INTRODUCTION
Dieulafoy’s lesion (DL), a condition first reported in 1884, and named in 1898, refers to dilated submucosal arterial malformation (width of 1-3 mm) that protrudes through overlying epithelium erosion accompanied with normal surrounding mucosa[1]. It can be found in any segment of the gastrointestinal (GI) tract with a preference for the lesser curvature of the stomach, 6 cm distally from the esophagogastric junction[2]. The incidence of DL is unknown because it is usually an asymptomatic condition that is often undiagnosed; however, its most common complication, GI hemorrhage, accounts for 2%-3% of all GI hemorrhage. A combination of abundant arterial bleeding with an inapproachable site of the lesion implies urgent and reliable treatment; the available endoscopic hemostatic methods are thermal, mechanical and injection[3,4]. To the best of our knowledge mini-loop ligation has never been reported as a treatment option in a case of DL.
CASE REPORT
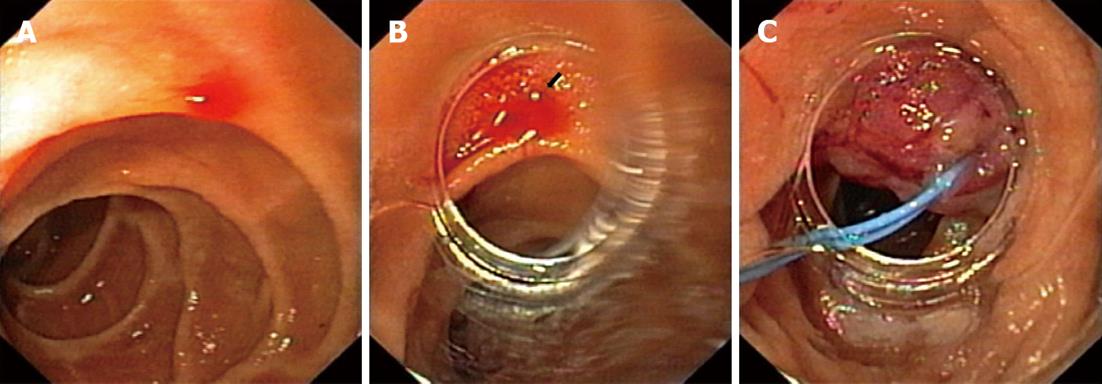
A 61-year-old woman was admitted to our emergency unit because of melenic stool without abdominal pain, nausea or vomiting. Her previous history indicated that she did not have any serious health problems, just arterial hypertension and hyperlipoproteinemia, which were adequately regulated with chronic therapy. There was no history of alcohol abuse or excess intake of non-steroidal anti-inflammatory drugs (NSAIDs). She was anemic, but showed no signs of hemodynamic instability. Although a nasogastric tube aspirate was clear after insertion, a rectal examination showed evidence of melena. Laboratory tests revealed low levels of erythrocytes 2.8 × 109/L and hemoglobin 79 g/L with normal coagulation parameters. After signing an informed consent, the patient underwent an urgent upper endoscopy (GIF Q160, Olympus Optical Co., Japan). Blood oozing from a pin-point defect with normal surrounding mucosa of the duodenum was visualized and that was suggestive of DL (Figure 1A). A detachable nylon ring was opened at the rim of a transparent ligation chamber attached to the tip of the endoscope (Figure 1B). The lesion was aspirated into the chamber and subsequently the mini-loop was closed and detached, achieving complete hemostasis (Figure 1C). A blood transfusion was administered later, together with pantoprazole in the infusion over a 48 h period. A repeated endoscopy showed that the mini-loop was located at the site of the lesion with no visible sign of acute hemorrhage. The patient was discharged from the hospital on the fourth day with a satisfactory complete blood count (erythrocytes 3.9 × 109/L and hemoglobin 98 g/L). On follow up examination two months later, no pathology of a duodenal mucosa was observed, such as ulceration on the site, and values of her complete blood count were normal.
Figure 1 Sixty-one-year-old woman was admitted to our emergency unit because of melenic stool without abdominal pain, nausea or vomiting.
A: Bleeding duodenal Dieulafoy's lesion; B: Visible bleeding Dieulafoy's lesion through the rim of a transparent ligation chamber attached to the tip of the endoscope; C: The mini-loop is closed and detached, achieving complete hemostasis.
DISCUSSION
DL of the duodenum is an uncommon cause of upper GI bleeding, currently experiencing an increase of its frequency because operators’ increased awareness of this condition. The duodenum is the second commonest site of DLs, accounting for 15% of patients, second only to the lesser curvature of the stomach, which accounts for around 70%[3,5]. Although the incidence of DL is unknown, it accounts for 2%-3% of all GI hemorrhages. Clinical presentations of this condition are extremely wide-ranging: from symptom free, and therefore, frequently undiagnosed cases, to signs of severe and often fatal GI hemorrhage. These lesions usually appear among the elderly, predominantly in men, and are associated with multiple co-morbidities such as chronic renal and heart failure. Other risk factors include the usage of NSAIDs, and antiplatelet and anticoagulant therapy. The patient we treated was an elderly woman with a history of arterial hypertension and hyperlipoproteinemia.
Upper endoscopy, the gold standard, is diagnostic in 90% of the cases; other available diagnostic techniques are angiography and endoscopic ultrasound (EUS). Selective catheter-directed embolization, especially in patients at high risk for surgery, is usually a salvage method in cases of unsuccessful endoscopic treatment, particularly if the lesion cannot be visualized because of excessive hemorrhage[6]. EUS is used to help detect the site of the submucosal vessel, especially in cases of spouting bleeding and to confirm ablation of a DL after therapy.
The endoscopic appearance of a DL is typically arterial spurting or oozing streaming from a minimal defect of the normal surrounding mucosa, or, less commonly, a protruding vessel without signs of active bleeding or with an adherent clot. In this case, blood oozing out of the normal mucosa was visualized without the possibility to distinguishing the underlying vessel. Once the diagnosis is established, several endoscopic techniques can be used, depending on the site of the lesion, severity of symptoms and previous operator experience. Available endoscopic procedures are electrocoagulation, heat probe coagulation, argon plasma coagulation, local epinephrine injection, sclerotherapy, banding and hemoclip. There is no consensus as to which is the most appropriate method. Based on the available data, endoscopic mechanical haemostatic methods seem to be superior to thermal or injection treatment methods[7,8]. However, the most commonly used methods are injection therapy and mechanical endoscopic therapy with hemoclips. In our case, a mechanical hemostatic method was used; the bleeding vessel with the surrounding mucosa was aspirated into the transparent ligation chamber and a preloaded nylon ring, called a mini-loop, was closed and detached with consequent complete hemostasis. A search of the published literature using PubMed with the phrase “Dieulafoy’s lesion” resulted in 211 articles, of which the first was dated to early 1978 reporting two cases of DL of the jejunum[9]. First report of a duodenal DL was published in 1993[10] and to the best of our knowledge, there have been no reports of mini-loop ligation as a choice of endoscopic treatment for DL. Despite the fact that the first choice therapy for the bleeding duodenal DLs among most endoscopists includes injection of epinephrine and electrocoagulation[11], mechanical techniques are used more frequently and endoscopic band ligation (EBL) has been demonstrated to be an effective treatment for bleeding esophageal, stomach, duodenal and rectal DLs[12,13]. Chung et al[14] and Yanar et al[15] demonstrated higher efficacy of mechanical hemostatic therapy in initial hemostasis and lowering re-bleeding rate with hemoclip and band ligation compared to injection therapy in patients with DL. On the other hand, EBL has been shown to be as effective as injection therapy with epinephrine, with or without thermal therapy, with a significantly longer length of hospitalization among patients treated with injection therapy or combined therapy with epinephrine and thermal therapy[16]. In a study that compared two mechanical methods, EBL and endoscopic hemoclip placement (EHP), no difference was detected in efficacy or safety in the management of bleeding gastric DLs and only one patient from each group experienced re-bleeding[17]. A larger study (66 patients) showed a lower recurrent bleeding rate in patients treated with EBL compared with EHP (3.1% vs 14.7%)[18]. A combined therapy, such as EBL with EUS, promises a new era in diagnostic efficacy in terms of establishing accurate diagnosis, guiding EBL and confirming success of the treatment[19].
Therapeutic endoscopy is the first line of treatment of DL. However, the choice of technique is not specified. In our case, endoscopic mini-loop ligation proved to be an effective, easy to use and safe method for the treatment of DL. Mini-loop ligation may become a method of choice in resolving bleeding DL; however, case reports with longer follow-up periods are needed for a definitive statement because of the substantial risk of re-bleeding from a residual aberrant artery.