Published online Jun 21, 2013. doi: 10.3748/wjg.v19.i23.3699
Revised: March 22, 2013
Accepted: April 27, 2013
Published online: June 21, 2013
Processing time: 136 Days and 6.4 Hours
Dissemination of gastric cancer may usually occur by direct spread through the perigastric tissues to adjacent organ, lymphatic spread, and hematogenous spread. We report a rare case of gastric cancer with mucosal metastastic lesion on the upper esophagus that was diagnosed by endoscopy and endosonography. A biopsy of the esophageal mass was performed and the pathologic findings with immunohistochemical stain for Mucin-5AC are proved to be identical to that of gastric adenocarcinoma, suggesting metastasis from main lesion of the gastric cancer. The lesion could not be explained by lymphatic or hematogenous spread, and its metastasis mechanism is considered to be different from previous studies. We suggest that the gastroesophageal reflux of cancer cells could be one of the possible metastatic pathways for metastasis of esophagus from an adenocarcinoma of the stomach.
Core tip: I believe the paper may be of particular interest to your readers because the reason is as follows. First, there has been rarity of case reports about esophageal metastasis from gastric cancer without any evidence of lymphatic involvement or direct spread from the primary lesion. Second, gastroesophageal reflux of cancer cells could be one of the possible metastatic pathways for metastasis of esophagus from an adenocarcinoma of the stomach, and this case proves the possibility of direct implantation of gastric adenocarcinoma cells refluxed on esophagus.
- Citation: Ki SH, Jeong S, Park IS, Lee DH, Lee JI, Kwon KS, Kim HG, Shin YW. Esophageal mucosal metastasis from adenocarcinoma of the distal stomach. World J Gastroenterol 2013; 19(23): 3699-3702
- URL: https://www.wjgnet.com/1007-9327/full/v19/i23/3699.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i23.3699
Dissemination of gastric cancer may usually occur through one of following three pathways: (1) direct spread through the perigastric tissues to adjacent organ; (2) lymphatic spread; and (3) hematogenous spread[1,2].
Herein we report a rare case of gastric cancer with mucosal metastastic lesion on the upper esophagus that was diagnosed by endoscopy and endosonography. The lesion could not be explained by lymphatic or hematogenous spread, and its metastasis mechanism is considered to be different from previous ones.
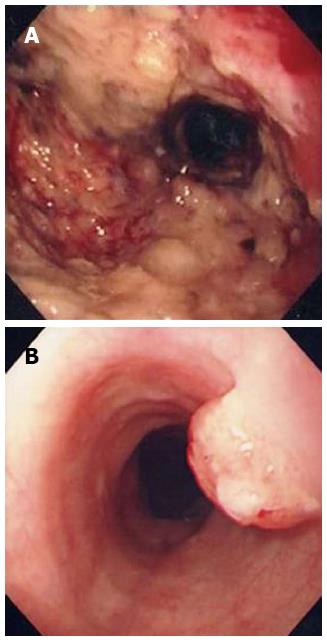
A 60-year-old man was admitted to our institution for systemic chemotherapy. Fourteen months prior to the admission, he was diagnosed with advanced gastric carcinoma on the antrum (Figure 1A). Palliative subtotal gastrectomy was performed with gastrojejunostomy to relieve pyloric obstruction, and the pathologic finding of surgically resected stomach disclosed adenocarcinoma.
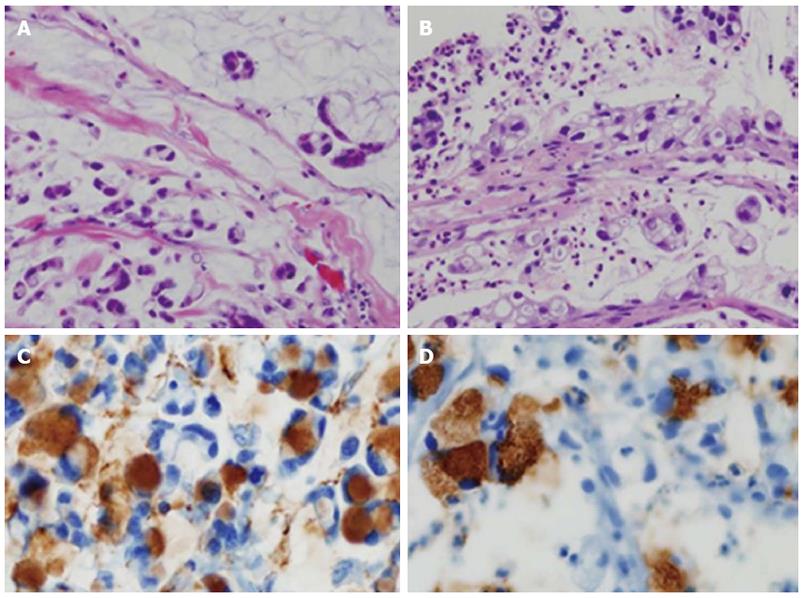
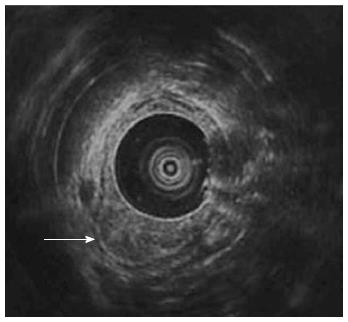
Follow-up abdominal computer tomography was done a week prior to the current admission which revealed multiple hepatic metastases. The patient complained of dysphagia, and therefore endoscopy was performed. The endoscopic examination of the upper digestive tract showed a single, 1 cm-sized, polypoid mass which located 26 cm below the upper incisor (Figure 1B). Evidence of tumor recurrence in remnant stomach was not found. A biopsy of the polypoid mass of esophagus was performed and the pathologic findings with immunohistochemical stain for Mucin-5AC are proved to be identical to that of gastric adenocarcinoma, suggesting metastasis from main lesion of the gastric cancer (Figure 2). Endoscopic ultrasonography (EUS) with a miniature probe of 20 mHz frequency revealed hypoechoic wall thickening of upper esophagus, confined only to mucosal layer without any lymph node enlargement around esophagus (Figure 3).
He was treated with second line of systemic chemotherapy, consisted of docetaxel and cisplatin. However the disease progressed even after 3 cycles of the chemotherapy.
Intramural spread of upper gastrointestinal tract tumors usually occurs via abundant lymphatic channels within the submucosal and subserosal layers of the gastric channel and prominent submucosal lymphatic plexus in esophagus. Regardless of the histologic type of the tumor, the tumor is able to infiltrate into submucosal or subserosal layer and spread to adjacent organ via lymphatic communication between stomach and esophagus[3-5]. It is considered that esophageal metastasis from the gastric cancers would also be seen as submucosal tumor in gross appearance since it shares same lymphatic channel. In our patient, follow-up endoscopy revealed polypoid mass in upper esophagus instead of appearance of submucosal tumor. EUS of the esophagus performed for assessment of the infiltration depth of the metastatic tumor evidently showed that the tumor was confined to mucosal layer and there was no disruption of muscularis mucosa or enlarged lymph nodes. If there was a pathologic confirmation such as endoscopic submucosal dissection or esophagectomy it must have been definite that the esophageal tumor was confined to mucosal layer. But the resection could not be performed, considering his performance is poor and the disease is markedly progressed. EUS is currently the most accurate means available for tumor staging and locoregional nodal staging[6,7]. Therefore we could conclude that the esophageal tumor was confined to mucosal layer using by EUS.
There has been a case report of gastric cancer with esophageal metastasis which showed very similar finding of esophagus in EUS[8]. The wall of the esophagus at the level of the polypoid lesion was hypo-echoic and thick due to thickened mucosa. In this case total gastrectomy and esophagectomy was performed and the esophageal polypoid lesion was proved to be adenocarcinoma, identical to the primary gastric cancer. In this case report the author speculated that esophageal implantation metastasis from the gastric adenocarcinoma might have taken place by the gastro-esophageal reflux since gastro-esophageal reflux has been documented in various numbers of patients after distal gastrectomy.
Symptoms of gastroesophageal reflux disease have been previously reported to occur in about 30% of patients undergoing distal gastrectomy with Billoth I reconstruction[9]. Distal gastrectomy with Billoth I reconstruction causes two anatomical changes which promote gastroesophageal reflux; the presence of abnormal findings in the cardia affected by the enlarged angle of Hiss and the high positioning of the remnant stomach in the supine position[9]. This patient underwent palliative subtotal gastrectomy and it is most likely that he had at least gastroesophageal reflux due to the anatomical alterations after surgery and it could have affected the direct implantation of gastric cancer cells on the esophagus.
According to previous study in adenocarcinoma of gastric cancer six patients among a total of 143 patients were verified to have intramural esophageal metastasis[10]. Most of these metastases would have been mediated by lymphatic channels between stomach and esophagus but few could have been done by direct implantation of tumor cells. All patients had gastric cancer from cardia with lymphatic invasion. The distance from the primary tumor of the metastases was 20-50 mm. The metastases were appeared as multiple small submucosal tumors with intact mucosa in some patients. Most of these metastases would have been mediated by lymphatic channels between stomach and esophagus but few could have been done by direct implantation of tumor cells.
In our case, previous multiple hepatic metastases can arouse another possible mechanism of esophageal metastasis, but the esophageal lesion could not be explained by lymphatic or hematogenous spread, and its metastasis mechanism is considered to be different from previous studies.
In hematogenous or lymphatic spread, the esophageal metastasis involves submucosa and usually present as multiple masses, but in our case, esophageal metastasis was single solitary mass and was confined only to mucosal layer without any lymph node enlargement around esophagus. Most intramural esophageal metastases from gastric cancer originate from gastric cardia via lymphatic channels. But in our case, gastric cancer had occurred from antrum that was not close to esophagus and esophageal tumor was located at mid esophagus, far from stomach. It was difficult to metastasize from gastric antrum to mid esophagus without adjacent invasion if it metastasized via lymphatic channels.
We confirmed that the esophageal mass was metastasized from gastric cancer by pathology using immunohistochemical stain. We suggest that the gastroesophageal reflux of cancer cells could be one of the possible metastatic pathways for metastasis of esophagus from an adenocarcinoma of the stomach.
There has been rarity of case reports about esophageal metastasis from gastric cancer without any evidence of lymphatic involvement or direct spread from the primary lesion.
We suggest that the gastroesophageal reflux of cancer cells could be one of the possible metastatic pathways for metastasis of esophagus from an adenocarcinoma of the stomach, and this case proves the possibility of direct implantation of gastric adenocarcinoma cells refluxed on esophagus.
P- Reviewers Rajeshwari K, Shehata MM S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Robbins SL, Cotran RS, Kumar V. Pathologic basis of disease. Philadelphia: WB Saunders 2005; 79-281. |
| 2. | Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine. 17th ed. New York: McGraw-hill 2008; 509-513. |
| 3. | DeVita VT, Lawrence TS, Rosenberg , Weinberg RA, Depinho RA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and practice of oncology. 8th ed. Philadelphia: Lippincott Williams and Wilkins 2008; 1045-1046. |
| 4. | Takano Y, Koyama S, Yokota H, Nakahara A, Fukutomi H, Osuga T, Horiuchi S, Todoroki K, Iwasaki Y, Ishidoh T. [A case of superficial esophageal cancer with an intramural metastasis to the gastric wall]. Gan No Rinsho. 1989;35:948-954. [PubMed] |
| 5. | Ebihara Y, Hosokawa M, Kondo S, Katoh H. Thirteen cases with intramural metastasis to the stomach in 1259 patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2004;26:1223-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Abdalla EK, Pisters PW. Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol. 2004;31:513-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol. 2004;31:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Szántó I, Vörös A, Gonda G, Nagy P, Cserepes E, Gamal EM, Kiss J. [Esophageal implantation metastasis from adenocarcinoma of the cardia]. Magy Seb. 2001;54:393-396. [PubMed] |
| 9. | Takahashi T, Yoshida M, Kubota T, Otani Y, Saikawa Y, Ishikawa H, Suganuma K, Akatsu Y, Kumai K, Kitajima M. Morphologic analysis of gastroesophageal reflux diseases in patients after distal gastrectomy. World J Surg. 2005;29:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Szántó I, Vörös A, Nagy P, Gonda G, Gamal EM, Altorjay A, Banai J, Kiss J. Esophageal intramural metastasis from adenocarcinoma of the gastroesophageal junction. Endoscopy. 2002;34:418-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |