as a thermal in situ destruction technique was proved to be a safe and effective treatment. It is possible for RFA to achieve complete necrosis for treatment of small HCC. In recent years, RFA has been accepted as one of the best treatment options for small HCC. However, due to the limitation of the ablation area, it is barely possible for RFA to achieve complete necrosis during treatment of a relatively large HCC[27,28]. RFA combined with TACE can resolve this problem effectively. According to the clinical studies and experience, we found that there were some synergistic effects between RFA and TACE in combined treatment: (1) TACE can reduce the cooling effect of hepatic blood flow by decreasing hepatic arterial flow, and improve the effect of percutaneous RFA thermal therapy, which plays a critic role in inducing tumor necrosis[14,20,21]; (2) edematous change in the tumors induced by ischemia and inflammation after TACE is expected to enlarge the area of tumor necrosis during RFA treatment; and (3) RFA combined with TACE can prevent HCC with hepatic artery portal fistula from invading the portal vein and provides a better prognosis for HCC patients[20,29].
The risk of publication bias in this meta-analysis was assessed by the symmetry of the funnel plot[30,31]. All the studies included in the current meta-analysis had comparative data for 1-year overall survival rate. In the analysis of the effect of 1-year overall survival rate, we found that the level of the symmetry of the funnel plot was judged to be high, which indicated that there was no significant publication bias in this meta-analysis. Eight randomized controlled trials were included in this study. The overall quality of the studies was detected using Review Manager from the Cochrane Collaboration and was judged to be high. This suggests that the studies included in the study had strong evidence to support the results of our meta-analysis.
To the best of our knowledge, no meta-analysis has been performed to compare the efficacy and safety of RFA combined with TACE and RFA monotherapy in terms of overall survival rate, recurrence-free survival rate, tumor progression, and major complications. Our meta-analysis is believed to be the first report to assess comprehensively combination of TACE and RFA compared with RFA alone for treatment of HCC. Additionally, we also analyzed and compared the effectiveness of RFA plus TACE and RFA monotherapy for treatment of small-, intermediate- and large-size HCC. The analysis of these studies is important and useful for precise and objective statistical assessment of the clinical efficacy of RFA plus TACE and RFA alone for treatment of HCC.
Our study had several limitations. First, the heterogeneity of the inclusion criteria (Child-Pugh class, number of tumors, tumor size, tumor stage, and treatment design) might have affected the consistency of the results and caused between-study heterogeneity, which could have affected the overall quality of our study. Second, the etiological factors of HCC such as viral hepatitis, alcoholic liver disease, and autoimmune liver disease, were not considered in the trials. Whether HCC patients with different etiological factors could obtain similar outcomes from treatment with RFA plus TACE needs further research.
In conclusion, this meta-analysis suggests that combination of RFA with TACE is more effective than RFA monotherapy in the treatment of patients with intermediate- and large-size HCC, with significantly higher survival rates achieved with the combined methods. The combination of interventional therapies may be applied more widely in the treatment of HCC.
Radiofrequency ablation (RFA) and transcatheter arterial chemoembolization (TACE) have been widely accepted as the established non-surgical treatment options for hepatocellular carcinoma (HCC). However, which is the superior treatment choice is still uncertain. Previous studies have suggested that the effectiveness of combination of TACE and RFA is much better than that of RFA monotherapy in HCC. However, other studies assessing the effectiveness of combination of TACE and PRFA compared with RFA alone have reported conflicting results. Hence, it is necessary to design a study to compare comprehensively the effectiveness and safety of TACE plus RFA and RFA monotherapy for treatment of patients with HCC.
In the current study, a meta-analysis was designed to compare comprehensively the overall survival rates, recurrence-free survival rates, and tumor progression rates for RFA plus TACE with those of RFA monotherapy. Additionally, based on the tumor size, the survival rates of patients with small-, intermediate- and large-size HCC underwent RFA plus TACE compared with those who underwent RFA alone.
The current analysis comprehensively compared the effectiveness and safety of RFA combined with TACE with that of RFA monotherapy in HCC. This analysis indicated that the overall and recurrence-free survival rates of patients treated with RFA plus TACE were significantly higher than those with RFA alone. The authors also found that although there were no significant differences between RFA plus TACE and RFA monotherapy for small HCC, the efficacy of RFA plus TACE was obviously better than that of RFA alone for treatment of intermediate- and large-size HCC.
The analysis showed that the effectiveness of RFA combined with TACE was much better than that of RFA monotherapy for treatment of intermediate- and large-size HCC. Comparison of these two treatments could provide the theoretical basis for the use of RFA plus TACE for treatment of HCC and help stratify the benefits of treatment choices for patients with HCC.
TACE is one of the most widely performed treatments for unresectable HCC, which is a type of interventional radiology. RFA is a thermal in situ destruction technique, and it has been proved to be a safe and effective treatment. RFA has been accepted as one of the best treatment options for small HCC.
This was an excellent study that addresses an important topic, particularly the part comparing the efficacy and safety of combination of RFA and TACE with RFA monotherapy for treatment of patients with HCC. The report is concise and informative.
P- Reviewers Shi HY, Sun ZH S- Editor Zhai HH L- Editor Kerr C E- Editor Xiong L
| 1. | Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 2. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 3. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2598] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 5. | El Awady MK, Bader El Din NG, Tabll A, El Hosary Y, Abdel Aziz AO, El Khayat H, Salama M, Abdelhafez TH. IL28B polymorphism and cytomegalovirus predict response to treatment in Egyptian HCV type 4 patients. World J Gastroenterol. 2013;19:290-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Buscarini L, Buscarini E, Di Stasi M, Quaretti P, Zangrandi A. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med. 1999;20:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 396] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Marelli L, Shusang V, Buscombe JR, Cholongitas E, Stigliano R, Davies N, Tibballs J, Patch D, Meyer T, Burroughs AK. Transarterial injection of (131)I-lipiodol, compared with chemoembolization, in the treatment of unresectable hepatocellular cancer. J Nucl Med. 2009;50:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, Lim YS, Lee HC, Chung YH, Lee YS. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Farinati F, Giacomin A, Vanin V, Giannini E, Trevisani F. TACE treatment in hepatocellular carcinoma: what should we do now? J Hepatol. 2012;57:221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 13. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1103] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 14. | Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, Yi CH. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Yang W, Chen MH, Wang MQ, Cui M, Gao W, Wu W, Wu JY, Dai Y, Yan K. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res. 2009;39:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, Kim PN. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81:e189-e193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 20. | Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452-5460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Yang P, Liang M, Zhang Y, Shen B. Clinical application of a combination therapy of lentinan, multi-electrode RFA and TACE in HCC. Adv Ther. 2008;25:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Kang CB, Xu HB, Wang SL, Rui B. Treatment of large hepatoma by TACE in combination with RFA. Zhonghua Gandan Waike Zazhi. 2007;13:828-830. [DOI] [Full Text] |
| 23. | Shen SQ, Xiang JJ, Xiong CL, Wu SM, Zhu SS. Intraoperative radiofrequency thermal ablation combined with portal vein infusion chemotherapy and transarterial chemoembolization for unresectable HCC. Hepatogastroenterology. 2005;52:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Zhang Z, Wu M, Chen H, Chen D, He J. [Percutaneous radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma]. Zhonghua Waike Zazhi. 2002;40:826-829. [PubMed] |
| 25. | Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2012;57:3026-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30388] [Article Influence: 779.2] [Reference Citation Analysis (0)] |
| 27. | Widmann G, Schullian P, Haidu M, Bale R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Meza-Junco J, Montano-Loza AJ, Liu DM, Sawyer MB, Bain VG, Ma M, Owen R. Locoregional radiological treatment for hepatocellular carcinoma; Which, when and how? Cancer Treat Rev. 2012;38:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Zhao M, Wang JP, Pan CC, Li W, Huang ZL, Zhang L, Fang WJ, Jiang Y, Li XS, Wu PH. CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement. Eur J Radiol. 2012;81:2717-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24774] [Article Influence: 1769.6] [Reference Citation Analysis (3)] |
| 31. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40440] [Article Influence: 1444.3] [Reference Citation Analysis (2)] |