Published online Jun 14, 2013. doi: 10.3748/wjg.v19.i22.3375
Revised: January 16, 2013
Accepted: January 23, 2013
Published online: June 14, 2013
Processing time: 218 Days and 6.6 Hours
Non-alcoholic fatty liver disease (NAFLD) is currently not a component of the diagnostic criteria for metabolic syndrome (MetS); however, the development of NAFLD has some common mechanisms with the development of MetS, as they share the pathophysiologic basis of insulin resistance. It is also recognized that NAFLD is the hepatic manifestation of MetS. To define MetS, the presence of at least three of the proposed criteria is required, and sometimes it is sufficient to have only one laboratory value, modified by diet or drugs, for the classification of MetS. Ultrasonographically-detected NAFLD (US-NAFLD) is more stable, only changing during the middle- to long-term. Although controversies over MetS continue, and considering that abdominal ultrasonography for diagnosing NAFLD has high specificity and guidelines to modify the natural course of NAFLD by diet composition or lifestyle have not yet been established, why should we not introduce US-NAFLD as a new criterion to define MetS?
- Citation: Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol 2013; 19(22): 3375-3384
- URL: https://www.wjgnet.com/1007-9327/full/v19/i22/3375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i22.3375
Non-alcoholic fatty liver disease (NAFLD) is characterized by the accumulation of fat in the liver when it exceeds 5%-10% of its weight[1]. In addition to leading to major histopathological alterations, it may be associated with elevated liver enzymes and abnormal liver function, ranging from steatosis to steatohepatitis, fibrosis, and cirrhosis[2,3]. Although diagnosed worldwide, its prevalence varies, reaching approximately 20%-30% in western countries[4]. In the United States, where 25% of the adult population is obese, the disease occurs in more than two-thirds of these individuals and in more than 90% of class III obese individuals[5]. It is estimated that 2% to 3% of the population has nonalcoholic steatohepatitis (NASH)[6,7].
Approximately 74%-90% of patients who undergo liver biopsy show alterations due to triacylglycerol accumulation[8]. The disease is highly prevalent (88.7%) in obese patients undergoing bariatric surgery[9], and the likelihood of developing steatohepatitis is increased in class III obesity, with 15%-20% of these patients diagnosed with NASH[10].
Some studies have shown an increased prevalence and higher incidence of cardiovascular disease (CVD) in individuals with NAFLD. These studies have shown hepatic steatosis as an independent risk factor for the development of this disease[11,12].
Metabolic syndrome (MetS), which involves the combination of risk factors for CVD such as insulin resistance, abdominal fat, dyslipidaemia, glucose intolerance, and hypertension, has often been associated with more severe liver abnormalities[13].
NAFLD is now considered to be the hepatic component of the MetS[14,15].
Conventional radiology studies used in the diagnosis of fatty liver include ultrasonography (US), computed tomography, and magnetic resonance imaging. Other than these radiological studies, there are no sensitive and low invasive screening methods for NAFLD. Alanine aminotransferase (ALT) > 30 IU/L is usually used as the cut off level for screening NAFLD[16,17]. This threshold had a sensitivity of 0.92 for detecting the fatty-fibrotic pattern proven by ultrasound among obese children[18]. However, ALT was within normal levels in 69% of those who had increased liver fat[19]. Similarly, in the Dallas Heart Study, 79% of the subjects with a fatty liver (liver fat content > 5.6%) had normal serum ALT[20]. This implies that a normal ALT does not exclude steatosis. Aspartate aminotransferase and gamma glutamyltransferase also correlate with liver fat content independent of obesity[21], but are even less sensitive than serum ALT.
It is well known that NAFLD mirrors insulin resistance, and patients with NAFLD tend to have the abnormal components of the MetS. However, the target for correctly detecting MetS has not yet been met.
MetS identifies patients at increased risk of developing CVD and type 2 diabetes mellitus. As it is a clustering of different risk factors, and its pathogenesis is not well understood, this has given rise to the development of multiple concurrent definitions. Central obesity and insulin resistance are acknowledged as important causative factors[22-24], together with other associated conditions, including physical inactivity[25], ageing[26] and hormonal imbalance[27,28] such as polycystic ovary syndrome or testosterone insufficiency.
The concept of clustering of risk factors was first described by Reaven[29], when the term “insulin resistance syndrome” was conceived. However, as the mechanisms underlying the link to CVD risk factors remain uncertain and insulin resistance is not easily measured in clinical practice, the more recent consensus, e.g., 2001 National Cholesterol Education Programme (NCEP) Adult Treatment Panel III (ATP III)[30] (Table 1) and the 2006 International Diabetes Federation (IDF) criteria[31] (Table 2), favours focusing on other clinical parameters that are easier to measure. It is, therefore, imperative to bear in mind that as the newer criteria do not include insulin resistance as one of its diagnostic criteria, individuals diagnosed as having the syndrome using these criteria may not necessarily be insulin resistant. This is in contrast to the 1999 World Health Organization (WHO)[32] and the 1999 European Group for the Study of Insulin Resistance[33] criteria, which emphasise insulin resistance. The IDF consensus, however, takes into account the importance of gender and ethnic differences in predicting early cardiovascular risk and may indeed be a better predictor for risk in women[34] and specific ethnic groups, e.g., South Asians (Indians), who appear to be more susceptible to the development of MetS at waist circumferences below that of the NCEP/ATP III cutpoints[35] (Table 3). It is also worth noting that the NCEP/ATP III criteria were revised in 2004, where the threshold for fasting glucose was lowered to ≥ 100 mg/dL (5.6 mmol/L) in concordance with American Diabetes Association criteria for impaired fasting glucose (IFG)[36]. Hence, in view of the various diagnostic criteria used, care will need to be exercised when interpreting clinical studies related to MetS.
| Risk factor | Defining level (AHA 2005) |
| Abdominal obesity (waist, inches) | |
| Men | > 40 |
| Women | > 35 |
| Triglycerides (mg/dL) | 150 (or Med) |
| HDL-C (mg/dL) | |
| Men | < 40 (or Med) |
| Women | < 50 |
| BP (mmHg) | 130/85 (or Med) |
| Fasting glucose (mg/dL) | 110 (100) |
| Central obesity plus any two of the following four factors | |
| Raised triglyceride | 150 mg/dL, or specific treatment for this lipid abnormality |
| Reduced HDL-C | < 40 mg/dL in men and < 50 mg/dL in women, or specific treatment for this lipid abnormality |
| Raised BP | Systolic BP 130 or diastolic BP 85 mmHg, or treatment of previously diagnosed hypertension |
| Raised FPG | 100 mg/dL, or previously diagnosed type 2 diabetes. If above 100 mg/dL, OGTT is strongly recommended but is not necessary to define presence of the syndrome |
| Country/ethnic group | WC |
| Europids (In the United States, the NCEP-ATP III values1 are likely to continue to be used for clinical purposes) | |
| Male | ≥ 94 cm (37 in) |
| Female | ≥ 80 cm (32 in) |
| South Asians (based on a Chinese, Malay, and Asian-Indian population) | |
| Male | ≥ 90 cm (35 in) |
| Female | ≥ 80 cm (32 in) |
| Chinese | |
| Male | ≥ 90 cm (35 in) |
| Female | ≥ 80 cm (32 in) |
| Japanese | |
| Male | ≥ 85 cm (34 in) |
| Female | ≥ 90 cm (32 in) |
MetS continues to be highly prevalent and contributes to a rapidly growing problem globally. About 40% of adults in the US population are estimated to have MetS by the age of 60 years[26,37]. At least one-fourth of the adult European population may have MetS[38-40], with a similar prevalence in Latin America[41]. MetS is also considered an emerging epidemic in developing Asian countries, including Singapore, China, Japan and South Korea, with a prevalence of 8%-13% in men and 2%-18% in women, depending on the population and definitions used[42-44].
There have been several proposed hypotheses for the development of MetS. One such widely quoted hypothesis suggests that adipose tissue dysfunction is the underlying cause, resulting in abnormal metabolism of free fatty acids and the release of adipocytokines which are responsible for the observed inflammatory changes and insulin resistance[45-47]. Adipose tissue is in itself an endocrine organ that is metabolically active, rather than purely an energy storage organ[48-51]. Adiponectin is secreted exclusively by adipocytes in adipose tissue, and low levels in individuals have consistently predicted the presence of MetS and CVD risk[52-56]. In fact, adiponectin can be measured reliably in a clinical setting; its circulating values do not umdergo diurnal fluctuation as much as other markers such as insulin, glucose or triglycerides, and only a small amount is required for its measurement, making this a potentially suitable biomarker for MetS[57,58]. Resistin[23,59,60] and visfatin[61-64] are the other adipocytokines implicated in the pathogenesis of MetS.
An alternative proposed aetiology suggests an underlying state of chronic, low-grade inflammation[65-67], leading to endothelial dysfunction and the release of inflammatory cytokines, which induce insulin resistance in adipose tissue and muscle[67,68]. Indeed, insulin-resistant individuals manifest evidence of low-grade inflammation even without an increase in total body fat[69].
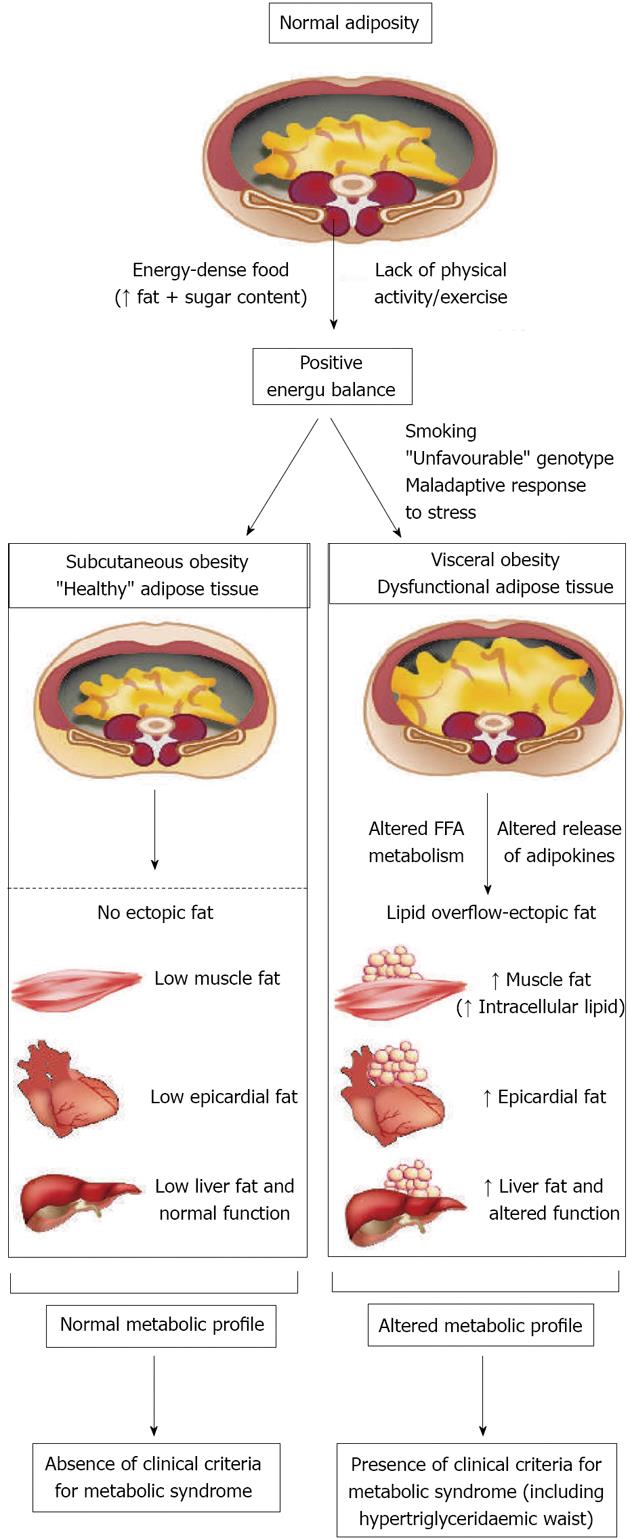
Excess visceral fat accumulation may be causally related to the features of insulin resistance, but might also be a marker of dysfunctional adipose tissue which is unable to appropriately store the energy excess (Figure 1). According to this model, the body’s ability to cope with the surplus of calories (resulting from excess caloric consumption, a sedentary lifestyle or, as is often the case, a combination of both factors) might, ultimately, determine the individual’s susceptibility to developing MetS. There is evidence to suggest that if the extra energy is channelled into insulin-sensitive subcutaneous adipose tissue, the individual, although in positive energy balance, will be protected against the development of MetS. However, in cases in which adipose tissue is absent, deficient or insulin resistant with a limited ability to store the energy excess, the triacylglycerol surplus will be deposited at undesirable sites such as the liver, the heart, the skeletal muscle and in visceral adipose tissue - a phenomenon described as ectopic fat deposition. Factors associated with the preferential accumulation of visceral fat and with features of insulin resistance include, among others, smoking, the well documented genetic susceptibility to visceral obesity[70] and a neuroendocrine profile related to a maladaptive response to stress[71]. The resulting metabolic consequences of this “defect” in energy partitioning include visceral obesity, insulin resistance, an atherogenic dyslipidaemia and a pro-thrombotic, inflammatory profile. These are defining features of MetS.
This constellation of abnormalities can be detected by the clinical criteria for MetS, the two simplest being the simultaneous presence of increased waist girth and fasting triacylglycerol levels, a condition that has been described as “hypertriglyceridaemic waist”[72].
It is noteworthy to stress that the identification of other risk factors might improve knowledge on the pathogenesis of NAFLD and open the way to new therapeutic approaches[73-75]. The debate surrounding the mechanisms inducing or favouring the presence/severity of NAFLD continues. In fact, some investigators have identified factors other that MetS to be associated with NAFLD[76-78].
Although the MetS has existed in various forms and definitions for more than eight decades, only in the past 5 years has real controversy about its definition and significance emerged[79,80]. The main controversy is that the syndrome has had too many definitions and there is a lack of clarity about its role and value in clinical practice[81]. It is fair to say, with exceptions[82], that most of the published reports indicate that the syndrome does not predict cardiovascular events or disease progression any better than the sum of its components[3,83,84]. The relative value in predicting type 2 diabetes remains uncertain[85]. The controversy, however, drove the need for a single global definition. Thus, came the initiative of the IDF and the American Heart Association/National Heart, Lung, and Blood Institute, joined by the World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity[79] to develop one unified definition[86].
The main difference between the NCEP ATP III (National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults)[87] and the IDF definitions[88] was that the IDF had a threshold value for waist circumference as obligatory. As a major step in consensus, this obligation has been reversed so that we now have a platform for standardised reporting in epidemiological and clinical research[81]. Yet, because the relation between waist circumference and CVD and diabetes risk differs globally, the definition for the expanded waist circumference remains unsettled. In the meantime, national or regional cutpoints for waist circumference can be used.
Insulin resistance continues to explain most, if not all of the MetS. In fact, no other mechanisms have emerged that come close to justifying the individual components or their clustering. Evidence now indicates that the MetS begins with excess central adiposity[89]. When β-cell function is responsive, hyperinsulinaemia results but fasting and postprandial glycaemia often remain normal for years. However, in those genetically predisposed, defects in insulin secretion and IFG and/or impaired glucose tolerance follow[90].
Most controversial has been the mechanism of hypertension under the tent of insulin resistance. However, not only are the effects of insulin on sodium reabsorption and sympathetic nervous system activation maintained despite insulin resistance, but increases in angiotensinogen, resistin, and leptin secretion from adipose tissue have also been implicated in the pathophysiology of hypertension in the syndrome[91]. Moreover, insulin resistance is closely associated with abnormalities in nitric oxide (NO) bioavailability and reduced phosphatidylinositol 3-kinase/protein kinase B signalling in the vascular wall, both of which have a crucial role in mobilisation of endothelial progenitor cells from bone marrow[91]. Not only do higher levels of free fatty acids directly reduce NO-dependent vasodilatation, but insulin resistance itself also results in structural or functional damage to the endothelium and apoptosis[91]. Reparative processes that regenerate injured endothelium might be increased by agents such as peroxisome proliferator-activated receptor γ agonists that enhance insulin sensitivity, an effect mediated by endothelial progenitor cells[92].
Genetic predisposition also relates to the MetS. A recent study found that a polymorphism in the multi-PDZ domain-containing adaptor protein, a protein that regulates the high-density lipoprotein-receptor scavenger-receptor type B class 1, was associated with the MetS[93]. Shift work, sleep deprivation, and bright-light exposure at night also relate to increased adiposity and prevalence of the MetS; clock genes are expressed in adipose tissue, and both their levels of expression and their genetic variants correlate with different components of the syndrome[94].
Another area of recent interest is vitamin D. Increasing evidence indicates that vitamin D deficiency is associated with the risk of CVD. Particularly relevant is a study that examined the association of serum vitamin D concentrations with risk factors for CVD in US adolescents[95].
The hypothesis that the MetS is an outgrowth of insulin resistance provides a strategy for management. Weight loss often reduces insulin resistance; and caloric restriction, weight-loss drugs, and bariatric surgery have been proved to be effective.
Although long-term weight reduction through dietary and pharmacological means is theoretically possible, most dietary and weight-loss drug studies have only continued for a few years. In contrast, in one 10-year follow-up after bariatric surgery[96], weight loss of 25% and improvement in the MetS were achieved; total mortality was also reduced. Even in the absence of weight loss, long-term physical activity, as measured by cardiorespiratory fitness, prevents the MetS[97], reduces cancer incidence and related mortality, and all-cause mortality[98].
Finally, one class of drugs that reduces insulin resistance and many of the components of the syndrome is the thiazolidinediones. These drugs act mainly in adipose tissue to favourably modify secretions of products that contribute to the pathophysiology of the MetS, including free fatty acids and adipocytokines. The major effect of thiazolidinediones is on dysglycaemia, which accounts for their use in the treatment of diabetes, yet the class as a whole has anti-inflammatory effects. At present, however, drug therapy for the MetS largely requires separate agents for the treatment of dysglycaemia, dyslipidaemia, and hypertension[99].
The MetS is a widely accepted concept that identifies the centrally obese patient with increased risk for CVD and diabetes. A global definition has now been proposed, insights into aetiology and mechanisms have been furthered, and, despite the controversies, lifestyle interventions remain the primary therapy. After lifestyle, residual risk for CVD needs to be treated with appropriate drugs.
US is currently the most common method for screening asymptomatic patients with elevated liver enzymes and suspected NAFLD[100]. US findings of fatty liver include hepatomegaly, diffuse increases in the echogenicity of the liver parenchyma, and vascular blunting.
Nonsteatotic hepatic parenchyma exhibits an echotexture similar to that of renal parenchyma, but becomes “brighter” when infiltrated with fat[101]. This hepatorenal contrast can be used to detect hepatosteatosis[102,103]. However, bright liver contrast associated with fibrosis is discussed in the literature[103]. US is easily performed and has a low cost, however, it also has limitations. It is operator dependent and subject to significant intra- and inter-observer variability[104]. It is impossible for US to provide quantitative information about the degree of fat accumulation. The sensitivity of US to detect steatosis decreases with a degree of fat infiltration less than 30%[105]. In obese patients, sensitivity lower than 40% has been reported in the detection of hepatosteatosis[106]. Finally, US has failed to prove efficacious in the detection of inflammation and fibrosis, therefore, it cannot be utilized to diagnose NASH and hepatic fibrosis[107]. However, in a recent study, Iijima et al[108] used an ultrasound contrast agent (Levovist; Sherling, Berlin, Germany) to distinguish between simple steatosis and NASH. Levovist contains galactose and palmitic acid and is taken up by hepatocytes[109]. These moieties participate in sugar and fat metabolism[110]. The uptake of Levovist was observed to significantly decrease in NASH patients, thus correlating with fibrosis rather than steatosis[108]. Larger studies are needed to evaluate the use of contrast US in the diagnosis of NASH characterised by inflammation and fibrosis, although there is a no absolute consensus in separating NASH from simple fatty liver as two distinct entities.
The MetS is associated with abdominal obesity and its criteria include waist circumference[30,31]. In addition, NAFLD has been reported to be associated with abdominal obesity[110].
The presence of multiple metabolic disorders such as diabetes mellitus, obesity, dyslipidaemia and hypertension is associated with a potentially progressive, severe liver disease[14,111-115]. Previous reports demonstrated that the prevalence of NAFLD increased to 10%-80% in individuals with obesity, 35%-90% in individuals with type 2 diabetes mellitus, 30%-56% in individuals with hypertension, and 26%-58% in individuals with dyslipidaemia[116-119].
It is clinically critical that a large number of patients with NAFLD were not diagnosed with the MetS, when we used today’s definition of the MetS[120]. Why not change the approach and use the presence of NAFLD as a new criterion for detecting the MetS?
Recently, it was shown that ultrasonographically-detected NAFLD (US-NAFLD) is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults[121]. Therefore, US-NAFLD may identify individuals with insulin resistance that cannot be identified by MetS in this population[121].
On this basis, we believe that this suggestion, i.e., the inclusion of NAFLD could help initiate weight control at the “earliest possible time” in the progression of disease, i.e., obesity/MetS, which means diagnosing NAFLD earlier rather than later, using the simplest method possible, i.e., at US[110].
NAFLD is highly prevalent and is considered the hepatic component of the MetS. The WHO, the NCEP-ATP III and the IDF have different criteria to define MetS. The MetS is associated with NAFLD, with the WHO definition being the best to determine its presence, probably due to the inclusion of insulin resistance as a main component. Unification of criteria is needed to adequately compare the prevalence of MetS and its relationship with NAFLD in different population, however, this is very hard task.
Further study will be needed to verify whether the inclusion of steatosis in the panel of MetS indicators will improve the predictive power of cardiovascular risk better than the current MetS criteria.
To define MetS, the presence of at least three of the proposed criteria is required, however, sometimes it is sufficient to have only one laboratory value, modified by diet or drugs, for the classification. US-NAFLD detection is more stable, and changes in the middle-to-long term. Although the controversy surrounding the utility of the MetS continue, considering that abdominal US in the diagnosis of NAFLD has a sensitivity of 91.7% and a specificity of 100%[122] and guidelines to modify the natural course of NAFLD by diet composition or lifestyle have not been established[123], why should we not introduce US-NAFLD as a new criterion to define MetS?
P- Reviewers Alberto P, Alessandro G S- Editor Jiang L L- Editor Webster JR E- Editor Li JY
| 1. | Finelli C, Tarantino G. Is visceral fat reduction necessary to favour metabolic changes in the liver? J Gastrointestin Liver Dis. 2012;21:205-208. [PubMed] |
| 2. | Tarantino G, Colao A, Capone D, Conca P, Tarantino M, Grimaldi E, Chianese D, Finelli C, Contaldo F, Scopacasa F. Circulating levels of cytochrome C, gamma-glutamyl transferase, triglycerides and unconjugated bilirubin in overweight/obese patients with non-alcoholic fatty liver disease. J Biol Regul Homeost Agents. 2011;25:47-56. [PubMed] |
| 3. | Tarantino G, Finelli C, Colao A, Capone D, Tarantino M, Grimaldi E, Chianese D, Gioia S, Pasanisi F, Contaldo F. Are hepatic steatosis and carotid intima media thickness associated in obese patients with normal or slightly elevated gamma-glutamyl-transferase? J Transl Med. 2012;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Colicchio P, Tarantino G, del Genio F, Sorrentino P, Saldalamacchia G, Finelli C, Conca P, Contaldo F, Pasanisi F. Non-alcoholic fatty liver disease in young adult severely obese non-diabetic patients in South Italy. Ann Nutr Metab. 2005;49:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 526] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 6. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 7. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2292] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 8. | De Ridder RJ, Schoon EJ, Smulders JF, van Hout GC, Stockbrügger RW, Koek GH. Review article: Non-alcoholic fatty liver disease in morbidly obese patients and the effect of bariatric surgery. Aliment Pharmacol Ther. 2007;26 Suppl 2:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;CD007340. [PubMed] |
| 10. | Scheen AJ, Luyckx FH. Obesity and liver disease. Best Pract Res Clin Endocrinol Metab. 2002;16:703-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S11-S16. [PubMed] |
| 12. | Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 13. | Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, Patel N, Madan A, Amarapurkar A. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Souza MR, Diniz Mde F, Medeiros-Filho JE, Araújo MS. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol. 2012;49:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999-2004. Gastroenterology. 2007;133:1814-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 17. | Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727-733. [PubMed] |
| 18. | Devadason CA, Scheimann AO. Overview of screening methods for fatty liver disease in children. World J Hepatol. 2012;4:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 543] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 20. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2695] [Article Influence: 128.3] [Reference Citation Analysis (3)] |
| 21. | Thamer C, Tschritter O, Haap M, Shirkavand F, Machann J, Fritsche A, Schick F, Häring H, Stumvoll M. Elevated serum GGT concentrations predict reduced insulin sensitivity and increased intrahepatic lipids. Horm Metab Res. 2005;37:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Anderson PJ, Critchley JA, Chan JC, Cockram CS, Lee ZS, Thomas GN, Tomlinson B. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int J Obes Relat Metab Disord. 2001;25:1782-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Sadashiv S, Paul BN, Kumar S, Chandra A, Dhananjai S, Negi MP. Over expression of resistin in adipose tissue of the obese induces insulin resistance. World J Diabetes. 2012;3:135-141. [PubMed] |
| 24. | Indulekha K, Surendar J, Mohan V. High sensitivity C-reactive protein, tumor necrosis factor-α, interleukin-6, and vascular cell adhesion molecule-1 levels in Asian Indians with metabolic syndrome and insulin resistance (CURES-105). J Diabetes Sci Technol. 2011;5:982-988. [PubMed] |
| 25. | Golbidi S, Mesdaghinia A, Laher I. Exercise in the metabolic syndrome. Oxid Med Cell Longev. 2012;2012:349710. [PubMed] |
| 26. | Gombet T, Longo-Mbenza B, Ellenga-Mbolla B, Ikama MS, Mokondjimobe E, Kimbally-Kaky G, Nkoua JL. Aging, female sex, migration, elevated HDL-C, and inflammation are associated with prevalence of metabolic syndrome among African bank employees. Int J Gen Med. 2012;5:495-503. [PubMed] |
| 27. | Malik SM, Traub ML. Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World J Diabetes. 2012;3:71-79. [PubMed] |
| 28. | Saad F, Gooren LJ. The role of testosterone in the etiology and treatment of obesity, the metabolic syndrome, and diabetes mellitus type 2. J Obes. 2011;2011:471584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2634] [Cited by in RCA: 2285] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 30. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [PubMed] |
| 31. | Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3852] [Cited by in RCA: 4244] [Article Influence: 223.4] [Reference Citation Analysis (0)] |
| 32. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [PubMed] |
| 33. | Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999;16:442-443. [PubMed] |
| 34. | Skilton MR, Moulin P, Sérusclat A, Nony P, Bonnet F. A comparison of the NCEP-ATPIII, IDF and AHA/NHLBI metabolic syndrome definitions with relation to early carotid atherosclerosis in subjects with hypercholesterolemia or at risk of CVD: evidence for sex-specific differences. Atherosclerosis. 2007;190:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Ajjan R, Carter AM, Somani R, Kain K, Grant PJ. Ethnic differences in cardiovascular risk factors in healthy Caucasian and South Asian individuals with the metabolic syndrome. J Thromb Haemost. 2007;5:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433-438. [PubMed] |
| 37. | Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2:180-193. [PubMed] |
| 38. | Martínez MA, Puig JG, Mora M, Aragón R, O’Dogherty P, Antón JL, Sánchez-Villares T, Rubio JM, Rosado J, Torres R. Metabolic syndrome: prevalence, associated factors, and C-reactive protein: the MADRIC (MADrid RIesgo Cardiovascular) Study. Metabolism. 2008;57:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Zanchetti A, Hennig M, Baurecht H, Tang R, Cuspidi C, Carugo S, Mancia G. Prevalence and incidence of the metabolic syndrome in the European Lacidipine Study on Atherosclerosis (ELSA) and its relation with carotid intima-media thickness. J Hypertens. 2007;25:2463-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Anagnostis P. Metabolic syndrome in the Mediterranean region: Current status. Indian J Endocrinol Metab. 2012;16:72-80. [PubMed] |
| 41. | Escobedo J, Schargrodsky H, Champagne B, Silva H, Boissonnet CP, Vinueza R, Torres M, Hernandez R, Wilson E. Prevalence of the metabolic syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: the CARMELA cross sectional study. Cardiovasc Diabetol. 2009;8:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Shen J, Goyal A, Sperling L. The emerging epidemic of obesity, diabetes, and the metabolic syndrome in china. Cardiol Res Pract. 2012;2012:178675. [PubMed] |
| 43. | Lee J, Heng D, Ma S, Chew SK, Hughes K, Tai ES. The metabolic syndrome and mortality: the Singapore Cardiovascular Cohort Study. Clin Endocrinol (Oxf). 2008;69:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Park HS, Kim SM, Lee JS, Lee J, Han JH, Yoon DK, Baik SH, Choi DS, Choi KM. Prevalence and trends of metabolic syndrome in Korea: Korean National Health and Nutrition Survey 1998-2001. Diabetes Obes Metab. 2007;9:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Dodson MV, Mir PS, Hausman GJ, Guan LL, Du M, Jiang Z, Fernyhough ME, Bergen WG. Obesity, metabolic syndrome, and adipocytes. J Lipids. 2011;2011:721686. [PubMed] |
| 46. | Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond). 2009;33:54-66. [PubMed] |
| 47. | Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1). J Clin Invest. 2003;111:453-461. [PubMed] |
| 48. | Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1151] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 49. | Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 898] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 50. | Frühbeck G. The adipose tissue as a source of vasoactive factors. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29:81-90. [PubMed] |
| 52. | Garg MK, Dutta MK, Mahalle N. Adipokines (adiponectin and plasminogen activator inhhibitor-1) in metabolic syndrome. Indian J Endocrinol Metab. 2012;16:116-123. [PubMed] |
| 53. | Alikaşifoğlu A, Gönç N, Özön ZA, Sen Y, Kandemir N. The relationship between serum adiponectin, tumor necrosis factor-alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: adiponectin is a marker of metabolic syndrome. J Clin Res Pediatr Endocrinol. 2009;1:233-239. [PubMed] |
| 54. | Bai YM, Chen TT, Yang WS, Chi YC, Lin CC, Liou YJ, Wang YC, Su TP, Chou P, Chen JY. Association of adiponectin and metabolic syndrome among patients taking atypical antipsychotics for schizophrenia: a cohort study. Schizophr Res. 2009;111:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Ahonen TM, Saltevo JT, Kautiainen HJ, Kumpusalo EA, Vanhala MJ. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2012;22:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Okamoto Y. Adiponectin provides cardiovascular protection in metabolic syndrome. Cardiol Res Pract. 2011;2011:313179. [PubMed] |
| 57. | Brooks NL, Moore KS, Clark RD, Perfetti MT, Trent CM, Combs TP. Do low levels of circulating adiponectin represent a biomarker or just another risk factor for the metabolic syndrome? Diabetes Obes Metab. 2007;9:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Hirose H, Yamamoto Y, Seino-Yoshihara Y, Kawabe H, Saito I. Serum high-molecular-weight adiponectin as a marker for the evaluation and care of subjects with metabolic syndrome and related disorders. J Atheroscler Thromb. 2010;17:1201-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Povel CM, Boer JM, Feskens EJ. Shared genetic variance between the features of the metabolic syndrome: heritability studies. Mol Genet Metab. 2011;104:666-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Mojiminiyi OA, Abdella NA. Associations of resistin with inflammation and insulin resistance in patients with type 2 diabetes mellitus. Scand J Clin Lab Invest. 2007;67:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Hajianfar H, Bahonar A, Entezari MH, Askari G, Yazdani M. Lipid Profiles and Serum Visfatin Concentrations in Patients with Type II Diabetes in Comparison with Healthy Controls. Int J Prev Med. 2012;3:326-331. [PubMed] |
| 62. | Taşkesen D, Kirel B, Us T. Serum visfatin levels, adiposity and glucose metabolism in obese adolescents. J Clin Res Pediatr Endocrinol. 2012;4:76-81. [PubMed] |
| 63. | Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur J Clin Invest. 2008;38:71-72. [PubMed] |
| 64. | Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 65. | Hanley AJ, Festa A, D’Agostino RB, Wagenknecht LE, Savage PJ, Tracy RP, Saad MF, Haffner SM. Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes. 2004;53:1773-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 561] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 67. | Haring R, Rosvall M, Völker U, Völzke H, Kroemer H, Nauck M, Wallaschofski H. A network-based approach to visualize prevalence and progression of metabolic syndrome components. PLoS One. 2012;7:e39461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O’Connell TM, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Newgard CB. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS One. 2012;7:e38812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Jeemon P, Prabhakaran D, Ramakrishnan L, Gupta R, Ahmed F, Thankappan K, Kartha C, Chaturvedi V, Reddy K. Association of high sensitive C-reactive protein (hsCRP) with established cardiovascular risk factors in the Indian population. Nutr Metab (Lond). 2011;8:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J Nutr. 1997;127:943S-947S. [PubMed] |
| 71. | Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 268] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 72. | Lemieux I, Poirier P, Bergeron J, Alméras N, Lamarche B, Cantin B, Dagenais GR, Després JP. Hypertriglyceridemic waist: a useful screening phenotype in preventive cardiology? Can J Cardiol. 2007;23 Suppl B:23B-31B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Green RM. NASH--hepatic metabolism and not simply the metabolic syndrome. Hepatology. 2003;38:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 444] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 75. | Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 76. | Yilmaz Y, Senates E, Ayyildiz T, Colak Y, Tuncer I, Ovunc AO, Dolar E, Kalayci C. Characterization of nonalcoholic fatty liver disease unrelated to the metabolic syndrome. Eur J Clin Invest. 2012;42:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Xu L, Xu CF, Yu CH, Miao M, Li YM. Haemoglobin and non-alcoholic fatty liver disease: further evidence from a population-based study. Gut. 2009;58:1706-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Yu C, Xu C, Xu L, Yu J, Miao M, Li Y. Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J Hepatol. 2012;56:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1380] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 80. | Saukkonen T, Jokelainen J, Timonen M, Cederberg H, Laakso M, Härkönen P, Keinänen-Kiukaanniemi S, Rajala U. Prevalence of metabolic syndrome components among the elderly using three different definitions: a cohort study in Finland. Scand J Prim Health Care. 2012;30:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 960] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 82. | Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2169] [Cited by in RCA: 1962] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 83. | Kang HM, Kim DJ. Metabolic Syndrome versus Framingham Risk Score for Association of Self-Reported Coronary Heart Disease: The 2005 Korean Health and Nutrition Examination Survey. Diabetes Metab J. 2012;36:237-244. [PubMed] |
| 84. | Koskinen J, Kähönen M, Viikari JS, Taittonen L, Laitinen T, Rönnemaa T, Lehtimäki T, Hutri-Kähönen N, Pietikäinen M, Jokinen E. Conventional cardiovascular risk factors and metabolic syndrome in predicting carotid intima-media thickness progression in young adults: the cardiovascular risk in young Finns study. Circulation. 2009;120:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31:1898-1904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 86. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10555] [Article Influence: 659.7] [Reference Citation Analysis (0)] |
| 87. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [PubMed] |
| 88. | Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5325] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 89. | Cameron AJ, Boyko EJ, Sicree RA, Zimmet PZ, Söderberg S, Alberti KG, Tuomilehto J, Chitson P, Shaw JE. Central obesity as a precursor to the metabolic syndrome in the AusDiab study and Mauritius. Obesity (Silver Spring). 2008;16:2707-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 676] [Cited by in RCA: 635] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 91. | Zhang Y, Sowers JR, Ren J. Pathophysiological insights into cardiovascular health in metabolic syndrome. Exp Diabetes Res. 2012;2012:320534. [PubMed] |
| 92. | Kahn MB, Yuldasheva NY, Cubbon RM, Smith J, Rashid ST, Viswambharan H, Imrie H, Abbas A, Rajwani A, Aziz A. Insulin resistance impairs circulating angiogenic progenitor cell function and delays endothelial regeneration. Diabetes. 2011;60:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Povel CM, Boer JM, Reiling E, Feskens EJ. Genetic variants and the metabolic syndrome: a systematic review. Obes Rev. 2011;12:952-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 94. | Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Reis JP, von Mühlen D, Miller ER, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124:e371-e379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 96. | Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1154] [Cited by in RCA: 1124] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 97. | LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 319] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 98. | Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1966] [Cited by in RCA: 2191] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 99. | Grundy SM. Drug therapy of the metabolic syndrome: minimizing the emerging crisis in polypharmacy. Nat Rev Drug Discov. 2006;5:295-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 100. | Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. [PubMed] |
| 101. | Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996;24:25-29. [PubMed] |
| 102. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 781] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 103. | Zardi EM, Caturelli E. May sonography distinguish between liver fibrosis and liver steatosis? Dig Liver Dis. 2007;39:790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 104. | Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320-W323. [PubMed] |
| 105. | Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 106. | Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L, Repetto G. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14:635-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 107. | Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 108. | Iijima H, Moriyasu F, Tsuchiya K, Suzuki S, Yoshida M, Shimizu M, Sasaki S, Nishiguchi S, Maeyama S. Decrease in accumulation of ultrasound contrast microbubbles in non-alcoholic steatohepatitis. Hepatol Res. 2007;37:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Iijima H, Moriyasu F, Miyahara T, Yanagisawa K. Ultrasound contrast agent, Levovist microbubbles are phagocytosed by Kupffer cells-In vitro and in vivo studies. Hepatol Res. 2006;35:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Tarantino G, Colicchio P, Conca P, Finelli C, Di Minno MN, Tarantino M, Capone D, Pasanisi F. Young adult obese subjects with and without insulin resistance: what is the role of chronic inflammation and how to weigh it non-invasively? J Inflamm (Lond). 2009;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1917] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 112. | Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol. 2007;13:4669-4672. [PubMed] |
| 113. | Tarantino G, Pizza G, Colao A, Pasanisi F, Conca P, Colicchio P, Finelli C, Contaldo F, Di Somma C, Savastano S. Hepatic steatosis in overweight/obese females: new screening method for those at risk. World J Gastroenterol. 2009;15:5693-5699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Labruna G, Pasanisi F, Nardelli C, Tarantino G, Vitale DF, Bracale R, Finelli C, Genua MP, Contaldo F, Sacchetti L. UCP1 -3826 AG+GG genotypes, adiponectin, and leptin/adiponectin ratio in severe obesity. J Endocrinol Invest. 2009;32:525-529. [PubMed] |
| 115. | Tarantino G, Caputi A. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J Gastroenterol. 2011;17:3785-3794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 116. | Tarantino G. Non-alcoholic fatty liver disease, obesity and other illnesses. Clin Invest Med. 2008;31:E290-E295. [PubMed] |
| 117. | Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5-10. [PubMed] |
| 118. | Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 119. | Fan JG, Peng YD. Metabolic syndrome and non-alcoholic fatty liver disease: Asian definitions and Asian studies. Hepatobiliary Pancreat Dis Int. 2007;6:572-578. [PubMed] |
| 120. | Hamaguchi M, Takeda N, Kojima T, Ohbora A, Kato T, Sarui H, Fukui M, Nagata C, Takeda J. Identification of individuals with non-alcoholic fatty liver disease by the diagnostic criteria for the metabolic syndrome. World J Gastroenterol. 2012;18:1508-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 121. | Sinn DH, Gwak GY, Park HN, Kim JE, Min YW, Kim KM, Kim YJ, Choi MS, Lee JH, Koh KC. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. 2012;107:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 122. | Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 673] [Article Influence: 37.4] [Reference Citation Analysis (3)] |
| 123. | Finelli C, Tarantino G. Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients? J Gastrointestin Liver Dis. 2012;21:293-302. [PubMed] |