Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3052
Revised: February 19, 2013
Accepted: March 8, 2013
Published online: May 28, 2013
Processing time: 259 Days and 15.3 Hours
AIM: To investigate whether neoadjuvant-intensified radiochemotherapy improved overall and disease-free survival in patients with locally advanced rectal cancer.
METHODS: Between January 2007 and December 2011, 80 patients with histologically confirmed rectal adenocarcinoma were enrolled. Tumors were clinically classified as either T3 or T4 and by the N stage based on the presence or absence of positive regional lymph nodes. Patients received intensified combined modality treatment, consisting of neoadjuvant radiation therapy (50.4-54.0 Gy) and infusional chemotherapy (oxaliplatin 50 mg/m2) on the first day of each week, plus five daily continuous infusions of fluorouracil (200 mg/m2 per die) from the first day of radiation therapy until radiotherapy completion. Patients received five or six cycles of oxaliplatin based on performance status, clinical lymph node involvement, and potential risk of a non-sphincter-conserving surgical procedure. Surgery was planned 7 to 9 wk after the end of radiochemotherapy treatment; adjuvant chemotherapy treatment was left to the oncologist’s discretion and was recommended in patients with positive lymph nodes. After treatment, all patients were monitored every three months for the first year and every six months for the subsequent years.
RESULTS: Of the 80 patients enrolled, 75 patients completed the programmed neoadjuvant radiochemotherapy treatment. All patients received the radiotherapy prescribed total dose; five patients suspended chemotherapy indefinitely because of chemotherapy-related toxicity. At least five cycles of oxaliplatin were administered to 73 patients. Treatment was well tolerated with high compliance and a good level of toxicity. Most of the acute toxic effects observed were classified as grades 1-2. Proctitis grade 2 was the most common symptom (63.75%) and the earliest manifestation of acute toxicity. Acute toxicity grades 3-4 was reported in 30% of patients and grade 3 or 4 diarrhoea reported in just three patients (3.75%). Seventy-seven patients underwent surgery; low anterior resection was performed in 52 patients, Miles’ surgery in 11 patients and total mesorectal excision in nine patients. Fifty patients showed tumor downsizing ≥ 50% pathological downstaging in 88.00% of tumors. Out of 75 patients surviving surgery, 67 patients (89.33%) had some form of downstaging after preoperative treatment. A pathological complete response was achieved in 23.75% of patients and a nearly pathologic complete response (stage ypT1ypN0) in six patients. An involvement of the radial margin was never present. During surgery, intra-abdominal metastases were found in only one patient (1.25%). Initially, 45 patients required an abdominoperineal resection due to a tumor distal margin ≤ 5 cm from the anal verge. Of these patients, only seven of them underwent Miles’ surgery and sphincter preservation was guaranteed in 84.50% of patients in this subgroup. Fourteen patients received postoperative chemotherapy. In the full analysis of enrolled cohort, eight of the 80 patients died, with seven deaths related to rectal cancer and one to unrelated causes. Local recurrences were observed in seven patients (8.75%) and distant metastases in 17 cases (21.25%). The five-year rate of overall survival rate was 90.91%. Using a median follow-up time of 28.5 mo, the cumulative incidence of local recurrences was 8.75%, and the overall survival and disease-free survival rates were 90.00% and 70.00%, respectively.
CONCLUSION: The results of this study suggest oxaliplatin chemotherapy has a beneficial effect on overall survival, likely due to an increase in local tumor control.
Core tip: Management of rectal cancer requires a multimodality treatment approach. The objective of this study was to determine whether neoadjuvant-intensified radiochemotherapy, using traditional radiation therapy in combination with oxaliplatin and 5-fluorouracil (5-FU), could improve the overall and disease-free survival rates in patients with locally advanced rectal cancer. Conventional chemotherapeutic strategies typically only use 5-FU infusion. The results from this study indicate that the addition of oxaliplatin to the chemotherapeutic regime enhances the 5-year overall survival rate, facilitates a high rate of sphincter preservation, and reduces the local recurrence rate relative to the traditional strategies previously reported in the literature. Furthermore, oxaliplatin addition was well tolerated by patients, demonstrating an acceptable level of toxicity.
- Citation: Musio D, De Felice F, Bulzonetti N, Guarnaccia R, Caiazzo R, Bangrazi C, Raffetto N, Tombolini V. Neoadjuvant-intensified treatment for rectal cancer: Time to change? World J Gastroenterol 2013; 19(20): 3052-3061
- URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3052.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3052
Management of rectal cancer requires a multimodality treatment approach. Significant progress has been made in the conventional modalities of surgery, radiotherapy and chemotherapy, typically used to treat this type of cancer. The frequent spread of neoplastic cells to mesorectal nodes and the consequent increase in local recurrence has led to surgical standardization using total mesorectal excision (TME). With standard TME surgery, the incidence of local recurrence in lymph node metastasis-negative, pN0, tumors is reduced. However, the local recurrence rate is still higher than 20% in patients with lymph node positive, pN+, disease and chemotherapy has remained the standard adjuvant care. Technical progress in radiation techniques, identified in major accuracy planning, and further analysis of accurate timing in sequential multimodality therapy has created improvements in local control, toxicity (acute and chronic), and sphincter preservation[1-3].
Previous work by Sauer et al[4] from the German Rectal Cancer Study Group identified preoperative radiochemotherapy as the standard treatment for patients with stage cT3-4 and/or N+ tumors. Based on empiric data and on the efficacy demonstrated in stage III colon cancer patients[5,6], the addition of a second chemotherapeutic agent in a neoadjuvant setting confirmed oxaliplatin (OXP) radiosensitizing properties both in vitro and in vivo[7].
The goal of this work was to determine whether neoadjuvant-intensified radiochemotherapy improved the overall and disease-free survival, which is typically only achieved with 5-fluorouracil (5-FU) treatment, in patients with locally advanced rectal cancer. In this study, tumor downstaging, pathological complete response (pCR), and negative radial (circumferential) margins of tumors were assessed as well as the overall and disease-free survival rates in a cohort of 80 patients, 51 of whom had already been evaluated as previously described[8]. The results indicate that oxaliplatin therapy, in addition to traditional radiation and 5-FU therapies, enhances the overall survival rate and reduces local recurrence in patients with rectal cancer.
Patients enrolled were positively diagnosed with rectal adenocarcinoma as shown by histological analysis. Tumors were within 12 cm from the anal margin and clinically classified as described below. The performance status (PS), age, normal blood parameters and normal renal function were also assessed. Patient exclusion criteria consisted of the presence of synchronous tumors, cardiovascular disease, history of neurological or psychiatric disorders, and previous pelvic radiotherapy. All patients were enrolled after providing informed consent.
The pre-treatment staging included obtaining the complete history and careful physical examination of the patient, digital rectal examination, rectocolonscopy, trans-rectal ultrasound and total body computerized tomography. Patient tumors were evaluated by ultrasound exam and tumor stage (T) was classified according to the American Joint Committee on Cancer tumor, nodes, metastasis (TNM) Staging System[9]. With the exception of one sample, all tumors were classified as either T3 or T4 and N+ if positive regional lymph nodes were detected without any distant metastases. In the case of an uncertain diagnosis, patients underwent abdominal-pelvic magnetic resonance imaging. The evaluation of clinically positive lymph nodes (N) was performed by trans-rectal ultrasound and TC; lymph nodes ≥ 1 cm were considered pathological.
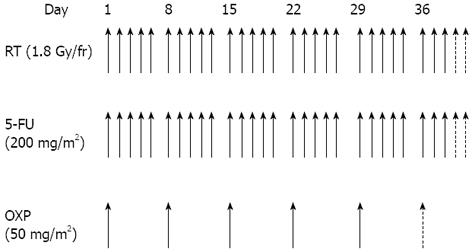
Radiotherapy: As the physical positioning of a patient must remain identical for both the initial localization of the tumor by computerised tomography (CT) scanning and during subsequent treatment, a planning CT scan was performed in the treatment position. Patients were treated in the prone position using a belly-board device to displace the small bowels out of the treatment field. A radio-opaque marker was placed on the anal verge. CT images were acquired from the level of L1 to 3 cm below the anal marker with 5 mm slice spacing. CT data were analyzed using Treatment Planning Software (Pinnacle®) for target volume definition and dose solutions. The planning target volume 1 (PTV1) encompassed the primary tumor, the mesorectal and posterior pelvic sub-regions, and the regional node. The presacral, obturator and internal iliac lymph nodes were monitored in all patients. The external iliac lymph nodes were monitored if clinically positive or in the case of T4 tumor. The inguinal lymph nodes were irradiated if there was major tumor extension to the internal and external anal sphincter. The superior field border was located at the bifurcation of the common iliac vessels (L5/S1 interspace); the inferior margin was 5 cm below the inferior edge of the tumor. The lateral extension was 2 cm outside of the pelvic bones. The posterior border was placed 1 cm behind the sacrum to include sacral hollows. The anterior limit was placed at the posterior margin (cT3) or anterior margin (cT4) of the symphysis.
The PTV2 included the tumor mass with a 2 cm 3-D margin. The organs at risk were bowel (Dmax < 55 Gy), bladder (V50 60%; V60 50%), femoral heads (V50 60%), and anal canal (Dmax < 55 Gy). Patients were set up daily, using sagittal and lateral tattoos and a laser to prevent lateral rotation. Electronic portal imaging was used to check treatment organization once a week from the start to the end of treatment; portal images were compared with digitally-reconstructed radiographs from the planning CT scan. Radiation therapy was delivered with a 3-D-conformational multiple field technique at a dose of 45 Gy (in 25 daily fractions of 1.8 Gy given in 5 wk) to the whole pelvis for PTV1 and 5.4-9.0 Gy (in 3-5 daily fractions of 1.8 Gy) to the tumor volume for PTV2 with 6-15 MV energy photons.
Chemotherapy: All patients received a central venous access (port-a-cath) for delivering chemotherapy. Chemotherapy consisted of a 2-h oxaliplatin infusion (50 mg/m2) on the first day of each week of radiotherapy, and five daily continuous infusions of 5-FU (200 mg/m2 per die). Patients received five or six cycles of oxaliplatin, dependent on PS, clinical lymph node involvement, and potential risk of a non-sphincter-conserving surgical procedure. Desamethasone (8 mg) and ondansetron (8 mg) were administered before the oxaliplatin infusion. Figure 1 shows the neoadjuvant treatment protocol.
Toxicity was evaluated using National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0[10]. Oxaliplatin and 5-FU dose reductions were not planned. For occurrence of hematological toxicity grade 3 or neurological toxicity grade 2, the oxaliplatin administration was interrupted; both chemotherapeutic agents were stopped if grade 3 toxicity was reached. If severe toxicity persisted, did not return to grade 1, or was classified as grade 4, chemotherapy was cancelled but radiotherapy was completed.
Surgery: Five weeks from the end of neoadjuvant treatment, each patient underwent digital rectal examination, total body TC, colonscopy and trans-rectal ecography to evaluate clinical response. Surgery was planned seven to nine weeks after the end of radiochemotherapy treatment. The surgeon chose the type of surgery to perform.
Adjuvant chemotherapy: Adjuvant chemotherapy treatment was left to the oncologist’s discretion and was recommended in patients with lymph node metastases.
Pathological staging was designated as pTNM and depended on the data acquired clinically in addition to surgical and pathologic findings. Radial margins were considered positive if there was evidence of microscopic invasion. Downstaging was defined as a reduction of at least one level in T or N staging between the baseline ultrasound exam and histopathological staging. Downsizing was defined as a reduction of lesion diameter between pre-treatment ultrasound evaluation and histopathological results. pCR was defined as the absence of any residual tumor cells detected in the operative specimen.
This study was an extension of a previous study of 51 patients[8]. Between January 2007 and December 2011, 29 new patients were enrolled for a total of 80 participants: 25 females (31.25%) and 55 males (68.75%). The patients’ ages ranged between 36-76 years (average = 63.55 years). Patients presented clinically with rectal bleeding (39/80, 48.75%) that may have been accompanied by a change in bowel habits, such as unexplained constipation and diarrhea (10/80, 12.50%). At a pre-treatment evaluation, 75.00% of patients showed pathological tumor-positive lymph nodes; 37.5% of patients were clinically staged as IIIB and 37.5% as IIIC. The distance of the inferior margin of the tumor lesion was located in the lower rectum at ≤ 5 cm from the anal verge in 56.25% of patients. The characteristics of patients are listed in Table 1.
| Characteristics | Patients |
| Performance status | |
| 0 | 52 (65.00) |
| 1 | 27 (33.75) |
| 2 | 1 (1.25) |
| Localization | |
| ≤ 5 cm from anal verge | 45 (56.25) |
| > 5 to ≤ 8 cm from anal verge | 19 (23.75) |
| > 8 cm from anal verge | 16 (20.00) |
| Stage | |
| IIA | 19 (23.75) |
| IIIA | 1 (1.25) |
| IIIB | 30 (37.50) |
| IIIC | 30 (37.50) |
After surgery, all patients were monitored at three-month intervals for the first year and at six-month intervals for the subsequent years. All patients were stratified into five cohorts according to the year of the last treatment. We defined “absolute permanence” as the greatest number of months of permanence in each cohort. Absolute permanence, overall survival (OS) and disease-free survival (DFS) were measured in months from the end of the neoadjuvant treatment.
Statistical analysis was performed using the following factors: 1 - sex, 2 - age, 3 - PS, 4 - TNM clinical staging, 5 - cranio-caudal extension of tumor lesion, 6 - tumor location, 7 - total radiotherapy dose, 8 - cycles of associated chemotherapy, 9 - interval between neoadjuvant treatment and surgery, 10 - type of surgery, 11 - toxicity, 12 - OS, 13 - DFS, 14 - pathologic downstaging, and 15 - pCR. Factors from 1 to 11 were considered “causal” or “predictive”; factors from 12 to 15 characterized the “considerable” results of therapy. Standard descriptive statistics were used to evaluate the distribution of each factor. OS and DFS were evaluated in each cohort. To determine the association between downstaging or pCR and predictive factors, the univariate analysis was performed using the non-parametric Bernard test. Statistical tests were one-sided. Changes of OS and DFS according to predictive factors were assessed using a logistic model in multivariate analysis. Statistical analysis was performed using MATLAB software, version 7.5.0.342 (R2007b), and SAS software, version 9.1.
Seventy-five patients completed the programmed radiochemotherapy treatment. All patients received the radiotherapy prescribed total dose of 50.4 Gy in 75 patients (93.75%) and 54 Gy in five patients (6.25%). Five patients suspended chemotherapy indefinitely because of chemo-related toxicity after the second cycle (one patient), third cycle (two patients), or fourth cycle (two patients). Twelve patients stopped the planned neoadjuvant treatment because of acute toxicity: five patients interrupted radiation therapy only and seven patients interrupted both treatments. In these patients, radiotherapy was stopped for an average period of 10.58 d (range 2-22 d).
Seventy-seven out of 80 patients underwent surgery. A “wait and see” approach was recommended to only one patient; he was unfit for surgery because of type II diabetes mellitus and pericarditis co-morbidities. After neoadjuvant treatment, he underwent pelvic RM that indicated a complete clinical regression of the tumor. One patient did not undergo surgery because of liver metastases. One patient had a myocardial infarction two weeks after the end of radiochemotherapy and died.
For those patients who were eligible, surgery was planned an average of 9.30 wk (range 5-24 wk) after the end of neoadjuvant treatment. Low anterior resection was performed in 52 patients, Miles’ surgery in 11 patients, and transanal endoscopic microsurgery in nine patients. Three patients had a different surgical approach. Two patients died of intra-operative complications. None had positive radial margins. At the beginning, 45 patients required an abdominoperineal resection due to a distal tumor margin distance of ≤ 5 cm from the anal verge. Only seven patients underwent Miles’ surgery, and sphincter preservation was guaranteed in 84.50% of this subgroup of patients. Post-operative complications were recorded in nine patients; the most common type was perianastomotic fistula (six patients) and other post-operative complications included fever (one patient), intestinal obstruction (one patient), and adhesion (one patient).
Downsizing and downstaging was evaluated by comparing clinical staging to pathological staging. Fifty patients showed tumor downsizing of ≥ 50% and associated downstaging in 88.00% of tumors. Out of 75 patients surviving surgery, 67 patients (89.33%) had some form of downstaging from preoperative treatment. After surgery, 25 patients (33.33%) harbored tumors that were classified as Stage I. Of these, 18 patients (72.00%) had clinical positive nodes at diagnosis. Pathologic complete response, defined as the absence of tumor cells in the operative specimen, was observed in 18 patients (22.50%); only six of them had clinical negative lymph nodes at diagnosis. Six patients had a nearly pathologic complete response (stage ypT1ypN0). During surgery, intra-abdominal metastases were found in one patient only (1.25%).
Fourteen patients received postoperative chemotherapy. Twelve patients had lymph node-positive tumors previously identified by trans-anal ultrasound (eight patients with cN2 and four patients with cN1). One patient had positive lymph nodes identified by histopathological examination and distant metastases were detected intraoperatively in one patient. Of 16 patients with ypT3ypN0 disease, only four were assigned to adjuvant chemotherapy.
Until January 2012, surviving patients were monitored with a follow-up program; the average follow-up time was 27.28 mo and the median follow-up time was 28.50 mo (range 2-58 mo). Twenty-four patients were followed for 25-36 mo, 18 patients followed for 37-48 mo, and 11 patients were followed for at least 49 mo.
In the full analysis of study cohort, eight of the 80 patients died. Seven deaths were related to rectal cancer and one death was caused by unrelated causes. Of these, one patient died before surgery, two died during surgery, and four deaths occurred during the follow-up program. Of the latter, two patients had local recurrence 6 and 9 mo, respectively, after the end of neoadjuvant treatment and two patients had distant metastases 13 and 15 mo, respectively, after the end of preoperative radiochemotherapy. Three out of the four patients had stage IIIB disease at diagnosis.
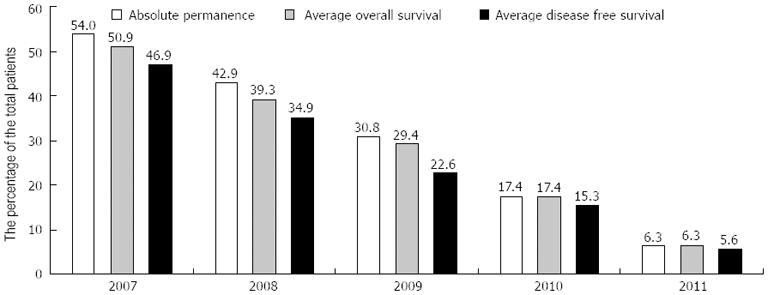
The absolute permanence (AP), OS, and DFS times were evaluated for each cohort (Table 2 and Figure 2), as well as the permanence/OS and permanence/DFS ratios and the total number of patients surviving (α) or deceased (Ω) at the time of analysis (Table 2). It is important to note that OS times were nearly equivalent to absolute permanence times; the permanence/OS ratios were greater than 90% in all years evaluated. Remarkably, comparisons between OS and DFS demonstrated that the DFS/OS ratio was always higher than 88%, except in the 2009 cohort.
| Cohort | α | Ω | AP, mo | OS, mo | DFS, mo | OS/AP % | DFS/OS % |
| 2007 | 16 | 3 | 54.00 | 50.90 | 46.90 | 94.28 | 92.14 |
| 2008 | 22 | 3 | 42.90 | 39.30 | 34.90 | 91.40 | 89.01 |
| 2009 | 18 | 2 | 30.80 | 29.40 | 22.60 | 95.53 | 76.97 |
| 2010 | 13 | 0 | 17.40 | 17.40 | 15.30 | 100.00 | 87.93 |
| 2011 | 11 | 0 | 6.30 | 6.30 | 5.60 | 100.00 | 88.79 |
| Total | 80 | 8 | 29.80 | 27.90 | 23.90 | 93.45 | 85.88 |
Of 19 patients with a complete response (18 pathological and one clinical), 18 patients are still disease-free survivors. One patient had local recurrence 19 mo after the end of neoadjuvant treatment. In the full analysis of the study participants, locoregional recurrence was observed in seven patients (8.75%) an average of 21.29 mo (range 6-39 mo) after the end of preoperative radiochemotherapy, six patients showed downstaging, and one patient had no benefit from neoadjuvant treatment. Local recurrence was located in the perianastomotic region (six cases) or pre-sacral region (one case). Distant metastases were recorded in 17 cases (21.25%) where eight patients presented pulmonary metastases after an average of 22.75 mo (range 7-38 mo) after the end of neoadjuvant treatment and six patients had liver metastases after an average of 6.83 mo (range 1-16 mo) after the end of radiochemotherapy. Individual cases of brain and ovarian metastases were recorded 6 and 12 mo, respectively, after the end of preoperative treatment. One patient presented distant metastases in multiple locations. Of these 17 patients, only five showed negative lymph nodes at diagnosis.
Acute toxicity: Table 3 summarizes the incidence of acute toxicity. Proctitis, grade G2, was the most common symptom (63.75%) and the earliest manifestation of acute toxicity. On average, proctitis symptoms appeared during the ninth day of radiation therapy (range 2-25 d). Grade G1-2 diarrhea was noted in 31.00% of patients. Grades 3-4 toxicity was seen only in nine patients, with symptoms of diarrhea (three patients), proctitis (four patients), and abdominal pain (two patients). Genitourinary toxicity was observed in 32.50% of patients. Symptoms included an increase in frequency and dysuria, usually during the end of third week of radiation therapy (range 3-26 d). Of the 23 patients with dysuria, 47.83% (11 patients) were classified as G2. Radiation dermatitis was reported in 30 patients; ten patients’ symptoms were graded as G1, 17 patients as G2, and three patients as G3. There was a significant correlation between radiation dermatitis and tumors located within 5 cm of the anal verge (P value, 0.0076). No hematological toxicity was observed. Hypersensitivity reactions were recorded in six patients. One patient stopped chemotherapy after the second cycle. One patient completed all five prescribed cycles of intensified chemotherapy, but during the last two cycles was administered 5-FU only. Four patients completed the prescribed treatment after taking a one-week break.
| G1 | G2 | G3 | |
| Blood-bone marrow | |||
| Neutrofilis-granulocytes | 1 (1.25) | ||
| Allergy-immunology | |||
| Allergic reaction-hypersensitivity | 6 (7.5) | ||
| Cardiac arrhythmia | |||
| Palpitation | 1 (1.25) | ||
| Constitutional symptoms | |||
| Fatigue | 10 (12.5) | 5 (6.25) | |
| Fever | 7 (8.75) | 1 (1.25) | |
| Dermatology-skin | |||
| Pruritus-itching | 2 (2.5) | ||
| Rash-desquamation | 2 (2.5) | 6 (7.5) | |
| Radiation-dermatitis | 10 (12.5) | 17 (21.25) | 3 (3.75) |
| Gastrointestinal | |||
| Constipation | 2 (2.5) | 15 (18.75) | |
| Diarrhea | 14 (17.5) | 11 (13.75) | 3 (3.75) |
| Nausea | 12 (15) | ||
| Proctitis | 6 (7.5) | 51 (63.75) | 4 (5) |
| Vomiting | 5 (6.25) | 1 (1.25) | |
| Neurology | |||
| Neuropathy: sensory | 14 (17.5) | 1 (1.25) | 1 (1.25) |
| Pain | |||
| Pelvic pain | 1 (1.25) | 3 (3.75) | |
| Abdominal pain or cramping | 6 (7.5) | 2 (2.5) | 2 (2.5) |
| Renal-genitourinary | |||
| Dysuria-painful urination | 12 (15) | 11 (13.75) | |
| Urinary frequency | 3 (3.75) | ||
| G3 | G4 | G5 | |
| Cardiovascular | |||
| Vascular thromboembolism | 7 (8.75) | 2 (2.5) | 2 (2.5) |
Sixteen patients presented neurologic toxicity due to oxaliplatin. Neuropathy was classified as G3 in only one patient, who had sensory loss and paresthesia during the end of the fifth cycle of chemotherapeutic treatment. In total, 11 patients (13.75%) had adverse cardiovascular events. Two patients experienced acute myocardial infarction (IMA), two patients experienced pulmonary embolism, and seven patients experienced deep vein thrombosis requiring anticoagulant therapy. Embolic events G3-4 arose after the end of neoadjuvant-intensified radiochemotherapy by an average of 4.42 mo (range 1-8 mo). One patient died 2 wk after the end of treatment; it was considered an IMA-related death. One patient died during surgery of cardiac infarction.
Late toxicity: Late toxicity was defined as long-term toxic effects occurring at least 6 mo after the end of radiochemotherapy treatment. Considering all enrolled patients, gastrointestinal toxicity was the most evident late side effect and was recorded in 37 patients (46.25%). Fecal incontinence was reported in 32.43% of patients and proctitis in 32.43% of patients. Four patients had diarrhea and five patients had an increase in stool frequency. Seven patients reported sexual dysfunction. Urinary incontinence was observed in three patients with colostomy. In 11 out of 60 patients with acute proctitis, the symptoms continually persisted as G1-2 grade. No correlation was observed between acute toxicity and late toxicity. Neuropathy with loss of deep tendon reflexes and paresthesia that did not interfere with activities of daily living was documented in five patients and acute neuropathic toxicity was reported in 16 patients.
We evaluated 11 “predictive” factors: (1): sex; (2): age; (3): PS; (4): TNM clinical staging; (5): cranio-caudal extension of the tumor lesion; (6): tumor location; (7): total radiotherapy dose; (8): cycles of associated chemotherapy; (9): interval between neoadjuvant treatment and surgery; (10): type of surgery; and (11): toxicity. Univariate analysis did not show any significant correlation between “predictive” factors and downstaging or pCR. There was no causal significant correlation between “predictive” factors and OS or DFS in the multivariate analysis.
Two additional statistical analyses were of interest and enhanced the evaluation of the data. First, the linear relationship between the infusion of six cycles of chemotherapy and tumor sizes smaller than 5 cm was statistically significant (P value, 0.0334). Second, a causal correlation was found between surgery performed seven weeks before the end of neoadjuvant treatment and OS or DFS, although the data was not stratified by the surgery characteristics.
In the past two decades, several clinical trials have been performed to determine the role of a multimodal approach in treating rectal cancer. These clinical trials faced three problems: (1) determination of temporal sequencing of treatment modalities; (2) integration of radiotherapy and chemotherapy; and (3) radiation dose fractionation. Testing and analysis of new approaches should be aimed at defining therapeutic strategies to improve local tumor control and overall survival benefit[11].
Year after year, local recurrence has represented the prevailing method of treatment failure. Now, with improvement in surgical and radiotherapeutic techniques, local tumor control rates have improved. Neoadjuvant radiotherapy and concurrent 5-FU based chemotherapy, as compared with the same protocol delivered after surgery, has improved local control[4]. The addition of 5-FU to radiation therapy also has been shown to significantly reduce the incidence of local recurrences[4,11,12]. Likewise, survival has improved with standardization of total mesorectal excision surgery[13-16]. The decrease in local recurrence rates emphasizes the need to investigate the risk of distant metastases and requires new treatment strategies to improve distant tumor control.
Although most patients achieve tumor downstaging after preoperative radiochemotherapy, the debate over monochemotherapy vs polichemotherapy still remains. Specifically, whether 5-FU infusion, the standard of care in rectal cancer, is better than multichemotherapeutic agents for reducing the risk of distant metastases. The prevalence of distant metastases (24%-30%) stresses the importance of a more effective systemic treatment[11].
In this study, the effects of a multimodal therapy approach for rectal cancer were evaluated. Disease-free survival was considered the most efficient indicator of the absence of disease and is the most robust indicator of effective treatment. In our previously reported study[8], we evaluated the toxicity and efficacy of preoperative-intensified radiochemotherapy, and the subsequent pathologic complete response, downstaging, and sphincter preservation rates. In the current study, the patient cohort from the previous study was expanded with 29 new cases and the overall survival and disease-free survival rates were evaluated and stratified per year.
The aim of this study was to verify efficacy of neodjuvant-intensified treatment. Oxaliplatin (50 mg/m2 per week) was added to the standard 5-FU chemotherapy, normally given in continuous infusion of 200 mg/m2 per die during each day of radiation therapy. Weekly administration of oxaliplatin, with the cumulative dose of 300 mg/m2, was chosen so that its toxic effects were reduced and to optimize its radiosensitizing properties. This intensified radiochemoterapy regime was used to test the hypothesis that it could produce greater OS and DFS within tolerable toxicity. An empirical analysis of the study results confirms our hypothesis; the treatment was well tolerated, with high compliance and a relatively good level of toxicity. All patients had received the total prescribed radiotherapy dosage and at least five cycles of oxaliplatin were administered to 91.25% of patients.
The compliance rate (93.75%) was slightly higher than rates registered in studies in which intensified radiochemoterapy regimes were adopted (range 64%-85%)[17-21]. Most of the acute toxic side effects observed in this study were classified as grade 1-2. Grade 3-4 acute toxicity was reported in 30% of patients and was only slightly higher than data from the STAR-01 trial (24%) and the ACCORD study (25%)[17,18]. Of note, cardiovascular toxicity represented 45.83% of grade 3-4 acute toxic effects.
Thromboembolic risk could be ascribed to the type of chemotherapy administrated or to surgery. In the literature, there is not enough available data describing this risk, although chemotherapy is recognized as an independent risk factor for a thromboembolic event[22]. Ng et al[23] examined the frequency and pattern of cardiotoxicity in 153 patients treated with capecitabine used in addition with oxaliplatin for advanced colorectal cancer and found that 6.7% of patients developed thromboembolic events. Randomized studies, aimed to assess the efficacy of neoadjuvant radiochemotherapy treatment in locally advanced rectal cancer, with or without oxalipaltin, did not analyze cardiovascular toxicity. Chua et al[24,25] reported that during induction chemotherapy using capecitabine and oxaliplatin, 10 patients (8.5%) had cardiac or thromboembolic events and four patients died in a phase II trial. The incidence rate of that study was slightly lower than the rate reported here (13.75%). The high rate of thromboembolic risk may be associated with properties of oxaliplatin that boost the 5-FU thrombogenetic effect[26]. Certainly, the rise in cardiovascular, chemo-related toxicity must be monitored carefully and kept under control. Because of the risk, neoadjuvant-intensified radiochemotherapy must be interrupted if cardiac symptoms appear.
Excluding cardiovascular toxicity, grade 3 or 4 acute toxic effects occurred only in 13 patients (16.25%) with diarrhea occurring just in three patients (3.75%). In both STAR-01 and ACCORD trials, addition of oxaliplatin to 5-FU based radiochemotherapy increased toxicity rates. In those studies, grade 3-4 diarrhea was recorded in 15% and 13% of patients given oxaliplatin vs 4% and 3% of those in the control group, respectively[17,18]. The lowest rates of acute gastrointestinal toxicity in this study were observed in the oxaliplatin regimen, given as 2-h infusion at a dose of 50 mg/m2 per week. Grade 3-4 long-term toxic effects were not recorded.
It was difficult to determine if the cause of fecal incontinence and sexual function was due to radiation therapy, surgery, or both treatments. Sixty-four patients, or 80% of the enrolled patients, underwent conservative surgery. Of these patients, 10.45% reported sexual dysfunction and 17.91% fecal incontinence.
The sphincter preservation rate, evaluated in the total patient cohort and in a subgroup of 45 patients with a tumor localization ≤ l5 cm from the anal verge, was 80% and 84.50%, respectively. These results are slightly better than the ACCORD trial, in which conservative surgery was performed in 77.20% of patients, and similar to the STAR-01 study, in which 82% of patients had sphincter preservation. In the literature, the incidence of pCR and negative radial margins, in patients who received preoperative radiochemotherapy with 5-FU and oxaliplatin, ranged from 10% to 24% and from 80.95% to 100%, respectively[18-20,24,25,27,28]. Randomized trials confirmed an improvement in the local tumor control rate. Neoadjuvant treatment has been associated with a reduction of local recurrence of 6%-15%, although the incidence of distant metastases was still 30%[29-32]. A retrospective study showed that pathologic downstaging and pCR were essential for an accurate prediction of disease-free survival and overall survival[33].
In 22.5% of patients, no residual cancer cells were identified and the tumor was staged as pT0N0. Pathological complete response rates reached 23.75% and were evaluated by post-treatment RM. One patient is still a disease-free survivor 21 mo after the end of radiochemotherapy. The majority of patients, 83.75%, had some form of tumor downstaging after preoperative treatment; involvement of the radial margin was never present.
The 5-year overall survival rate (90.91%) in this study was better than results observed in randomized trials of locally advanced rectal cancer, which only observed survival rates of 65%-68%[12,13,26]. The results of this study indicate that intensification of radiochemotherapy using oxaliplatin is effective in preoperative treatment and showed that a strong response to neoadjuvant treatment increased the overall patient survival.
Considering a median follow-up of 28.5 mo (range 2-58 mo) in the total cohort, the cumulative incidence of local recurrences was 8.75% and the incidence of OS and DFS was 90% and 70%, respectively. The most common type of first recurrence was pulmonary metastases, which arose in eight patients. This observation confirmed the need to include CT thorax controls during the follow-up program. Although some questions remain, such as the need for adjuvant chemotherapy and whether oxaliplatin boosts the 5-FU trombogenetic effect, the radiochemotherapy regime used (OXP 50 mg/m2 and 5-FU 200 mg/m2) seems to be appropriate to produce good clinical results and to maintain toxicity at tolerable levels.
In conclusion, the results of this study indicate that neoadjuvant-intensified radiochemotherapy in patients with rectal cancer carcinoma improved pathological complete response, negative radial margins, and sphincter preservation. The chemotherapy regime of OXP 50 mg/m2 and 5-FU 200 mg/m2 was well tolerated, with few severe toxic effects. Despite the small number of patients enrolled, this study suggests that the addition of oxaliplatin has a beneficial effect on overall survival, likely due to an increase in local control, although there was not a clear increase in distant metastases control. On the basis of these results, the importance and validity of intensified radiochemotherapy to reduce local recurrence should be emphasized. Currently, we are waiting for longer follow-up data from the randomized phase III trials. Future studies will address the treatment and management of recurrent distant metastases in rectal cancer.
Management of rectal cancer needs a multimodality treatment approach. Significant progress has been made in the conventional modalities of surgery, radiotherapy and chemotherapy used to treat this type of cancer. The decrease in local recurrence rates emphasizes the need to investigate the risk of distant metastases and indicates that new treatment strategies are necessary to improve distant control.
Although most patients achieve a tumor downstaging after preoperative radiochemotherapy, the question of monochemotherapy versus polichemotherapy still remains. Infusion of 5-fluorouracil (5-FU), the standard of care in rectal cancer, is better than multi-chemotherapeutic agents for reducing the risk of distant metastases. The incidence of distant metastases (24%-30%) stresses the importance of a more effective systemic treatment.
The objective of this study was to determine whether neoadjuvant-intensified radiochemotherapy improved the overall and disease-free survival rates in patients with locally advanced rectal cancer. Typically, improved survival rates have only been achieved with 5-FU treatment.
Oxaliplatin is a chemotherapeutic agent. Studies in vitro and in vivo confirm its radiosensitizing properties.
This is a retrospective review in a significant number of patients with favourable results and worth publication.
P- Reviewer Cheung HYS S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, Hermans J, van de Velde CJ, Leer JW, van Krieken JH. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19:1976-1984. [PubMed] |
| 2. | Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. [PubMed] |
| 3. | Dolinsky CM, Mahmoud NN, Mick R, Sun W, Whittington RW, Solin LJ, Haller DG, Giantonio BJ, O’Dwyer PJ, Rosato EF. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol. 2007;96:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4409] [Article Influence: 210.0] [Reference Citation Analysis (1)] |
| 5. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [PubMed] |
| 6. | Sanoff HK, Carpenter WR, Martin CF, Sargent DJ, Meyerhardt JA, Stürmer T, Fine JP, Weeks J, Niland J, Kahn KL. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J Natl Cancer Inst. 2012;104:211-227. [PubMed] |
| 7. | Hospers GA, Punt CJ, Tesselaar ME, Cats A, Havenga K, Leer JW, Marijnen CA, Jansen EP, Van Krieken HH, Wiggers T. Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer. A phase I-II multicenter study of the Dutch Colorectal Cancer Group. Ann Surg Oncol. 2007;14:2773-2779. [PubMed] |
| 8. | Dionisi F, Musio D, Raffetto N, Codacci-Pisanelli G, Iannacone E, Caiazzo R, Banelli E. Preoperative intensified radiochemotherapy for rectal cancer: experience of a single institution. Int J Colorectal Dis. 2011;26:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Gherardi G, Bianchi F. Colon and rectum. AJCC Cancer Staging Atlas. 6th ed. New York: Springer 2007; 107-117. |
| 10. | Cancer Therapy Evaluation Program (2006) Common Terminology Criteria for Adverse Events, Version 3.0. Available from: http://ctep.cancer.gov. |
| 11. | Valentini V, Lambin P, Myerson RJ. Is it time for tailored treatment of rectal cancer? From prescribing by consensus to prescribing by numbers. Radiother Oncol. 2012;102:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2002] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 13. | Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1260] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 14. | Havenga K, Grossmann I, DeRuiter M, Wiggers T. Definition of total mesorectal excision, including the perineal phase: technical considerations. Dig Dis. 2007;25:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Bolognese A, Cardi M, Muttillo IA, Barbarosos A, Bocchetti T, Valabrega S. Total mesorectal excision for surgical treatment of rectal cancer. J Surg Oncol. 2000;74:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Piso P, Dahlke MH, Mirena P, Schmidt U, Aselmann H, Schlitt HJ, Raab R, Klempnauer J. Total mesorectal excision for middle and lower rectal cancer: a single institution experience with 337 consecutive patients. J Surg Oncol. 2004;86:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 569] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 18. | Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 566] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 19. | Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Machiels JP, Duck L, Honhon B, Coster B, Coche JC, Scalliet P, Humblet Y, Aydin S, Kerger J, Remouchamps V. Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadiOxCape study. Ann Oncol. 2005;16:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Zhao L, Bai C, Shao Y, Guan M, Jia N, Xiao Y, Qiu H, Zhang F, Yang T, Zhong G. A phase II study of neoadjuvant chemoradiotherapy with oxaliplatin and capecitabine for rectal cancer. Cancer Lett. 2011;310:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 625] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 23. | Ng M, Cunningham D, Norman AR. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur J Cancer. 2005;41:1542-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, Tebbutt N, Hill M, Ross PJ, Massey A. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 25. | Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, Tait D, Massey A, Tebbutt NC, Chau I. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 26. | Mezi S, Musio D, Orsi E, de Felice F, Verdinelli I, Morano F, Raffetto N, Tombolini V. Incidence of thromboembolic events in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. Acta Oncol. 2013;52:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Carlomagno C, Farella A, Bucci L, D’Armiento FP, Pesce G, Pepe S, Cannella L, Pacelli R, De Stefano A, Solla R. Neo-adjuvant treatment of rectal cancer with capecitabine and oxaliplatin in combination with radiotherapy: a phase II study. Ann Oncol. 2009;20:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Ofner D, Devries AF, Schaberl-Moser R, Greil R, Rabl H, Tschmelitsch J, Zitt M, Kapp KS, Fastner G, Keil F. Preoperative oxaliplatin, capecitabine, and external beam radiotherapy in patients with newly diagnosed, primary operable, cT₃NxM0, low rectal cancer: a phase II study. Strahlenther Onkol. 2011;187:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Gosens MJ, Klaassen RA, Tan-Go I, Rutten HJ, Martijn H, van den Brule AJ, Nieuwenhuijzen GA, van Krieken JH, Nagtegaal ID. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res. 2007;13:6617-6623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Créhange G, Bosset JF, Maingon P. [Preoperative radiochemotherapy for rectal cancer: forecasting the next steps through ongoing and forthcoming studies]. Cancer Radiother. 2011;15:440-444. [PubMed] |
| 31. | Gaedcke J, Liersch T, Hess C, Becker H, Rödel C, Ghadimi BM. [Rectal cancer: current status of multimodal therapy--when and how?]. Zentralbl Chir. 2011;136:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 403] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 33. | Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 855] [Article Influence: 45.0] [Reference Citation Analysis (0)] |