Published online Jun 14, 2013. doi: 10.3748/wjg.v19.i22.3433
Revised: January 22, 2013
Accepted: February 5, 2013
Published online: June 14, 2013
Processing time: 268 Days and 1.2 Hours
AIM: To study sleep aspects and parameters in cirrhotic patients and assess the role of liver dysfunction severity in polysomnographic results.
METHODS: This was a case-control study. Patients with a diagnosis of liver cirrhosis were consecutively enrolled in the study. Clinical examinations and laboratory liver tests were performed in all patients, and disease severity was assessed using the Child-Pugh score. The control group consisted of age- and gender-matched healthy volunteers. All individuals answered a questionnaire about habits, behaviors, and complaints related to sleep and were submitted to polysomnography. Sleep parameters were compared between the two groups, and separate analyses were performed among classes of Child-Pugh classification in the cirrhotic group.
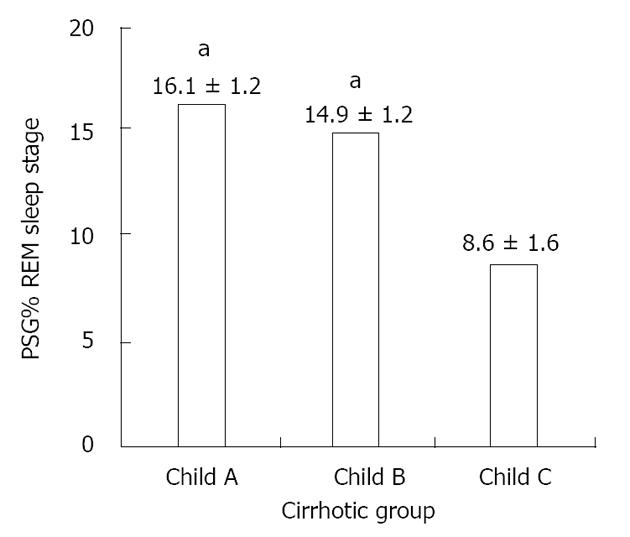
RESULTS: Forty-two cirrhotic patients and forty-two controls were enrolled. Compared to the control group, the cirrhotic group exhibited lower sleep efficiency (mean ± SD: 73.89% ± 14.99% vs 84.43% ± 8.55%, P < 0.01), increased latency (151.27 ± 93.24 min vs 90.62 ± 54.74 min, P < 0.01) and a lower percentage of rapid eye movement (REM) sleep (14.04% ± 5.64% vs 20.71% ± 6.77%, P < 0.05) as well as a higher frequency of periodic limb movements (10.56 ± 2.85/h vs 2.79 ± 0.61/h, P < 0.01). The comparison of sleep parameters among Child A, B and C cirrhotic patients revealed a significant reduction of REM sleep stage occurrence in individuals with severe liver disease (Child C patients) compared to Child A/B patients (polysomnography percentage of REM sleep stage of patients Child A: 16.1% ± 1.2%; Child B: 14.9% ± 1.2%; Child C: 8.6% ± 1.6%, P < 0.05).
CONCLUSION: Cirrhosis was associated with shorter sleep time, reduced sleep efficiency, increased sleep latency, increased REM latency and reduced REM sleep. Additionally, disease severity influences sleep parameters.
- Citation: Teodoro VV, Júnior MAB, Lucchesi LM, Cavignolli D, Mello MT, Kondo M, Tufik S. Polysomnographic sleep aspects in liver cirrhosis: A case control study. World J Gastroenterol 2013; 19(22): 3433-3438
- URL: https://www.wjgnet.com/1007-9327/full/v19/i22/3433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i22.3433
Although modern sleep research has a short forty-year history, interest in the impact of sleep quality on bio-regulatory functions and human health dates back to ancient times[1-3]. The development of polysomnography (PSG) provided the ability to assess sleep structure and led to thorough characterizations of different sleep stages and sleep disorders[4]. The rapid eye movement (REM) sleep stage has acquired increasing relevance; it is considered fundamental to the maintenance of important intellectual functions such as memory, attention and mood[5]. The REM stage is characterized by cortical activation; this is evidenced by a rapid transition to higher frequency rhythms with rapid, low-voltage, irregular activity on electroencephalogram (EEG). It is also characterized by irregular breathing, heart rate and muscle atonia[2]. Time spent in REM sleep is markedly reduced in several organic dysfunctions and is associated with cardiovascular adverse events, such as systemic arterial hypertension[6-8]. Similarly, spontaneous or induced REM sleep deprivation was previously correlated with higher death rates[9].
Sleep disturbances are commonly reported in liver cirrhosis (LC), particularly in patients with hepatic encephalopathy (HE)[10,11]. There are few clinical[12,13] and experimental[14] studies assessing the sleep patterns of LC patients without overt HE; however, to our knowledge, an approach based on full-night PSG (level 1) has never been performed[15-17].
A study that used sleep questionnaires, neuropsychological tests and actigraph monitoring suggested that sleep complaints are an early sign of HE[17] and that insomnia and excessive daytime sleepiness (EDS) are often described in patients with liver disease[10,11,13,17]. Conversely, Vignatelli et al[18] found only a small percentage of cirrhotic patients with EDS. Although clinical reports vary, one possible explanation for sleep dysfunction in LC patients is a disruption in melatonin circadian rhythms[13,14]; one study reported a delayed onset and peak of melatonin secretion, which caused a sleep phase delay and possibly EDS[19,20].
Detailed information regarding PSG sleep aspects in LC patients is lacking. Moreover, no study to date has attempted to determine the influence of disease severity on sleep structure. This study aimed to characterize sleep patterns of LC patients using a full-night PSG-based approach focusing on the following: (1) sleep structure in LC; (2) sleep pattern variations associated with liver disease severity; and (3) detection of possible sleep disorders linked to LC. We then conducted a case-control study to compare PSG sleep aspects between LC patients and healthy volunteers.
Patients with a diagnosis of LC by either liver biopsy or analysis of clinical and laboratory data were enrolled in the cirrhotic group. They were invited to participate in the study upon reporting to the Gastroenterology Outpatient Clinic of the Universidade Federal de São Paulo (UNIFESP). The exclusion criteria were the following: younger than 18 years, alcohol consumption or gastrointestinal bleeding in the last 6 mo, serum creatinine levels higher than 2.0 mg/dL, psychoactive drug intake in the last 2 wk and overt clinical HE in the initial assessment. Those who fulfilled the selection criteria had venous blood drawn, and a clinical evaluation was performed to determine liver disease severity according to the Child-Pugh score. At the same time, arterial blood was collected to determine arterial ammonia levels. LC etiology was determined in all patients. Selected patients were submitted to a full-night PSG examination in the same week as the initial assessment.
The control group was composed of age- and gender-matched volunteers from the UNIFESP-Instituto do Sono laboratories, who met the criteria listed above and were considered healthy according to clinical and laboratorial evaluations. They also completed a PSG examination.
The research protocol was reviewed and approved by the institutional research ethics committee (protocol number 1503/04), and all participants provided informed consent before enrollment in the study.
All PSG recordings were performed in the Instituto do Sono. Before PSG, all study participants completed a questionnaire about habits, behaviors, and complaints related to sleep. The questionnaire was developed by the internal staff of the Instituto do Sono[21] and validated for the local population.
Full-night PSGs were performed in all participants using the sleep laboratory digital system EMBLA S7000® (Embla Systems Inc., Broomfield, CO, United States). The subjects were instructed to go to sleep at their usual bedtime. The following physiological variables were simultaneously and continuously recorded during PSG: (1) four channels to EEG; (2) two channels to electrooculogram; (3) channels placed in the submentonian region, the anterior tibial muscle, the masseter region and the seventh intercostal space to the electromyogram; (4) electrocardiogram; (5) two channels for airflow monitoring (one for the thermocouple and the other for nasal pressure measurement); (6) the detection of respiratory efforts of the thorax (one channel) and the abdomen (one channel) for inductance plethysmography; (7) snore monitoring; (8) one channel for monitoring body position; and (9) oxy-hemoglobin saturation measurement.
All PSG sessions were monitored by trained technicians and visually scored according to standardized criteria[22]. EEG arousals and leg movement episodes were scored according to the “Manual for Scoring Sleep and Associated Events”[23,24]; apnea and hypopnea episodes as well as other detected sleep events were also scored and classified using recognized rules[25].
The following PSG sleep parameters were recorded and systematically evaluated: (1) total recording time (TRT): the entire period under PSG monitoring; (2) total sleep time (TST): the entire PSG recorded while sleeping; (3) wake: the entire PSG recorded while the patient was awake; (4) sleep efficiency: the TST/TRT ratio, expressed as percentage; (5) sleep latency: the length of time to sleep onset; (6) latency to REM sleep: the latency of REM sleep stage onset; (7) sleep stages 1, 2, 3 + 4 and REM sleep stage (S1, S2, S3 + 4 and REM): the percentage (%) of time patients spent in sleep stages 1, 2, 3 + 4 or REM sleep stage, respectively; (8) apnea-hypopnea index (AHI): an index to express the mean number of apneas or hypopneas in a 1-h period; (9) periodic leg movements of sleep per hour (PLMS/h): the average number of PLM events in a one hour period; (10) arousals/h: the average number of arousals in a one hour period; (11) mean SpO2: the mean oxy-hemoglobin saturation; and (12) nadir SpO2: minimal oxy-hemoglobin saturation recorded during PSG. These parameters were analyzed and scored by a blinded specialist before between-groups comparisons were made.
Statistical analyses were performed using STATISTICA software, version 5.1. The Student’s t-test was used to compare the quantitative PSG parameters between groups. Analysis of variance and Tukey’s post-hoc test were used to compare the sleep parameters among the three classes of liver disease according to the Child-Pugh score. Results were considered statistically significant if P < 0.05.
Forty-two cirrhotic patients and forty-two controls who satisfied the selection criteria were evaluated between May 2003 and August 2005. The cirrhotic group consisted of 29 males (69%) with a mean age of 50.0 ± 8.5 years, and the general demographic characteristics of cirrhotic patients did not differ significantly from those of the control group (Table 1). LC etiology and Child-Pugh scores are also shown in Table 1. The arterial ammonia measurements in cirrhotic patients were (mean ± SD) 166.38 ± 26.10 mol/L (normal value: 9-33 mol/L).
| Variables | Control group (n = 42) | Cirrhotic group (n = 42) | P value |
| Age (yr) | 48.4 ± 8.3 | 50.0 ± 8.5 | 0.32 |
| Males | 29 (69.0) | 33 (78.5) | 0.70 |
| BMI (kg/m2) | 25.3 ± 3.4 | 26.3 ± 4.4 | 0.40 |
| LC etiology | |||
| Alcohol | - | 15 (38) | - |
| HCV | - | 12 (30) | - |
| Alcohol + HCV | - | 8 (20) | - |
| HBV | - | 2 (4) | - |
| Alcohol + HBV | - | 1 (2) | - |
| Cryptogenic | - | 4 (6) | - |
| Child-Pugh | |||
| Child A | - | 16 (38) | - |
| Child B | - | 17 (40) | - |
| Child C | - | 9 (22) | - |
LC patients reported more sleep difficulties on the questionnaire, including trouble initiating sleep, non-restorative sleep and more episodes of napping during the day. Although the TRT and AHI were similar in both groups, a significant reduction in the TST, sleep efficiency and wake time were observed in the cirrhotic group. Increased sleep latency, REM latency, PLMS index and a decreased REM sleep percentage were also observed in the cirrhotic group (Table 2).
| Variables | Control group (n = 42) | Cirrhotic group (n = 42) | P value |
| TRT (min) | 421.98 ± 38.20 | 445.65 ± 50.64 | 0.07 |
| TST (min) | 357.18 ± 53.42 | 329.67 ± 76.62 | < 0.05 |
| Wake (min) | 51.86 ± 29.91 | 115.05 ± 67.92 | < 0.01 |
| SE | 84.43% ± 8.55% | 73.89% ± 14.99% | < 0.01 |
| Sleep Lat (min) | 13.39 ± 14.40 | 28.41 ± 29.28 | < 0.01 |
| REM Lat (min) | 90.62 ± 54.74 | 151.27 ± 93.24 | < 0.01 |
| S1 | 5.14% ± 3.26% | 5.90% ± 3.12% | 0.77 |
| S2 | 57.00% ± 8.73% | 61.72% ± 7.38% | 0.29 |
| S3 + 4 | 17.31% ± 7.00% | 18.32% ± 6.64% | 0.73 |
| REM | 20.71% ± 6.77% | 14.04% ± 5.64% | < 0.05 |
| AHI | 7.33 ± 1.01 | 5.16 ± 0.80 | 0.09 |
| PLMS/h | 2.79 ± 0.61 | 10.56 ± 2.85 | < 0.01 |
| Arousals/h | 15.88 ± 5.46 | 11.78 ± 7.06 | < 0.01 |
| Mean SpO2 | 94.80% ± 1.62% | 94.38% ± 2.05% | 0.06 |
| Nadir SpO2 | 87.45% ± 5.24% | 85.87% ± 8.98% | < 0.05 |
Comparison of sleep parameters among Child A, B and C cirrhotic patients revealed a significant reduction of REM sleep stage occurrence in individuals with severe liver disease (Child C patients) (Figure 1).
To assess the influence of alcoholism on sleep parameters, we divided the patients into two groups: those who had alcoholic etiology (alone or associated with viral hepatitis; n = 24) and those who had no history of alcoholism (n = 18). No significant differences were detected between the two groups in our study for any sleep parameter except sleep latency, which was longer in the group without alcoholic etiology (21.68 ± 15.38 min vs 40.53 ± 42.74 min for the alcoholic etiology group and without alcoholic etiology group respectively, P = 0.04).
The sleep parameters evaluated by PSG in this study indicated worsening sleep in the cirrhotic group compared to the control group. This was due to decreased sleep efficiency, increased time to initiate sleep, increased latency of REM sleep, reduced REM sleep percentage and higher PLMS indices. As our data suggest, disease severity influenced sleep parameters, especially when data were classified by the patients’ Child scale rating.
The sleep questionnaire results showed that the cirrhotic patients had more complaints about their sleep, such as difficulty initiating sleep and non-restorative sleep. These findings are consistent with the PSG results indicating lower sleep efficiency and higher sleep latency. Other studies have also described a higher number of sleep complaints in LC patients[12,26]. Moreover, LC patients report more episodes of napping during the day[10,19,27]. EDS appears to be attributable to a dysfunction of the neural circuit responsible for the maintenance of wakefulness and sleep states. The monoaminergic system, the systems of the locus coeruleus (noradrenergic) and the raphe nuclei (serotonergic) are important for directing attention and recruiting the cortex for processing external sensory stimuli[28]. High levels of ammonia can reduce serotonin and noradrenaline levels in the central nervous system, resulting in low alertness and attention[29,30].
The higher incidence of PLMS in our sample of LC patients was not related to any clinical disorder or laboratory finding commonly associated with this phenomenon. This would include conditions such as anemia, renal failure, or low levels of iron or plasma transferritin[31]. PLMS is also associated sleep complaints, including difficulty falling asleep, multiple arousals and EDS[32,33]. These complaints were also reported by our group of patients and objectively confirmed by the PSG findings of higher sleep latency and higher arousal index.
This study did not find statistically significant differences between groups regarding AHI. This is in contrast with two previous studies[15,26]; however, it is consistent with the findings of Nikaina et al[16]. Differing results may be explained by the presence or absence of ascites[34]. In fact, Nikaina et al[16] found no significant differences in the index of respiratory events between controls and patients with compensated cirrhosis without ascites.
It is widely recognized that chronic alcohol abuse influences sleep parameters[35] and many patients in this study experienced cirrhosis of alcoholic etiology. However, in our study, we did not confirm this influence, perhaps due to the fact that patients had been in withdrawal for at least six months. The absence of differences suggests that LC may be seen as a determining factor of the sleep parameters observed for study in this group. The only difference that was found, in sleep latency, which was longer in the group without alcoholic etiology, cannot be easily explained; considering the effects of alcohol, the expected result would be the opposite of what was actually observed[35].
The intense electrical and metabolic activity observed in REM sleep is the best argument supporting sleep as an active phenomenon[2,3]. The system responsible for the generation of REM sleep encompasses several specific nerve structures and follows a model of reciprocal interaction between the co-organizers and suppressor structures, which are located mainly in the brainstem and midbrain[2,3]. In the current study, both the latency and percentage of REM sleep of LC patients differed from those of controls, suggesting the hyperfunction of REM sleep suppressor mechanisms. Evidence from sleep deprivation studies suggests a role for dopamine during REM sleep[36], and these studies have described the relationship of REM sleep in terms of dopamine D2 receptors[37]. Specific changes in the dopamine receptors of the brain of LC patients have been previously described[38,39]. Dopamine receptor dysfunction is associated with low concentrations of serotonin, which may suppress REM sleep and constitute a possible explanation for the PSG findings in our LC group.
It is possible that our findings of sleep impairment in subjects with cirrhosis are common to all forms of metabolic encephalopathy. However, our study’s strength resides in the fact that we found differences in both subjective and objective parameters. In our observational study, LC patients had longer sleep latencies, shorter sleep time, worse sleep efficiency, increased REM sleep latencies and lower REM sleep percentages. The latter finding was negatively related to the severity of the disease. Therefore, we need to draw clinician`s attention to the importance of sleep complaints and parameters regarding prognosis in cirrhotic patients.
The authors would like to thank Altay Lino de Souza and Ana Amelia Benedito Silva for their valuable suggestions and statistical analyses. All the efforts of the Associação Fundo de Incentivo a Pesquisa staff, particularly those of the sleep technicians, are deeply appreciated.
Liver cirrhosis (LC) is currently a serious clinical problem, and is considered a very common disease with a major impact on public health worldwide. It is a chronic and irreversible process characterized by progressive replacement of the normal structure of the liver by fibrosis as a response to a continuous injury to this organ, leading to various clinical consequences, as alterations in sleep. In fact, sleep disturbances are commonly reported in LC.
Polysomnography provided the ability to assess sleep structure and led to a thorough characterization of sleep stages and sleep disorders. Sleep is divided into rapid eye movement (REM) and non-REM sleep stages. REM sleep has acquired increasing relevance because it is considered fundamental to the maintenance of important intellectual functions, such as memory, attention and mood. Time spent in REM is markedly reduced in several organic dysfunctions and is associated with cardiovascular adverse events, such as systemic arterial hypertension.
There is a lack of detailed information regarding polysomnographic sleep aspects in patients with LC. Moreover, no study has attempted to determine the influence of disease severity on sleep structure. This study aimed to characterize the sleep patterns of patients with LC with a full-night polysomnographic based approach focusing on the following: (1) sleep structure in LC; (2) sleep pattern variations associated with liver disease severity; and (3) detection of possible sleep disorders linked to LC. Authors then compared polysomnographic sleep aspects between patients with LC and healthy volunteers.
The study results suggest that hepatic cirrhosis was associated with shorter sleep time, reduced sleep efficiency, increased sleep latency, increased REM latency and reduced REM sleep. Additionally, disease severity influenced sleep parameters. Therefore, authors need to draw clinician’s attention to the importance of sleep complaints and parameters regarding prognosis in cirrhotic patients.
In this paper, the authors study sleep aspects and parameters in cirrhotic patients and assess the role of liver dysfunction severity on polysomnographic results. This study is interesting and suggests that cirrhosis is associated with sleep disturbances and that disease severity influences sleep parameters.
P- Reviewer Yang YF S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Dement WC. History of sleep physiology and medicine. Principles and practice of sleep medicine. 5th ed. Philadelphia: WB Saunders 2011; . |
| 2. | Swick TJ. The neurobiology of sleep. Sleep Med Clin. 2011;6:1-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Carskadon MA. Basics for polygraphic monitoring of sleep. Sleeping and walking disorders: indications and techniques. Boston: Butterworth Publishers 1982; 1-16. |
| 5. | Wamsley EJ, Stickgold R. Memory, Sleep and Dreaming: Experiencing Consolidation. Sleep Med Clin. 2011;6:97-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Radulovacki M, Trbovic SM, Carley DW. Cardiopulmonary interactions following REM sleep deprivation in Sprague-Dawley rats. Exp Neurol. 1997;145:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Endeshaw YW, Bloom HL, Bliwise DL. Sleep-disordered breathing and cardiovascular disease in the Bay Area Sleep Cohort. Sleep. 2008;31:563-568. [PubMed] |
| 8. | Wolk R, Somers VK. Cardiovascular consequences of obstructive sleep apnea. Clin Chest Med. 2003;24:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 285] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Baldy-Moulinier M, Besset A, Calvet B, Michel H. [24 hour polygraphic study of the waking-up and falling asleep periods in patients with hepatic encephalopathy (author’s transl)]. Rev Electroencephalogr Neurophysiol Clin. 1981;11:123-132. [PubMed] |
| 11. | Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, Schalm SW. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 355] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Tarter RE, Hegedus AM, Van Thiel DH, Schade RR, Gavaler JS, Starzl TE. Nonalcoholic cirrhosis associated with neuropsychological dysfunction in the absence of overt evidence of hepatic encephalopathy. Gastroenterology. 1984;86:1421-1427. [PubMed] |
| 14. | Jiménez-Anguiano A, Díaz-Medina V, Farfán-Labonne BE, Giono-Chiang G, Kersenobich D, García-Lorenzana M, Gutiérrez-Ruiz MC, Velázquez-Moctezuma J. Modification of sleep architecture in an animal model of experimental cirrhosis. World J Gastroenterol. 2009;15:5176-5180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Ogata T, Nomura M, Nakaya Y, Ito S. Evaluation of episodes of sleep apnea in patients with liver cirrhosis. J Med Invest. 2006;53:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Nikaina I, Pastaka C, Zachou K, Dalekos GN, Gourgoulianis K. Sleep apnoea syndrome and early stage cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2006;18:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Quero JC, Schalm SW. Subclinical hepatic encephalopathy. Semin Liver Dis. 1996;16:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Vignatelli L, Mattarozzi K, Zanatta C, Stracciari A. Cognitive function and Epworth Sleepiness Scale in ‘minimal’ hepatic encephalopathy. Eur J Neurol. 2001;8:369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123:274-277. [PubMed] |
| 20. | Ardizzi A, Grugni G, Saglietti G, Morabito F. [Circadian rhythm of melatonin in liver cirrhosis]. Minerva Med. 1998;89:1-4. [PubMed] |
| 21. | Bittencourt LR, Silva RS, Santos RF, Pires ML, Mello MT. [Excessive daytime sleepiness]. Rev Bras Psiquiatr. 2005;27 Suppl 1:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Rechtschaffen A, Kales A. Manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute/UCLA 1968; . |
| 23. | EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173-184. [PubMed] |
| 25. | Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667-689. [PubMed] |
| 26. | Crespo J, Cifrián J, Pinto JA, Jiménez-Gómez A, Pons-Romero F. Sleep apnea obstructive syndrome: a new complication previously undescribed in cirrhotic patients with ascites. Am J Gastroenterol. 2003;98:2815-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Mostacci B, Ferlisi M, Baldi Antognini A, Sama C, Morelli C, Mondini S, Cirignotta F. Sleep disturbance and daytime sleepiness in patients with cirrhosis: a case control study. Neurol Sci. 2008;29:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 2006;117:1885-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 628] [Cited by in RCA: 432] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 29. | Baldessarini RJ, Fischer JE. Serotonin metabolism in rat brain after surgical diversion of the portal venous circulation. Nat New Biol. 1973;245:25-27. [PubMed] |
| 30. | Lozeva-Thomas V. Serotonin brain circuits with a focus on hepatic encephalopathy. Metab Brain Dis. 2004;19:413-420. [PubMed] |
| 31. | Montplaisir J, Michaud M, Lavigne GJ. Periodic limb movements in sleep. Sleep and movement disorders. Philadelphia: Butterworth Heinemann 2003; . |
| 32. | Esteves AM, de Mello MT, Pradella-Hallinan M, Tufik S. Effect of acute and chronic physical exercise on patients with periodic leg movements. Med Sci Sports Exerc. 2009;41:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Nicolas A, Lespérance P, Montplaisir J. Is excessive daytime sleepiness with periodic leg movements during sleep a specific diagnostic category? Eur Neurol. 1998;40:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Saleh AM, Mohamed H, El Bendary M, lsayad S. Sleep disorder breathing in liver cirrhosis: a cross sectional study based on child classification. Sleep. 2008;31:A497. |
| 35. | Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Nunes Júnior GP, Tufik S, Nobrega JN. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res Bull. 1994;34:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26:10577-10589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Weissenborn K, Berding G, Köstler H. Altered striatal dopamine D2 receptor density and dopamine transport in a patient with hepatic encephalopathy. Metab Brain Dis. 2000;15:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Watanabe Y, Kato A, Sawara K, Butterworth RF, Sasaki T, Terasaki K, Sera K, Suzuki K. Selective alterations of brain dopamine D(2) receptor binding in cirrhotic patients: results of a (11)C-N-methylspiperone PET study. Metab Brain Dis. 2008;23:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |