Published online Jun 14, 2013. doi: 10.3748/wjg.v19.i22.3481
Revised: March 23, 2013
Accepted: March 28, 2013
Published online: June 14, 2013
Processing time: 209 Days and 2.2 Hours
AIM: To investigate the efficacy and safety of combined de novo lamivudine (LAM) and adefovir dipivoxil (ADV) therapy in hepatitis B virus (HBV)-related decompensated liver cirrhosis patients.
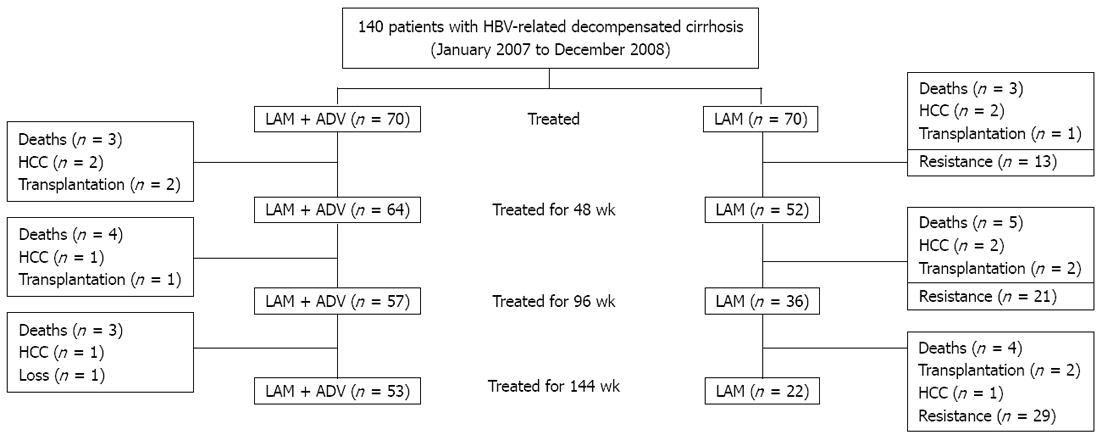
METHODS: One hundred and forty patients with HBV-related decompensated cirrhosis were recruited, 70 patients were treated with combined LAM and ADV de novo therapy, and the other 70 patients were treated with LAM alone as controls. The follow-up period was 144 wk. All patients with LAM resistance were shifted to ADV.
RESULTS: The percentage of HBV-related decompensated cirrhosis patients with undetectable HBV DNA in de novo combination group was 51.6% (33/64), 84.2% (48/57), and 92.3% (49/53) by weeks 48, 96, and 144, respectively. In monotherapy group, HBV DNA negativity rate was 46.1% (30/65), 56.1% (32/57), and 39.2% (20/51) by weeks 48, 96 and 144, respectively. There was a significant difference between the two groups by weeks 96 and 144 (P = 0.012 and 0.001). The hepatitis B e antigen seroconversion rate was 28.1% (9/32), 40.0% (12/30), and 53.6% (15/28) in the combination group by weeks 48, 96 and 144, respectively, and 24.2% (8/33), 31.0% (9/29), and 37.0% (10/27) by weeks 48, 96 and 144, respectively, in monotherapy group. A total of 68.6% (44/64), 84.2% (48/57), and 92.5% (49/53) patients achieved alanine aminotransferase (ALT) normalization by weeks 48, 96 and 144, respectively in the combination group. In monotherpy group, the ALT normalization rate was 64.6% (42/65) by week 48, 73.7% (42/57) by week 96, and 80.4% (41/51) by week 144. No patients in the combination group exhibited detectable resistance for at least 144 wk. The cumulative resistance rate in monotherapy group at weeks 48, 96, and 144 was 20.0%, 36.8%, and 56.9%. Both combination group and monotherapy group demonstrated an improvement in Child-Turcotte Pugh and Model for End-Stage Liver Disease scores at weeks 48, 96, and 144. All patients tolerated both combination and monotherapy. The ceratinine levels and glomerular filtration rate remained normal in all patients during the follow-up period.
CONCLUSION: In HBV-related decompensated liver cirrhosis patients, the combined de novo LAM and ADV therapy is more efficacious and safer compared to LAM alone.
Core tip: Present treatment guidelines advocate oral nucleos(t)ide analogues in decompensated chronic hepatitis B (CHB) patients. Studies with lamivudine (LAM) have demonstrated decreased mortality and improved liver function in CHB decompensated patients. However, LAM resistance mutations emerging during monotherapy can negate therapeutic benefit. Adefovir dipivoxil had no cross resistance with LAM. Consistent with outcomes in patients with LAM-resistance, no patient in de novo combination therapy group showed detectable resistance up to 144 wk in this study. The de novo combination therapy markedly improved liver function, reduced Child-Turcotte Pugh and Model for End-Stage Liver Disease scores in hepatitis B virus-related decompensated cirrhosis patients.
- Citation: Lv GC, Yao JM, Yang YD, Zheng L, Sheng JF, Chen Y, Li LJ. Efficacy of combined therapy in patients with hepatitis B virus-related decompensated cirrhosis. World J Gastroenterol 2013; 19(22): 3481-3486
- URL: https://www.wjgnet.com/1007-9327/full/v19/i22/3481.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i22.3481
It is estimated that over 350 million people worldwide are chronically infected with hepatitis B virus (HBV). The majority of these individuals reside in the Asia-Pacific region[1]. Chronic hepatitis B (CHB) infection is the principal cause of liver cirrhosis and hepatocellular carcinoma (HCC). In cirrhotic patients, the 5-year probability of decompensation is 15%-20%, and the risk increases as the HBV DNA level increases[2]. The 5-year survival rate in decompensated cirrhosis patients is 14%-35%, compared to 84% for those with compensated cirrhosis[3].
Previous studies have shown that high serum HBV DNA is a major risk factor for disease progression to cirrhosis or HCC. Lamivudine (LAM) is the first nucleoside analog widely prescribed for CHB patients due to its antiviral efficacy and safety profile. LAM is found effective for patients with HBV-related decompensated liver cirrhosis[4,5]. However, LAM is associated with a high risk of drug resistance and virological breakthrough[6]. Adefovir dipivoxil (ADV) exhibits specificity, low drug resistance, and no cross resistance with other nucleoside analogs, which has been strongly considered as a rescue therapeutic agent to combat resistance[7,8]. In China, as the first approved drug for CHB patients, LAM has been widely prescribed for its clinical efficacy and low cost. Clinical trials have demonstrated the superiority of combined LAM and ADV therapy compared to ADV monotherapy in LAM resistant patients[9,10]. In this study, LAM and ADV are utilized as de novo combination treatment. To date, no study has been performed to systematically evaluate the efficacy and safety of de novo combined therapy in patients with HBV-related decompensated liver cirrhosis.
In this study, we aimed to compare the efficacy and safety of de novo combination therapy with monotherapy in patients with HBV-related decompensated liver cirrhosis.
From January 2007 to December 2008, 140 consecutive nucleoside analogs treatment-naïve patients with HBV-related decompensated cirrhosis were enrolled in this study in the First Affiliated Hospital of Zhejiang University.
Diagnostic criteria: The diagnosis of decompensated liver cirrhosis was based on clinical, laboratory, previous histological, ultrasonographic and radiological signs of cirrhosis with Child-Turcotte-Pugh (CTP) score. The inclusion criteria for this study were as follows: aged 18-65 years, with HBV DNA ≥ 103 copies/mL, a CTP score of 7-12 (inclusive), calculated serum creatinine clearance ≥ 50 mL/min, hemoglobin ≥ 75 g/L, total white blood cell ≥ 2.5×109/L, platelet count ≥ 30 × 109/L, α-fetoprotein ≤ 20 ng/mL, and no evidence of HCC.
Exclusion criteria: Patients were excluded for resistance to LAM, co-infection with hepatitis C virus, hepatitis D virus, hepatitis E virus or human immunodeficiency virus, and autoimmune hepatitis, alcoholic cirrhosis, hepatorenal syndrome, grade 3 or 4 hepatic encephalopathy, or spontaneous bacterial peritonitis, and severe heart, renal, brain diseases.
Among 140 patients with HBV-related decompensated cirrhosis, 70 patients were treated with de novo combination therapy of 100 mg/d LAM and 10 mg/d ADV; the other 70 patients were treated with 100 mg/d LAM alone as controls. The duration of the treatment was 144 wk. All patients who exhibited LAM resistance were administered ADV.
All patients were followed up every 3-6 mo with examinations of liver and renal function, prothrombin time (PT), international normalized ratio (INR), serum HBV DNA, hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), hepatitis B e antigen (HBeAg), hepatitis B e antibody (anti-HBe), and hepatitis B core antibody (anti-HBc), as well as ultrasonographic or computerized tomography examination. Routine biochemical and hematological tests, and clinical examination were performed at all clinical visits.
Biochemistry, hematology, PT, INR, and urinalysis were analyzed immediately. Serum hepatitis B viral markers, including HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc were detected using commercially available enzyme immunoassays (Abbott Laboratories, Chicago, IL, United States). Serum HBV DNA was measured by polymerase chain reaction with a linear range between 1 × 103 and 5 × 108 copies/mL (Shanghai ZJ Bio-Tech Co., Ltd., China). LAM and ADV associated mutations were assessed by direct sequencing.
Informed consent for inclusion in the study was obtained from all patients before recruitment. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University.
Statistical analysis was conducted using SPSS (version 16.0, IL, United States). Continuous variables were expressed as mean ± SD. Continuous variables were examined using the Students’s t test or Mann-Whitney U test. Categorical variables were compared by χ2 test. A P value < 0.05 was considered statistically significant.
A total of 140 patients were recruited: 70 received LAM and ADV de novo combination therapy and 70 received LAM monotherapy. Baseline characteristics were comparable between the two groups and are shown in Table 1. In de novo combination therapy group, 37 (52.9%) patients exhibited ascites, 9 (12.8%) exhibited episodes of hepatic encephalopathy, and 15 (21.4%) exhibited variceal bleeding. In LAM monotherapy group, 36 (51.4%) patients presented ascites, 8 (11.4%) presented episodes of hepatic encephalopathy, and 16 (22.9%) exhibited variceal bleeding. No patent in either group discontinued antiviral therapy during the study period. The characteristics of the patients during the 144 wk are shown in Figure 1.
| Characteristics | De novo combination group (n = 70) | Lamivudine group (n = 70) | P value |
| Age (yr) | 46.8 ± 10.3 | 47.1 ± 10.9 | 0.91 |
| Male/female | 41/29 | 40/30 | 0.89 |
| ALT (IU/L) | 134.6 ± 101.3 | 132.4 ± 120.8 | 0.78 |
| TBIL (μmol/L) | 38.5 ± 12.1 | 37.8 ± 11.9 | 0.84 |
| Albumin (g/dL) | 3.2 ± 0.7 | 3.3 ± 0.6 0.34 | |
| Prothrombin time (s) | 17.1 ± 4.5 | 17.6 ± 3.1 | 0.61 |
| HBV DNA (log10 copies/mL) | 6.87 ± 1.21 | 6.94 ± 1.15 | 0.73 |
| HBeAg positive | 34 (48.6) | 33 (47.1) | 0.94 |
| Platelet count (× 109/L) | 78.9 ± 24.2 | 76.7 ± 32.3 | 0.67 |
| Creatinine (μmol/L) | 89.7 ± 12.3 | 88.6 ± 13.1 | 0.45 |
| GFR (mL/min) | 115.2 ± 34.5 | 113.5 ± 22.9 | 0.68 |
| CTP score | 8.9 ± 2.1 | 8.8 ± 1.7 | 0.83 |
| CTP class | |||
| A | 6 (8.6) | 7 (10) | 0.65 |
| B | 48 (68.6) | 49 (70) | 0.77 |
| C | 16 (22.9) | 14 (20) | 0.59 |
| MELD score | 12.4 ± 3.7 | 11.9 ± 2.5 | 0.75 |
The percentage of HBV related decompensated cirrhosis patients with undetectable HBV DNA in the de novo combination group was 51.6% (33/64), 84.2% (48/57) and 92.3% (49/53) by weeks 48, 96, and 144, respectively. In the monotherapy group, the rate of HBV DNA negativity was 46.1% (30/65), 56.1% (32/57) and 39.2% (20/51) by weeks 48, 96, and 144, respectively. A significant difference was observed between the two groups by weeks 96 and 144 (P = 0.012 and 0.001).
Patients who were HBeAg-positive at baseline, 34/70 (48.6%) in de novo combination group and 33/70 (47.1%) in monotherapy group, exhibited HBeAg seroconversion: 28.1% (9/32) vs 24.2% (8/33) by week 48 (P = 0.372), 40.0% (12/30) vs 31.0% (9/29) by week 96 (P = 0.021), and 53.6% (15/28) vs 37.0% (10/27) by week 144 (P = 0.014), respectively.
Of the 70 decompensated cirrhosis patients receiving de novo combination therapy, 68.6% (44/64), 84.2% (48/57) and 92.5% (49/53) of the patients achieved alanine aminotransferase (ALT) normalization by weeks 48, 96 and 144, respectively. In monotherpy group, the ALT normalization rate was 64.6% (42/65) by week 48, 73.7% (42/57) by week 96, and 80.4% (41/51) by week 144, respectively. Compared to de novo combination therapy group, ALT normalization rates were lower in monotherapy group by weeks 96 and 144 (P = 0.026 and 0.037).
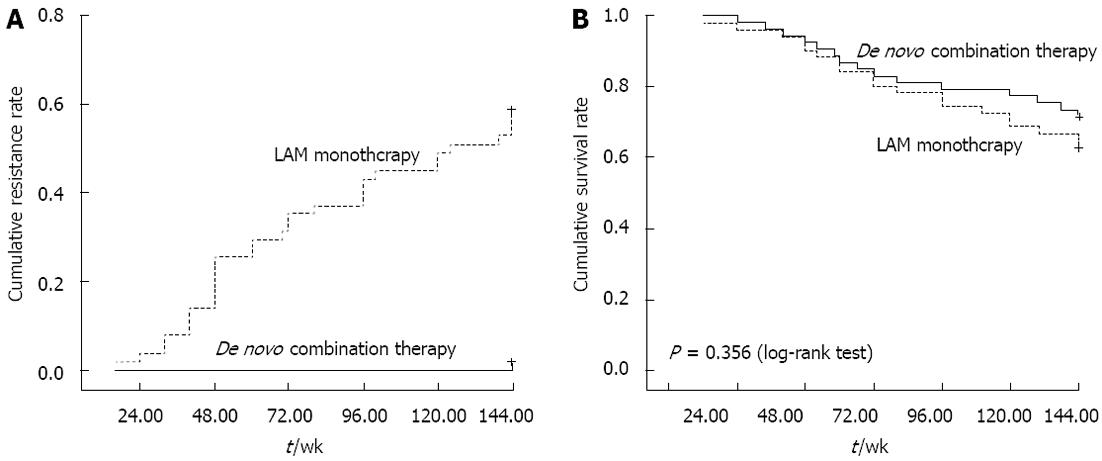
No patient in the de novo combination group exhibited detectable resistance within 144 wk. The cumulative resistance rate in monotherapy group at weeks 48, 96 and 144 was 20%, 36.8%, and 56.9%, respectively as shown in Figure 2A, significantly higher compared to de novo combination group.
Through week 144, both de novo combination group and monotherapy group achieved improvement in hepatic function, as evaluated by change from baseline in serum albumin, total bilirubin, prothrombin time; the degree of improvement in de novo combination group for albumin and prothrombin at week 144 was superior to monotherapy group, as shown in Table 2.
| Characteristics | De novo combination group | Lamivudine monotherapy group | ||||||
| Baseline | 48 wk | 96 wk | 144 wk | Baseline | 48 wk | 96 wk | 144 wk | |
| Hepatic function | ||||||||
| Albumin (g/dL) | 3.2 ± 0.7 | 3.5 ± 0.4 | 3.8 ± 0.1 | 4.1 ± 0.3a | 3.3 ± 0.6 | 3.4 ± 0.3 | 3.6 ± 0.8 | 3.8 ± 0.3c |
| TBIL (μmol/L) | 38.5 ± 12.1 | 24.3 ± 11.4 | 20.4 ± 10.8 | 16.1 ± 6.7a | 37.8 ± 11.9 | 26.5 ± 12.3 | 23.2 ± 10.5 | 18.1 ± 12.3 |
| PT (s) | 17.1 ± 4.5 | 14.5 ± 5.3 | 13.3 ± 9.1a | 11.4 ± 5.8a | 17.6 ± 3.1 | 15.4 ± 3.5 | 14.5 ± 6.9 | 13.4 ± 5.8c |
| CTP score | 8.9 ± 2.1 | 7.0 ± 1.2 | 6.3 ± 2.7a | 5.7 ± 1.9a | 8.8 ± 1.7 | 7.6 ± 2.4 | 6.6 ± 1.9 | 6.1 ± 3.8 |
| MELD score | 12.4 ± 3.7 | 10.0 ± 6.5 | 9.6 ± 5.3 | 8.8 ± 2.7a | 11.9 ± 2.5 | 10.8 ± 3.7 | 8.9.7 ± 4.8 | 8.9 ± 4.3 |
| Renal function | ||||||||
| BUN (mmol/L) | 6.7 ± 0.7 | 6.8 ± 1.4 | 7.1 ± 1.1 | 6.9 ± 0.3 | 6.8 ± 0.6 | 7.4 ± 1.3 | 7.2 ± 0.8 | 7.0 ± 1.1 |
| Cr (μmol/L) | 96.1 ± 12.3 | 98.3 ± 8.9 | 97.8 ± 10.7 | 99.7 ± 9.6 | 95.6 ± 12.1 | 97.8 ± 7.9 | 98.6 ± 12.7 | 99.5 ± 9.7 |
| GFR (mL/min) | 115.2 ± 34.5 | 112.8 ± 56.3 | 103.4 ± 76.1 | 109.1 ± 21.5 | 113.5 ± 22.9 | 107.6 ± 13.4 | 112.4 ± 45.5 | 109.4 ± 13.4 |
Both de novo combination and monotherapy groups demonstrated an improvement in CTP and Model for End-Stage Liver Disease (MELD) scores at weeks 48, 96 and 144. The mean change from baseline in CTP scores was -1.9, -2.6, and -3.2 at weeks 48, 96 and 144, respectively, for de novo combination group, and -1.7, -2.2 and -2.7 at weeks 48, 96, and 144, respectively, for monotherapy group. As a result, 45 (84.9%) of cirrhosis patients in de novo combination therapy group achieved CTP class A (score 5 or 6) after 144 wk of treatment, whereas in monotherapy group 68.6% (35/51) of patients achieved CTP class A after 144 wk.
The mean change from baseline in MELD scores was -2.4, -3.2, and -4.0 at weeks 48, 96, and 144, respectively, for de novo combination group, and -1.8, -2.3, and -3.0 at weeks 48, 96 and 144, respectively, for monotherapy group as shown in Table 2.
The 70 patients with decompensated cirrhosis in de novo combination therapy group exhibited a cumulative HCC incidence of 3.1% at week 48, 5.3% at week 96, and 7.5% at week 144, respectively; four patients developed HCC during the follow-up period. In monotherapy group, the cumulative incidence of HCC was 3.1%, 7.0% and 9.8% at weeks 48, 96 and 144, respectively; five patients developed HCC during the follow-up period. In addition, the cumulative mortality or orthotopic liver transplantation rate was 8.6%, 18.4% and 24.3% at weeks 48, 96 and 144, respectively in de novo combination group, and 9.4%, 18.4% and 27.2% at weeks 48, 96 and 144, respectively, in the monotherapy group, as shown in Figure 2B.
All patients in this study tolerated both the de novo combination therapy and monotherapy. No patient in either group discontinued the treatment during the follow-up period. In the de novo combination group, the blood urine nitrogen (BUN) of three patients increased to 8.6, 9.1 and 9.7 mmol/L respectively, without a concomitant increase in creatinine levels. BUN level of these patients were normalized during the follow-up period. The creatinine levels and glomerular filtration rate remained normal in all patients during the follow-up period, as shown in Table 2.
A consensus on the benefit of antiviral therapy for HBV-related cirrhosis has been achieved. Liaw et al[11] reported that continuous treatment with LAM delays clinical progression in patients with CHB and advanced fibrosis or cirrhosis by significantly reducing the incidence of hepatic decompensation and risk of HCC. However, LAM exhibits a high incidence of resistance mutations compared to other nucleos(t)ide analogs. The current CHB treatment guidelines advocate initial combination therapy or agents with a high genetic barrier for patients with a high risk of developing drug resistance and potentially life-threatening associated diseases, such as HBV-related liver cirrhosis[12-14]. A combination with other drugs that do not share cross resistance may promote or exhibit synergistic antiviral effects; most importantly, it may exhibit the potential to prevent resistance. Ghany et al[15] recently reported that extended combined therapy with LAM and ADV was associated with a high rate of long-term virological and biochemical response; none of the 22 combination therapy treated CHB patients developed resistance for up to 192 wk. Fan et al[16] demonstrated that de novo combination therapy with LAM and ADV was superior to add-on combination therapy in terms of CTP score, virus inhibition, and renal function. In this study, for patients with HBV-related decompensated liver cirrhosis, de novo combination therapy resulted in a superior virological response than monotherapy at weeks 96 and 144. The cumulative tyrosinemethionine-aspartic acid-aspartic acid resistance rate was higher in monotherapy group by weeks 48, 96 and 144, whereas none of the de novo combination therapy treated patients developed resistance during the follow-up period.
Higher HBeAg seroconversion rates and ALT normalization rates were also observed in de novo combination group compared to LAM monotherapy group at weeks 96 and 144. It is well known that HBeAg seroconversion is accompanied by biochemical and histological regression of liver diseases. Previous studies have indicated that combination therapy is efficacious in preventing drug resistance, but does not improve virological and serological response[17]. Our results and other studies suggest that de novo combination therapy can not only prevent drug resistance, but also improve virological and serological response[15,16]. Further elucidation concerning the mechanisms underlying the higher negative rates of HBV DNA and higher rates of HBeAg seroconversion by de novo combination therapy is warranted. In general, patients with CHB-related liver cirrhosis exhibit lower levels of HBV DNA and HBeAg compared with patients with CHB. Alternatively, during the course of HBV-related liver cirrhosis, the antiviral immune response is vigorously activated. In concordance with the findings of previous studies, the results that we present in this study suggest that de novo combination therapy is potentially suitable as an initial treatment for HBV-related decompensated liver cirrhosis in order to reduce resistance.
Previous clinical studies have demonstrated the positive effects of LAM therapy on the functional improvement in patients with HBV-related decompensated liver cirrhosis[4,5,18]. However, these benefits were offset due to drug-resistance. The CTP and MELD scores, both of which reflect components of liver function, including serum albumin, total bilirubin, and prothrombin time, were markedly improved during de novo combination therapy. These results clearly indicate that due to inhibition of HBV replication, de novo combination treatment potentially prevents clinical progression to liver failure, reduces complication risk, and delays or avoids the need for liver transplantation.
Renal impairment is a known risk of severe liver disease and is considered a potential side effect of de novo combination therapy. In this study, both de novo combination and monotherapy were well-tolerated, with no case of renal failure attributable to combination therapy.
This study has several limitations. It was not randomized or blinded, as this would be difficult for patients with CHB-related decompensated liver cirrhosis. This may contribute to the discord in results among previous studies.
In conclusion, this study demonstrated that de novo combination therapy is superior to monotherpy in suppressing HBV DNA and achieving ALT normalization and HBeAg seroconversion by weeks 96 and 144. Both treatments improved liver function as measured by reduction of CTP and MELD scores. In HBV-related decompensated liver cirrhosis patients, de novo combination therapy has also been proven safe. Our findings strongly support de novo combination therapy as a viable and effective therapeutic strategy in patients with HBV-related decompensated liver cirrhosis.
The mortality rate of hepatitis B virus (HBV)-related decompensated cirrhosis is very high. Recommended treatment options are nucleos(t)ide analogues. There are few reports regarding the issue of treatment for these patients with de novo combination therapy or monotherapy.
In China, tenofovir is not available yet and entecavir is expensive for most patients. Combination therapy with lamivudine (LAM) and adefovir dipivoxil (ADV) is better than ADV in LAM-resistance chronic hepatitis B (CHB) patients. But in patients with HBV-related decompensated live cirrhosis, it remains unclear whether de novo combination therapy is better than monotherapy. In this study, the authors demonstrate that LAM and ADV de novo combination therapy is more effective than LAM monotherapy for patients with HBV-related decompensated liver cirrhosis.
Many clinical studies showed that the combined LAM and ADV therapy is more effective than ADV monotherapy after LAM-resistance. However, the efficacy of de novo LAM and ADV combination therapy in patients with HBV-related decompensated liver cirrhosis is unclear. This is the first study to report that de novo LAM and ADV is more effective than LAM monotherapy, especially the combination therapy can reduce the drug resistance.
By understanding that LAM and ADV de novo combination therapy is more effective than LAM monotherapy in patients with HBV-related decompensated liver cirrhosis, this study may represent a future strategy for therapeutic intervention in CHB patients with HBV-related decompensated liver cirrhosis.
De novo combination therapy means combination with two or more drugs form the beginning of the treatment. The diagnosis of decompensated liver cirrhosis was based on clinical, laboratory, previous histological, ultrasonographic and radiological signs of cirrhosis with Child-Turcotte-Pugh (CTP) score. The CTP score is a system to assess the disease stage for decompensated cirrhotic patients.
This is a good clinical study in which the authors compared the effect of de novo LAM and ADV combination therapy with LAM monotherapy. The authors concluded that LAM and ADV should be combined at the beginning for treatment of the patients with HBV-related decompensated liver cirrhosis.
P- Reviewer Bruha R S- Editor Huang XZ L- Editor Ma JY E- Editor Xiong L
| 1. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1700] [Cited by in RCA: 1712] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 2. | McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25 Suppl 1:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Huber R, Orzeszyna M, Pokorny N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav Evol. 1997;50 Suppl 1:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 993] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 4. | Yao FY, Bass NM. Lamivudine treatment in patients with severely decompensated cirrhosis due to replicating hepatitis B infection. J Hepatol. 2000;33:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, Anschuetz G, Davis R, Gardner SD, Brown NA. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology. 2002;123:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Yang H, Westland CE, Delaney WE, Heathcote EJ, Ho V, Fry J, Brosgart C, Gibbs CS, Miller MD, Xiong S. Resistance surveillance in chronic hepatitis B patients treated with adefovir dipivoxil for up to 60 weeks. Hepatology. 2002;36:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, Seo YS, Chung HJ, Moon MS, Kim SO. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55:1488-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Rescue therapy for lamivudine-resistant chronic hepatitis B: comparison between entecavir 1.0 mg monotherapy, adefovir monotherapy and adefovir add-on lamivudine combination therapy. J Gastroenterol Hepatol. 2010;25:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Vassiliadis TG, Giouleme O, Koumerkeridis G, Koumaras H, Tziomalos K, Patsiaoura K, Grammatikos N, Mpoumponaris A, Gkisakis D, Theodoropoulos K. Adefovir plus lamivudine are more effective than adefovir alone in lamivudine-resistant HBeAg- chronic hepatitis B patients: a 4-year study. J Gastroenterol Hepatol. 2010;25:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 12. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 13. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 14. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 15. | Ghany MG, Feld JJ, Zhao X, Heller T, Doo E, Rotman Y, Nagabhyru P, Koh C, Kleiner DE, Wright EC. Randomised clinical trial: the benefit of combination therapy with adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther. 2012;Mar 26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Fan XH, Geng JZ, Wang LF, Zheng YY, Lu HY, Li J, Xu XY. De novo combination therapy with lamivudine and adefovir dipivoxil in chronic hepatitis B patients. World J Gastroenterol. 2011;17:4804-4809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1173] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 18. | Villeneuve JP, Condreay LD, Willems B, Pomier-Layrargues G, Fenyves D, Bilodeau M, Leduc R, Peltekian K, Wong F, Margulies M. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology. 2000;31:207-210. [PubMed] |