Published online May 14, 2013. doi: 10.3748/wjg.v19.i18.2818
Revised: March 14, 2013
Accepted: March 23, 2013
Published online: May 14, 2013
Processing time: 182 Days and 15.4 Hours
AIM: To assess CD163 expression in plasma and peripheral blood and analyze its association with disease in acute-on-chronic hepatitis B liver failure (ACHBLF) patients.
METHODS: A retrospective study was conducted from January 1, 2011 to January 1, 2012. Forty patients with ACHBLF (mean age 44.48 ± 12.28 years, range 18-69 years), 40 patients with chronic hepatitis B (CHB) (mean age 39.45 ± 12.22 years, range 21-57 years) and 20 age- and sex-matched healthy controls (mean age 38.35 ± 11.97 years, range 28-60 years) were included in this study. Flow cytometry was used to analyze the frequency of CD163+ peripheral blood mononuclear cells (PBMCs) and surface protein expression of CD163. Real-time transcription-polymerase chain reaction was performed to assess relative CD163 mRNA levels in PBMCs. Plasma soluble CD163 (sCD163) levels were measured by enzyme-linked immunosorbent assay. Clinical variables were also recorded. Comparisons between groups were analyzed by Kruskal-Wallis H test and Mann-Whitney U test. Statistical analyses were performed using SPSS 15.0 software and a P value < 0.05 was considered statistically significant.
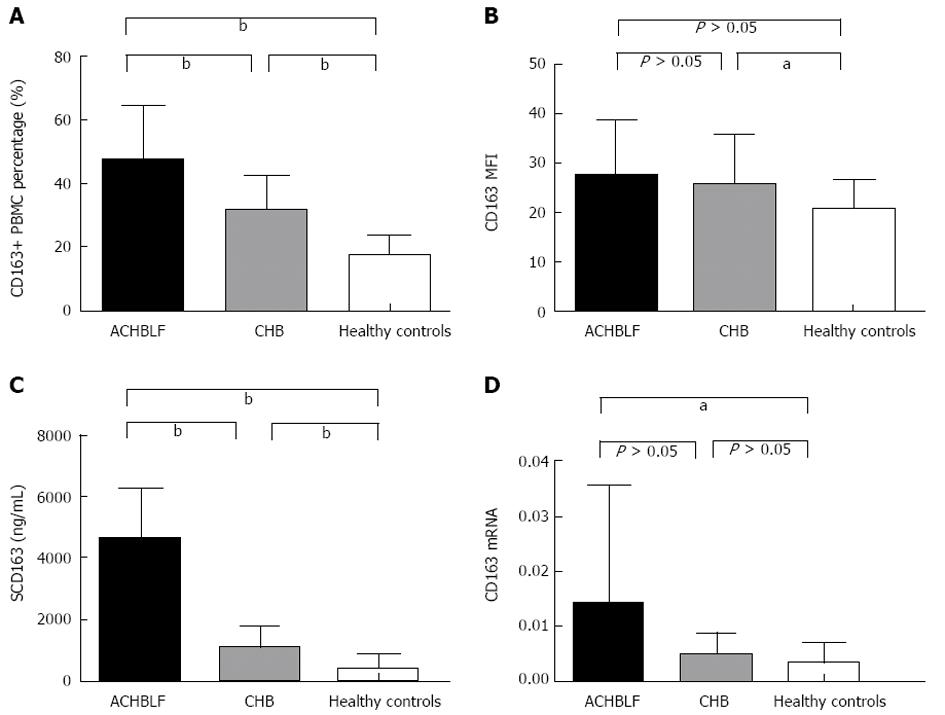
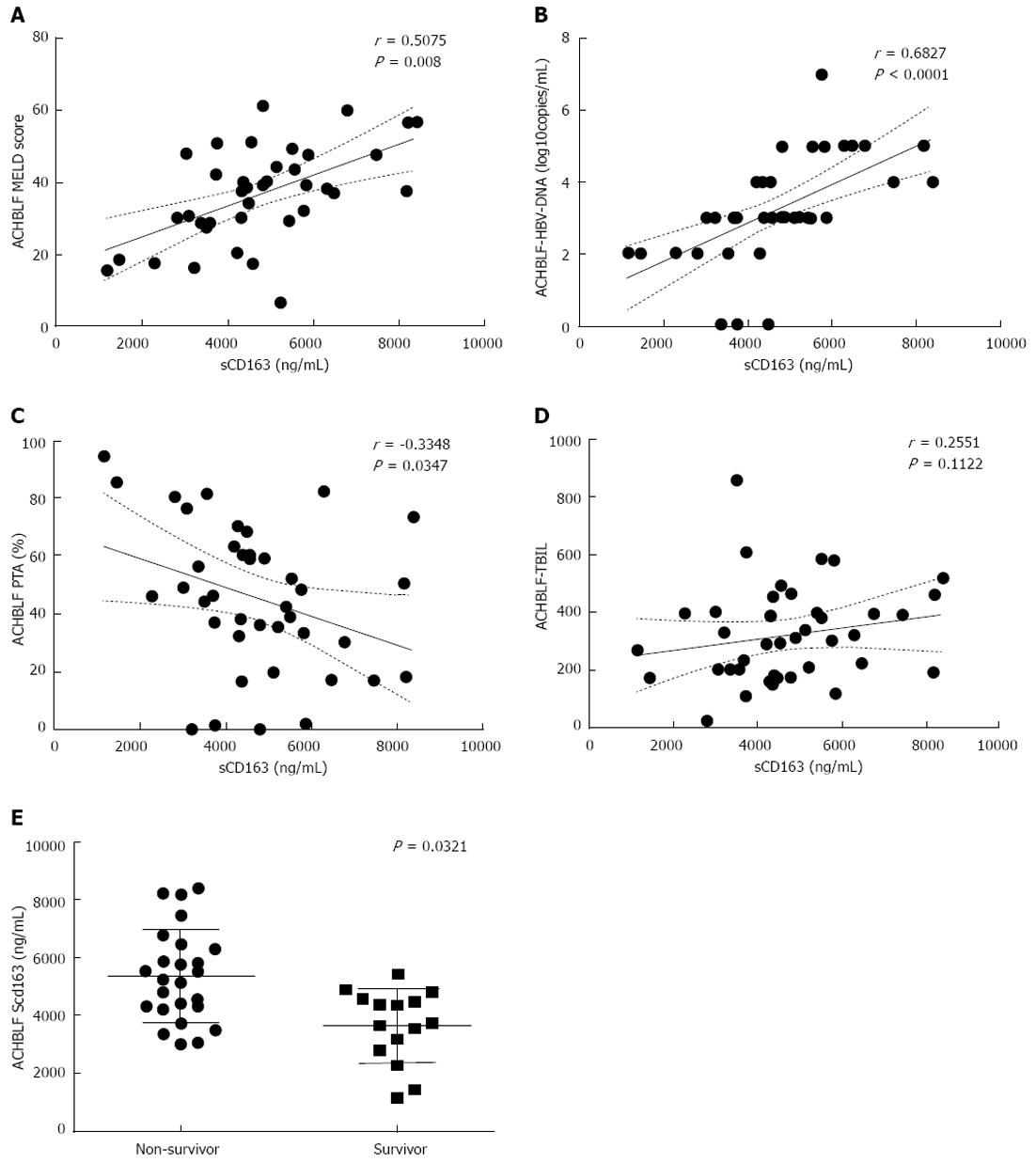
RESULTS: Flow cytometry showed that the population of CD163+ PBMCs was significantly greater in ACHBLF patients than in CHB patients and healthy controls (47.9645% ± 17.1542%, 32.0975% ± 11.0215% vs 17.9460% ± 6.3618%, P < 0.0001). However, there were no significant differences in mean fluorescence intensity of CD163+ PBMCs within the three groups (27.4975 ± 11.3731, 25.8140 ± 10.0649 vs 20.5050 ± 6.2437, P = 0.0514). CD163 mRNA expression in ACHBLF patients was significantly increased compared with CHB patients and healthy controls (1.41 × 10-2± 2.18 × 10-2, 5.10 × 10-3± 3.61 × 10-3vs 37.0 × 10-4± 3.55 × 10-4, P = 0.02). Plasma sCD163 levels in patients with ACHBLF were significantly increased compared with CHB patients and healthy controls (4706.2175 ± 1681.1096 ng/mL, 1089.7160 ± 736.8395 ng/mL vs 435.9562 ± 440.8329 ng/mL, P < 0.0001). In ACHBLF patients, plasma sCD163 levels were significantly positively associated with model for end-stage liver disease scores (r = 0.5075, P = 0.008), hepatitis B virus-DNA (r = 0.6827, P < 0.0001), and negatively associated with prothrombin activity (r = -0.3348, P = 0.0347), but had no correlation with total bilirubin (r = 0.2551, P = 0.1122). Furthermore, sCD163 was obviously elevated in non-surviving patients compared with surviving patients with ACHBLF (5344.9080 ± 1589.5199 ng/mL vs 3641.7333 ± 1264.5228 ng/mL, P = 0.0321).
CONCLUSION: CD163 and sCD163 may be related to disease severity and prognosis in ACHBLF patients.
Core tip: This study included three groups, acute-on-chronic hepatitis B liver failure (ACHBLF) patients, chronic hepatitis B (CHB) patients and healthy controls. Flow cytometry was used to analyze the frequency of CD163+ peripheral blood mononuclear cells (PBMCs) and surface protein expression of CD163. Real-time transcription-polymerase chain reaction was performed to assess relative CD163 mRNA levels in PBMCs. The population of CD163+ PBMCs was significantly larger in ACHBLF patients than in CHB patients and healthy controls. CD163 mRNA expression in ACHBLF patients was significantly increased compared with healthy controls. Plasma soluble CD163 (sCD163) levels were markedly increased and correlated with disease severity and prognosis in ACHBLF patients. CD163 and sCD163 may be useful biomarkers for ACHBLF.
- Citation: Ye H, Wang LY, Zhao J, Wang K. Increased CD163 expression is associated with acute-on-chronic hepatitis B liver failure. World J Gastroenterol 2013; 19(18): 2818-2825
- URL: https://www.wjgnet.com/1007-9327/full/v19/i18/2818.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i18.2818
Hepatitis B virus (HBV) infection is a major health problem worldwide. It is thought to be one of the main causes of liver-related chronic and acute diseases[1]. With severe acute exacerbation of the disease, some chronic hepatitis B (CHB) patients may progress to liver failure. We define this progression as acute-on-chronic hepatitis B liver failure (ACHBLF), and constitutes about 70% of all acute-on-chronic liver failure in areas with a high incidence of hepatitis B[2]. ACHBLF has an extremely poor prognosis due to a lack of understanding of its pathogenesis. Currently, clinical observations have shown that ACHBLF may be related to strong immune responses. Therefore, it is important to fully understand the course of the immune pathogenesis of ACHBLF. If we can identify efficient markers which predict disease progression, this may be of great help in the treatment of ACHBLF[3] .
Innate immune cells such as macrophages can be activated by acute and chronic inflammation and produce various cytokines. These immunological cytokines induce and cause liver tissue injury. Activated macrophages play important roles in the production of these immune cytokines. Monocytes and macrophages can be activated into M1 and M2 subpopulations in response to different environmental signals which are mainly derived from inflammatory diseases. Those of the M2 subpopulation inhibit the inflammatory state[4-6].
CD163 is a member of a scavenger receptor family and is expressed mainly on activated macrophages. It is a specific M2 macrophage marker. Soluble CD163 (sCD163) emerges from the shedding of CD163 from the cell surface in the plasma[6-9]. It is now evident that sCD163 and CD163 are very useful as biomarkers of macrophage activation in various inflammatory diseases. It is strongly indicated that CD163 and sCD163 may be involved in the pathogenesis of liver failure. However, the exact expression of CD163 and sCD163 in ACHBLF patients has not been fully elucidated[10,11].
This study aimed to evaluate peripheral blood CD163 and plasma sCD163 expression in macrophages from patients with ACHBLF.
Forty patients with ACHBLF, 40 patients with CHB and 20 healthy controls from Qilu Hospital of Shandong University, were included in this retrospectively study.
Blood samples collected from January 2011 to January 2012 at the Department of Hepatology, Qilu Hospital of Shandong University, were separated into plasma and stored at -70 °C until use.
Patients with CHB had more than twice the normal alanine aminotransferase level. All groups were matched for sex and age. ACHBLF patients had a history of CHB, with plasma total bilirubin (TBIL ≥ 85 μmol/L), prothrombin activity (PTA) < 40%, and complications such as hepatic encephalopathy (no less than grade II), ascites or hepato-renal syndrome. According to the Asian Pacific Association for the Study of the Liver guideline, all ACHBLF patients received inpatient treatment. We excluded patients who underwent liver transplantation, had hepatocellular carcinoma or other metastatic liver tumors or who had received immunotherapy or anti-viral treatment within 6 mo. Patients with a history of alcohol abuse, intravenous drug abuse, pregnancy, concomitant chronic hepatitis C, human immune deficiency virus infection or autoimmune hepatitis were also excluded. Hepatitis B surface antigen in healthy controls (n = 20) (age-, sex- and race-matched) was negative. Experiments and procedures were conducted with the guidance of the Helsinki Declaration of 1975[12]. The study was approved by the local Ethical Committee of Qilu Hospital of Shandong University. Prior to the collection of blood, informed consent was obtained from each patient. The characteristics of the enrolled subjects are summarized in Table 1.
| Patients | ACHBLF | CHB | Healthy control |
| Cases | 40 | 40 | 20 |
| Age, yr | 44.48 ± 12.28 | 39.45 ± 12.22 | 38.35 ± 11.97 |
| Sex (male/female) | 23/17 | 26/14 | 12/8 |
| HBeAg (+)/HBeAg (-) | 19/21 | 28/12 | NA |
| PTA, % | 45.36 ± 25.14 | 98.70 ± 17.56 | 108.00 ± 13.56 |
| TBIL, μmol/L | 322.08 ± 166.00 | 33.39 ± 66.35 | 18.65 ± 6.55 |
| HBV DNA, log10copies/mL | 322.08 ± 166.00 | 3.20 ± 1.44 | NA |
| Encephalopathy grade III/IV | 12.12% | 0% | 0% |
| Ascites | 30.30% | 0% | 0% |
| Mortality | 62.5% | 0% | 0% |
Six milliliters of peripheral venous blood were collected from each subject and Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) was used for isolation of PBMCs. Total RNA in PBMCs was extracted using Trizol (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. Two micrograms of total RNA were converted into cDNAs using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Lithuania) and real-time polymerase chain reaction (RT-PCR) was carried out on a Lightcycler (Roche Diagnostics, Germany). RT-PCR amplification mixtures (20 μL) contained 75 ng template cDNA, 10 μL 2 9 SYBR@ Premix Ex Taq™ (Takara, Japan) and 200 nmol/L forward and reverse primer. The real-time PCR reaction was performed as follows: the initial step was 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 60 °C for 30 s and 72 °C for 30 s. β-actin was used as the endogenous control for the normalization of RNA quantity and quality differences in all samples.
The primers were as follows: CD163 forward 5’-TCTGGCTTGACAGCGTTTC-3’; CD163 reverse 5’-TGTGTTTGTTGCCTGGATT-3’; β-actin forward 5’-CGGGAAATCGTGCGTGACATT-3’; β-actin reverse 5’-GGAGTTGAAGGTAGTTTCGTGG-3’ (Fermentas, Lithuania). Gene specific amplifications were demonstrated with melting curve data and gel-migration analyses.
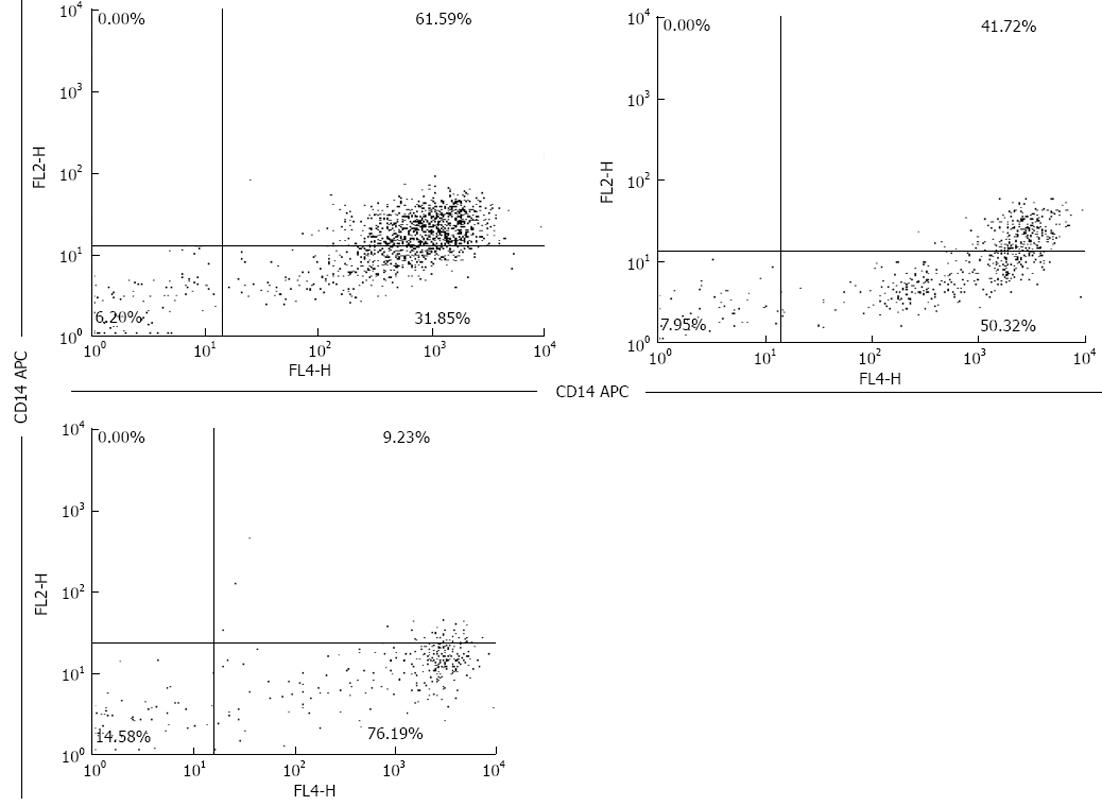
Freshly drawn peripheral whole blood samples of 200 μL were stained with isotype-matched control antibody or a relevant antibody (CD14, CD163) for 30 min at room temperature in the dark. Anti-human CD163 PE, anti-human CD14 APC and isotype-matched control antibody were purchased from eBioscience (San Diego, CA, United States). Following incubation, erythrocytes were lysed with RBC lysis buffer (Whole Blood Lysing Kit, R and D Systems, United States). Finally, the cells were washed three times, resuspended in 500 μL of PBS containing 0.5% formaldehyde, and analyzed using a FACS Calibur (BD Bioscience, PharMingen). The samples were analyzed with a Becton-Dickinson FACS Calibur machine. Separate gates were established for the macrophages. The amount of CD163 in peripheral blood was assessed using flow cytometry (Coulter counter)[13-15] (Figure 1).
SCD163 was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R and D, Minneapolis, MN, United States). All samples were analyzed in duplicate following the manufacturer’s protocol. The sensitivity of the assay was 0.1 ng/mL, and the intra-assay and inter-assay coefficients of variation were less than 3% and 6%, respectively.
Measurement of liver function tests: TBIL and hematological tests including PTA were performed using standard methods in a clinical setting. Hepatitis B markers were tested using a commercially available radioimmunoassay (Abbott). The level of HBV DNA was quantified using a DNA assay (sensitivity > 500 copies/mL). Model for end-stage liver disease (MELD) scores were calculated according to the Malinchoc formula: r = 9.57 × loge [creatinine (mg/dL)] + 3.78 × loge [bilirubin (mg/dL)] + 11.2 × loge (INR) + 6.43 × (etiology: 0 if cholestatic or alcoholic, 1 otherwise)[16,17].
Statistical analysis were performed using SPSS 15.0 software (SPSS Inc., Chicago, IL, United States). Comparisons between groups were analyzed by Kruskal-Wallis H test and Mann-Whitney U test. The Spearman rank correlation test was used for correlation analysis. All statistical analysis were two-sided, and a P value < 0.05 was considered statistically significant.
We determined the frequency of CD163+ PBMCs using flow cytometry and found that in ACHBLF patients, the frequency was markedly higher than that in the healthy control group and CHB patients, respectively (Figure 2A). There was a significant difference in the frequency of CD163+ PBMCs within the three groups (47.9645% ± 17.1542%, 32.0975% ± 11.0215% vs 17.9460% ± 6.3618%, P < 0.0001). We also evaluated the average mean fluorescence intensity (MFI) of CD163+ PBMCs using flow cytometric analysis. However, there were no significant differences in MFI in CD163 + PBMCs within the three groups (27.4975 ± 11.3731, 25.8140 ± 10.0649 vs 20.5050 ± 6.2437, P = 0.0514) (Figure 2B).
We evaluated the plasma levels of sCD163 by ELISA. The results showed that the levels of sCD163 in ACHBLF patients were markedly higher than those in CHB patients and the healthy control group. Significant differences in plasma sCD163 were found within ACHBLF patients, CHB patients and healthy controls (4706.2175 ± 1681.1096 ng/mL, 1089.7160 ± 736.8395 ng/mL vs 435.9562 ± 440.8329 ng/mL, P < 0.0001 ) (Figure 2C).
We also determined the mRNA level of CD163 in PBMCs using RT-PCR. No significant differences in the mRNA level of CD163 were found within the three groups (1.41 × 10-2± 2.18 × 10-2, 5.10 × 10-3± 3.61 × 10-3vs 37.0 ×10-4± 3.55 × 10-4, P = 0.02). The mRNA levels of CD163 in ACHBLF patients and CHB patients were significantly higher than those in healthy controls. No significant difference in the mRNA level of CD163 was observed in ACHBLF and CHB patients (P > 0.05) (Figure 2D).
To determine whether the increase in plasma levels of sCD163 correlated with liver injury, we analyzed the plasma levels of sCD163 in ACHBLF patients with clinical and laboratory parameters which indicated liver function or DNA replication in ACHBLF. The results showed that plasma levels of sCD163 were positively correlated with MELD score (r = 0.5075, P = 0.008), plasma HBV-DNA levels (r = 0.6827, P < 0.0001) and negatively correlated with PTA (r = -0.3348, P = 0.0347), but had no correlation with TBIL (r = 0.2551, P = 0.1122) (Figure 3A-D).
ACHBLF patients were grouped into survivors and non-survivors after 3 mo of follow up. The plasma levels of sCD163 were increased in non-survivors compared with survivors (5344.9080 ± 1589.5199 ng/mL vs 3641.7333 ± 1264.5228 ng/mL, P = 0.0321) (Figure 3E).
Although sCD163 is thought to play an important role in the pathogenesis of liver failure, this is the first study on the frequency of CD163+ PBMCs and CD163 mRNA level in ACHBLF patients[18,19]. Increased plasma sCD163 levels were correlated with disease severity in ACHBLF patients. These results strongly suggest that the role of CD163 in the immune response might affect the prognosis of ACHBLF.
CD163 is a scavenger receptor, and its expression has been proved to be associated with inhibition of inflammation. Furthermore, during the innate immune response, CD163 plays an important role in the host defense against inflammation[5,20,21]. The soluble form of CD163 is also a biomarker associated with inflammation[18]. In the present study, we first found that the frequency of CD163+ PBMCs and CD163 mRNA expression in PBMCs were dramatically increased in ACHBLF patients compared with healthy controls. These results could be interpreted together with previous reports to suggest that CD163 might have a key position in the immunolesion of ACHBLF.
There were no significant differences in MFI of CD163+ PBMCs within the three groups. This result is very interesting. From previous literature, which mainly included randomly selected patients, CD163 was inversely correlated with sCD163 plasma levels[22]. Lipopolysaccharide and other pro-inflammatory cytokines induce shedding of CD163 from the surface of isolated monocytes. During infection, the expression of monocyte surface CD163 decreases. In contrast, some studies have shown that with chronic and acute inflammation, the expression of surface CD163 increased. Through CD163 shedding, decreased surface CD163 was followed by recovery and induction of surface CD163 to higher levels[23-25]. We speculate that surface CD163 expression might vary according to different inflammatory states in patients and different stages of inflammation. More research is needed to confirm this.
In this study, we observed that peripheral mRNA levels of CD163 in ACHBLF patients were high and there was a significant difference between the three groups, however, mRNA levels of CD163 in ACHBLF patients were not significantly different to those in CHB patients. During inflammation, we speculate that plasma sCD163 levels might increase dramatically, but mRNA levels increase slowly[26-28].
In the present study, we found that plasma sCD163 levels were significantly increased in patients with ACHBLF compared to CHB patients and healthy controls. Plasma sCD163 levels were positively associated with HBV DNA levels, MELD scores and were negatively correlated with PTA. In addition, there were no associations with TBIL. It was demonstrated that plasma sCD163 correlated with the severity of ACHBLF[29-31].
The MELD scoring system is widely accepted and is used to predict prognosis in patients with liver failure. We chose the MELD scoring system to evaluate the prognosis of ACHBLF patients over a three month period. The results strongly indicated the potential role of plasma sCD163 levels in forecasting the prognosis of ACHBLF patients. Furthermore, plasma sCD163 levels were obviously elevated in ACHBLF patients who did not survive compared with surviving ACHBLF patients[16,17].
We found significant associations between plasma sCD163 levels and HBV DNA in ACHBLF patients. These results indicate that HBV may contribute to the aggravated macrophage immune response besides activation of HBV[32,33]. HBV might play a key role in promoting disease progression during host immune responses caused by the infection in ACHBLF patients. The exact role of HBV in CD163 immune response requires further clarification[34-36].
The present study has limitations, and it would be very interesting to determine the dynamic and histologic expression of CD163 in a future study.
In conclusion, our study demonstrated three major findings. First, the population of CD163+ PBMCs was significantly greater in ACHBLF patients than in CHB patients and healthy controls. Second, CD163 mRNA expression in ACHBLF patients was significantly increased compared with CHB patients and healthy controls. Third, plasma sCD163 levels were markedly increased and correlated with disease severity and prognosis in ACHBLF patients. Our findings indicate that CD163 and sCD163 may serve as useful biomarkers and new therapeutic targets for ACHBLF.
Acute-on-chronic hepatitis B liver failure (ACHBLF) constitutes about 70% of all acute-on-chronic liver failure (ACLF) in areas with a high incidence of hepatitis B. ACHBLF has an extremely poor prognosis due to a lack of understanding of its pathogenesis.
ACHBLF has an extremely poor prognosis due to a lack of understanding of its pathogenesis. It is important to fully understand the course of the immune pathogenesis of ACHBLF. It may be very helpful to identify a new biomarker which indicates the prognosis of ACHBLF.
This is the first study on the frequency of CD163+ peripheral blood mononuclear cells and CD163 mRNA level in ACHBLF patients. Authors found that increased plasma soluble CD163 (sCD163) levels were correlated with disease severity in ACHBLF patients. These findings strongly suggest that the role of CD163 and sCD163 in the immune response might affect the prognosis of ACHBLF.
Their findings indicate that CD163 and sCD163 may serve as useful biomarkers and new therapeutic targets for ACHBLF. These biomarkers should be determined as early as possible in ACHBLF patients.
ACLF was first used in 1995 to describe a rapid deterioration of liver function due to acute insult to the liver in patients with ongoing or chronic liver disease. Diagnosis of ACLF may be based on the following: acute hepatic insult manifesting as jaundice (serum bilirubin ≥ 85 μmol/L and coagulopathy international normalized ratio > 1.5 or prothrombin activity < 40%) complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease. ACHBLF is ACLF with acute or chronic hepatitis B.
Overall, this paper is of a very good quality. Study design is appropriate and clear, and helps to answer a clinical relevant question. Data analysis is sufficient. Manuscript is well structured, language is of sufficient quality.
P- Reviewer He JY S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Kitab B, Essaid El Feydi A, Afifi R, Trepo C, Benazzouz M, Essamri W, Zoulim F, Chemin I, Alj HS, Ezzikouri S. Variability in the precore and core promoter regions of HBV strains in Morocco: characterization and impact on liver disease progression. PLoS One. 2012;7:e42891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 643] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 3. | Huang K, Hu JH, Wang HF, He WP, Chen J, Duan XZ, Zhang AM, Liu XY. Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2011;17:3448-3452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Wang LY, Meng QH, Zou ZQ, Fan YC, Han J, Qi ZX, Ge J, Xu AL, Wang SK, Wang K. Increased frequency of circulating Th17 cells in acute-on-chronic hepatitis B liver failure. Dig Dis Sci. 2012;57:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. ScientificWorldJournal. 2011;11:2391-2402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Kowal K, Silver R, Sławińska E, Bielecki M, Chyczewski L, Kowal-Bielecka O. CD163 and its role in inflammation. Folia Histochem Cytobiol. 2011;49:365-374. [PubMed] |
| 7. | Lin LN, Zhu Y, Che FB, Gu JL, Chen JH. Invasive fungal infections secondary to acute-on-chronic liver failure: a retrospective study. Mycoses. 2013;Feb 1; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu YJ, Wang FS. Severe dendritic cell perturbation is actively involved in the pathogenesis of acute-on-chronic hepatitis B liver failure. J Hepatol. 2008;49:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Yang B, Wang Y, Zhao C, Yan W, Che H, Shen C, Zhao M. Increased Th17 cells and interleukin-17 contribute to immune activation and disease aggravation in patients with chronic hepatitis B virus infection. Immunol Lett. 2013;149:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 11. | Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 12. | Shephard DA. The 1975 Declaration of Helsinki and consent. Can Med Assoc J. 1976;115:1191-1192. [PubMed] |
| 13. | Etzerodt A, Maniecki MB, Graversen JH, Møller HJ, Torchilin VP, Moestrup SK. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. J Control Release. 2012;160:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Tippett E, Cheng WJ, Westhorpe C, Cameron PU, Brew BJ, Lewin SR, Jaworowski A, Crowe SM. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS One. 2011;6:e19968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Higashi-Kuwata N, Jinnin M, Makino T, Fukushima S, Inoue Y, Muchemwa FC, Yonemura Y, Komohara Y, Takeya M, Mitsuya H. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther. 2010;12:R128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | He WP, Hu JH, Zhao J, Tong JJ, Ding JB, Lin F, Wang HF. Comparison of four prognostic models and a new Logistic regression model to predict short-term prognosis of acute-on-chronic hepatitis B liver failure. Chin Med J (Engl). 2012;125:2272-2278. [PubMed] |
| 17. | Zheng MH, Shi KQ, Fan YC, Li H, Ye C, Chen QQ, Chen YP. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol. 2011;9:351-356.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 19. | Møller HJ, Grønbaek H, Schiødt FV, Holland-Fischer P, Schilsky M, Munoz S, Hassanein T, Lee WM. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671-676. [PubMed] |
| 20. | Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells. Adv Exp Med Biol. 2011;714:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Onofre G, Kolácková M, Jankovicová K, Krejsek J. Scavenger receptor CD163 and its biological functions. Acta Medica (Hradec Kralove). 2009;52:57-61. [PubMed] |
| 22. | Davis BH, Zarev PV. Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom. 2005;63:16-22. [PubMed] |
| 23. | Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72:711-717. [PubMed] |
| 24. | Weaver LK, Pioli PA, Wardwell K, Vogel SN, Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol. 2007;81:663-671. [PubMed] |
| 25. | Kneidl J, Löffler B, Erat MC, Kalinka J, Peters G, Roth J, Barczyk K. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell Microbiol. 2012;14:914-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Piatkowski A, Grieb G, Das R, Bozkurt A, Ulrich D, Pallua N. Soluble CD163: A novel biomarker for the susceptibility to sepsis in severe burn injuries. Indian J Plast Surg. 2011;44:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 28. | Jude C, Dejica D, Samasca G, Balacescu L, Balacescu O. Soluble CD163 serum levels are elevated and correlated with IL-12 and CXCL10 in patients with long-standing rheumatoid arthritis. Rheumatol Int. 2013;33:1031-1037. [PubMed] |
| 29. | Fan HL, Yang PS, Chen HW, Chen TW, Chan DC, Chu CH, Yu JC, Kuo SM, Hsieh CB. Predictors of the outcomes of acute-on-chronic hepatitis B liver failure. World J Gastroenterol. 2012;18:5078-5083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Feng L, Zhou X, Su LX, Feng D, Jia YH, Xie LX. Clinical significance of soluble hemoglobin scavenger receptor CD163 (sCD163) in sepsis, a prospective study. PLoS One. 2012;7:e38400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Matsushita N, Kashiwagi M, Wait R, Nagayoshi R, Nakamura M, Matsuda T, Hogger P, Guyre PM, Nagase H, Matsuyama T. Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin Exp Immunol. 2002;130:156-161. [PubMed] |
| 32. | Yan Y, Mai L, Zheng YB, Zhang SQ, Xu WX, Gao ZL, Ke WM. What MELD score mandates use of entecavir for ACLF-HBV HBeAg-negative patients? World J Gastroenterol. 2012;18:4604-4609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Zhang Z, Zhang JY, Wang LF, Wang FS. [The relationship of HBeAg status with HBV DNA loads, MELD scores in patients with acute-on-chronic hepatitis B liver failure during terminal phases]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2012;26:51-53. [PubMed] |
| 34. | Valisena S, Palumbo M, Parolin C, Palú G, Meloni GA. Relevance of ionic effects on norfloxacin uptake by Escherichia coli. Biochem Pharmacol. 1990;40:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Su L, Feng L, Liu C, Jiang Z, Li M, Xiao K, Yan P, Jia Y, Feng D, Xie L. Diagnostic value of urine sCD163 levels for sepsis and relevant acute kidney injury: a prospective study. BMC Nephrol. 2012;13:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Ingels C, Møller HJ, Hansen TK, Wouters PJ, Vanhorebeek I, Van den Berghe G. Circulating levels of the shed scavenger receptor sCD163 and association with outcome of critically ill patients. J Clin Immunol. 2013;33:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |