Published online Apr 21, 2013. doi: 10.3748/wjg.v19.i15.2419
Revised: March 15, 2013
Accepted: March 21, 2013
Published online: April 21, 2013
Processing time: 177 Days and 8.4 Hours
AIM: To determine the effects of gastric juice on the development of esophageal adenocarcinoma (EAC).
METHODS: A animal model of duodenogastroesophageal reflux was established in Sprague-Dawley rats undergoing esophagoduodenostomy. The development of EAC and forestomach adenocarcinoma was investigated 40 wk after the treatment. Intraluminal pH and bile of the forestomach were measured.
RESULTS: There were no significant differences in pH (t = 0.117, P = 0.925) or bile (χ2 = 0.036, P = 0.85) in the forestomach before and 40 wk after esophagoduodenostomy. There were also no significant differences between the model and controls during esophagoduodenostomy or 40 wk after esophagoduodenostomy. The incidence of intestinal metaplasia (88%) and intestinal metaplasia with dysplasia and adenocarcinoma (28%) in the esophagus in the model was higher than in the controls 40 wk after surgery (χ2 = 43.06, P < 0.001 and χ2 = 9.33, P = 0.002, respectively) and in the forestomach in the model (χ2 = 32.05, P < 0.001 and χ2 = 8.14, P = 0.004, respectively). The incidence rates of inflammation in the esophagus and forestomach were 100% and 96%, respectively (χ2 = 1.02, P = 0.31) in the model, which was higher than in the esophageal control (6.8%) (χ2 = 42.70, P < 0.001).
CONCLUSION: Gastric juice exposure may not cause intestinal metaplasia with dysplasia or adenocarcinoma of the forestomach and may not be related to EAC.
Core tip: The incidence of esophageal adenocarcinoma (EAC) has rapidly increased, which may be related to the increased incidence of gastroesophageal reflux disease. A better understanding of how refluxate contributes to development of EAC will help decrease the incidence of cancer. We surgically developed a rat model of duodenogastroesophageal reflux and found that although exposure of the forestomach to gastric juice may induce inflammation and mild metaplasia, it does not lead to the development of metaplasia with dysplasia or adenocarcinoma. It is concluded that gastric juice may not be related to the development of EAC.
- Citation: Cheng P, Li JS, Zhang LF, Chen YZ, Gong J. Exposure to gastric juice may not cause adenocarcinogenesis of the esophagus. World J Gastroenterol 2013; 19(15): 2419-2424
- URL: https://www.wjgnet.com/1007-9327/full/v19/i15/2419.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i15.2419
The incidence rate of esophageal adenocarcinoma (EAC) has recently increased more quickly than that of any other malignancies, which has attracted attention[1]. The rapid increase in the incidence of EAC might be related to the increase in that of gastroesophageal reflux disease (GERD) and Barrett’s esophagus[2,3]. The presence of Barrett’s metaplasia with specialized intestinal epithelium is the main risk factor for these tumors. This epithelium is an acquired condition after a particular type of healing from esophageal mucosal injury resulting from reflux disease[4]. Reflux of gastric acid and duodenal juice is the main cause of GERD, and gastric acid has always been regarded as the major risk element in GERD; the main clinical treatment of which is acid suppression[5]. However, because of the rapid increase in the incidence of EAC, the role of gastric acid in the development of GERD remains controversial.
Gastric juice that has refluxed into the esophagus in patients with GERD also contains biliary and pancreatic secretions that have refluxed into the stomach from the duodenum. Early studies have shown that reflux of combined duodenal and gastric juices into the esophagus causes severe esophagitis[6]. Reflux of duodenal juice results in the same degree of esophageal injury in gastrectomized animals[7]. Evidence from both animal models[8-11] and human clinical studies[12] has implicated esophageal exposure to duodenal juice as a key factor in the genesis of specialized intestinal metaplasia and the development of adenocarcinoma. Some researchers believe that acid resistance may be related to the obvious increase in the incidence of EAC[13]. With the development of the dynamic surveying system of duodenal juice, the role of duodenal juice reflux in the pathological process has attracted increasing attention. One study has even confirmed that duodenal juice reflux could induce Barrett’s esophagus and EAC in rats[10].
Therefore, the roles of gastric juice and of bile and pancreatic juice regurgitation in duodenal juice reflux in the development of EAC without exogenous carcinogens should be studied in an animal model of duodenogastroesophageal reflux. The aim of the current study was to investigate the role of gastric juice in the genesis of intestinal metaplasia and EAC in this rat model.
Sixty healthy 8-wk-old Sprague-Dawley rats weighing 200-250 g were purchased from the Experimental Animal Center of Xi’an Jiao Tong University. The paired male and female rats were randomly divided into two groups: sham-operated control (n = 30) and model (n = 30) groups.
A Sprague-Dawley rat model of duodenogastroesophageal reflux was created in accordance with the method of Zhang et al[14] and a sham-operated group was used as the control group. Surgical diversion of duodenal secretions into the esophagus was induced by end-to-side esophagoduodenostomy in the experimental group. All operated rats underwent esophagoduodenostomy. The esophagus was separated from the posterior vagal trunk and left gastric vessels, tied with silk at the gastroesophageal junction, and divided 2 mm proximal to the tie. The anterior vagus nerve was divided when the esophagus was cut and sutured with 16 interrupted stitches of 7-0 polypropylene.
Esophagoduodenostomy was the only procedure performed in 30 animals. The purpose of the anastomosis was to induce reflux of both gastric and duodenal juice into the esophagus. The anterolateral wall of the distal duodenum was opened longitudinally 1 cm from the pylorus, and the broken ends of the esophagus were anastomosed to the duodenal incision.
The sham-operated group included 30 rats. After the rats were paunched, only the lower esophagus and first portion of the duodenum were dissociated.
Operations were performed after an acclimatization period of 4 d. Rats were kept in hanging cages on a 12 h light-dark cycle at a temperature of 21 °C and humidity of 60%. Water and standard chow were given ad libitum. Food was discontinued in the evening before surgery or sacrifice, and water was discontinued in the morning of surgery. Rats were anesthetized with an intramuscular injection of xylazine hydrochloride (18 mg/kg) and ketamine (72 mg/kg), with further doses administered intraperitoneally during surgery as required. Before closure, 0.5 mL-1.5 mL 0.9% sodium chloride was instilled into the peritoneal cavity. Water was permitted when the rats awoke, and chow was provided on the next day. The rats were housed in cages at 22 °C-25 °C with free access to standard rat pellet food and water for 40 wk. Rats were treated following the Guidelines for the Care and Use of Laboratory Animals of the National Animal Welfare Committee.
Intraluminal pH and bile of the forestomach were measured during esophagoduodenostomy with a portable glass electrode pH monitor (Digitrapper MK; Medtronic Synectics, Stockholm, Sweden) and a portable bile monitor (Bilitec 2000; Medtronic Synectics). These parameters were also measured after rats were sacrificed 40 wk after the operation. For duodenal gastric reflux, the 2-min period was considered reflux positive if the bilirubin optical density was > 0.14 and lasted 5 s. An absorbance > 0.14 was used as the Bilitec threshold value[15].
The rats were sacrificed 40 wk after surgery. The esophagus and forestomach were opened longitudinally, and gross pathological changes were examined macroscopically. The samples of the esophagus and forestomach were then fixed in formalin, made into paraffin sections after numbering, and stained with hematoxylin-eosin. The characteristics of the pathological tissues were then observed under a light microscope.
The incidence rates of inflammation, intestinal metaplasia, intestinal metaplasia with dysplasia, and adenocarcinoma in the esophagus and forestomach were analyzed and compared using χ2 tests with SPSS software. Intraluminal pH of the forestomach was compared using t tests. Intraluminal bile of the forestomach was compared using χ2 tests. The level of significance was set at P < 0.05.
Twenty-five model and 29 control rats survived. Six rats died, and the mortality rate was 10%.
There were no significant differences in intraluminal pH (t = 0.117, P = 0.925) (Table 1) or bile (χ2 = 0.036, P = 0.85) (Table 1) in the forestomach between the time of esophagoduodenostomy and 40 wk after the operation in the model rats. There were also no significant differences in pH (t = 0.006, P = 0.99 and t = 0.20, P = 0.87) (Table 1) or bile (χ2 = 0.218, P = 0.64 and χ2 = 0.466, P = 0.495) (Table 1) in the forestomach between model and control rats at the time of esophagoduodenostomy and 40 wk after the operation.
| Model (n = 25) | Control (n = 29) | |||
| pH (mean ± SD) | Bile positive (n) | pH (mean ± SD) | Bile positive (n) | |
| At the time of esophagoduodenostomy | 3.22 ± 0.29 | 2 | 3.23 ± 0.29 | 3 |
| 40 wk after esophagoduodenostomy | 3.24 ± 0.31 | 2 | 3.25 ± 0.25 | 4 |
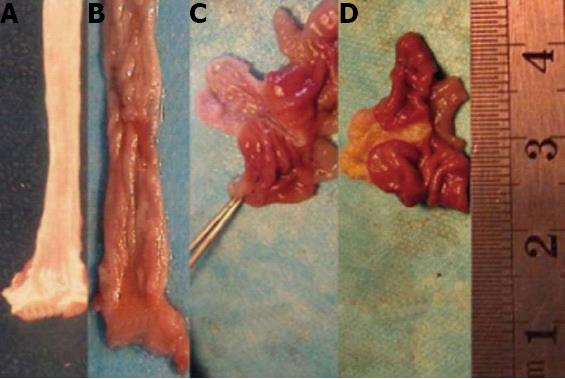
In the sham-operated group, the esophagus and forestomach walls were thin, the mucosa was smooth, and the blood vessels below the mucous membrane were visible with occasional changes consistent with congestive inflammation. In the animal models, inflammation and intestinal metaplasia differed in the esophagus and forestomach. Inflammation appeared as mucosal hyperplasia characterized by a thickened, rough surface with both small and large kernels or mild erosion and ulceration. Intestinal metaplasia appeared as a smooth and velvet-like surface. Adenocarcinoma in the forestomach had not developed. However, adenocarcinoma in the esophagus had developed and was characterized by nodular hyperplasia, ulceration, and a fish-like appearance (Figure 1).
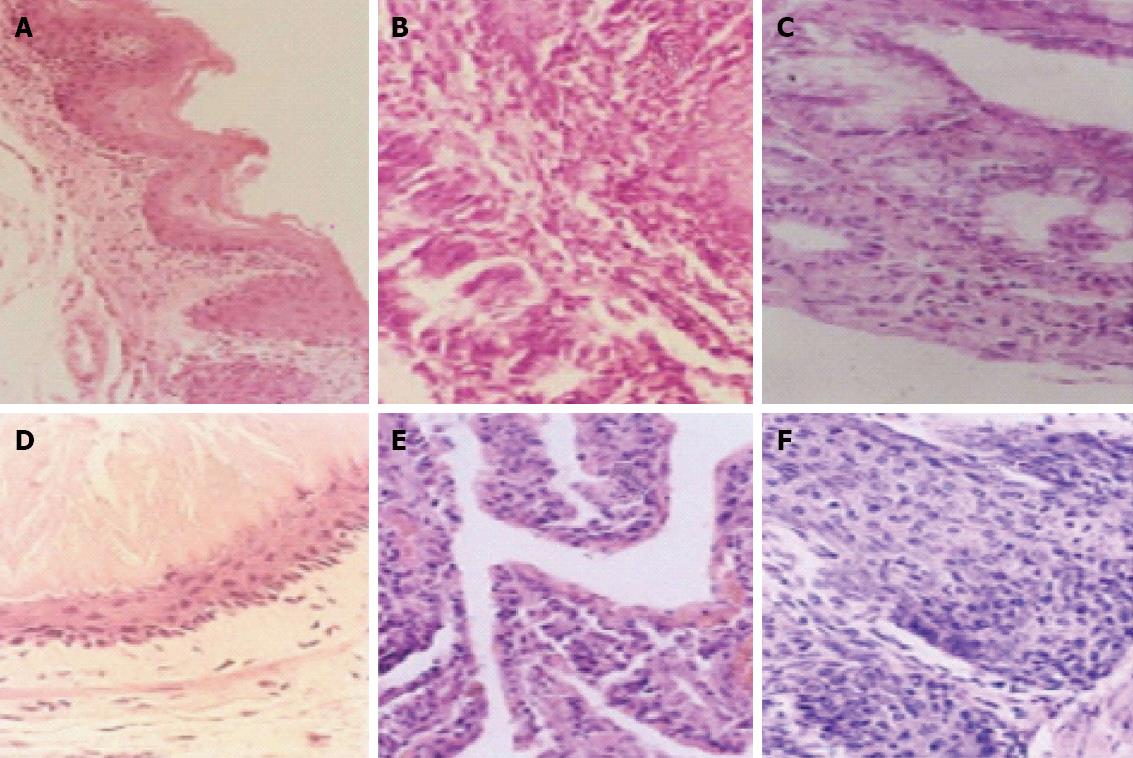
Normal forestomach and esophageal epithelia appeared as stratification of squamous epithelium in neat rows, and some showed keratinization. Inflammation in the forestomach and esophagus appeared as hyperplasia of scaly epithelial basal cells, excessive keratinization of papillomatosis, visible neutrophilic granulocytes, infiltration of lymphoepithelioid cells, and mucosal erosion and edema of the submucosa and lower layer of the mucosa. Intestinal metaplasia was characterized by replacement of the squamous mucosa with simple columnar epithelium. EAC was characterized by severe intestinal metaplasia with dysplasia, pathological invasion of the basilar membrane, and some invasion of the blood or lymphatic vessels (Figure 2).
The incidence of intestinal metaplasia (88%) or intestinal metaplasia with dysplasia and adenocarcinoma (28%) in the esophagus in model rats was higher than in the control rats 40 wk after surgery (χ2 = 43.06, P < 0.001 and χ2 = 9.33, P = 0.002, respectively) (Table 2). In model rats, the incidence of inflammation in the esophagus and forestomach was 100% and 96%, respectively (χ2 = 1.02, P = 0.31). However, the rates of intestinal metaplasia (8%) and intestinal metaplasia with dysplasia and adenocarcinoma (0%) in the forestomach were lower than those in the esophagus (χ2 = 32.05, P < 0.001 and χ2 = 8.14, P = 0.004, respectively) (Table 2). In a comparison of model and control rats 40 wk after creating the models, the incidence of inflammation in the forestomach was 96% and 6.8%, respectively (χ2 = 42.70, P < 0.001), and the incidence of intestinal metaplasia was 12% and 3.4%, respectively (χ2 = 1.43, P = 0.32).
| Inflammation | Intestinal metaplasia | Intestinal metaplasia with dysplasia | Adenocarcinoma | |
| Control esophagus (n = 29) | 2 (6.8) | 1 (3.4) | 0 | 0 |
| Model esophagus (n = 25) | 24 (96) | 22 (88) | 5 (20) | 2 (8) |
| Control forestomach (n = 29) | 2 (6.8) | 1 (3.4) | 0 | 0 |
| Model forestomach (n = 25) | 25 (100) | 2 (8) | 0 | 0 |
Rat stomach has a nonglandular forestomach and glandular portions separated by the limiting ridge. The forestomach, which is the proximal compartment of the stomach in many animal species, is especially well developed in rats. Its function is storage and predigestion of food, and histologically it is covered with esophageal-type mucosa; thus, the rodent forestomach is considered to be a dilation of the lower esophagus. In small laboratory rodents that are commonly used for carcinogenicity studies (rats, mice and hamsters), the forestomach comprises about 50% of the gastric surface[16].
The issues discussed above raise obvious questions about the predictive value of forestomach carcinogenesis. Indeed, the chronic animal study is regarded as the most predictive test for carcinogenicity in humans, and anatomical or physiological interspecies differences that might result in different tumor patterns are generally tolerated without seriously affecting the weight of evidence[17]. It would be provocative to consider the forestomach as a model for the human esophagus from anatomical and histological points of view; especially for studying the mechanism of action of human esophageal cancer[18]. However, several attempts to demonstrate similar reactivity for the esophageal and forestomach mucosa in various species have been unsuccessful.
GERD refers to conditions in which gastric and duodenal contents are regurgitated into the esophagus, which causes pathological mucosal lesions and esophageal changes[19]. Gastroesophageal reflux could cause EAC. The incidence rate of the latter has increased significantly in recent years and has taken the lead among all tumors[20]. The yearly increase in the incidence of GERD has been accompanied with an increasing trend in the incidence of EAC. Clinical epidemiology has shown that gastroesophageal reflux correlates closely with EAC[21].
The mechanism of induction of EAC by gastroesophageal reflux has been a hot research topic[22]. Recent studies have shown that reflux of both gastric and duodenal juice can damage the esophageal mucosa[23]. However, which contents are related to induction of EAC by gastroesophageal reflux is still controversial[24].
Gastric acid is considered to be an important factor in GERD[25]. Gastric acid and duodenal juice reflux is the main cause of GERD, and gastric acid has always been regarded as the major risk element in GERD; the main clinical treatment of which is acid suppression[5]. However, because of the rapid increase in the incidence of EAC, the role of gastric acid in the development of GERD remains controversial. Proton-pump inhibitors (PPIs) have not prevented recent increases in EAC[26]. Three large studies have examined PPI usage and EAC risk in Barrett’s esophagus patients; each reporting a strong inverse correlation. Two studies have shown a decreased risk with longer duration of PPIs, and one an increased risk with delayed PPI use[27].
We investigated the effects of gastric acid on intestinal metaplasia with dysplasia and malignant transformation of stratified squamous epithelium in the forestomach to study the specific factors involved in the induction of EAC through the surgical establishment of an animal model of duodenogastric reflux.
Surgical establishment of a duodenogastroesophageal reflux rat model showed that the forestomach developed abnormal changes. Most of the lesions were inflammatory (including mucosal damage); very few had intestinal metaplasia, and none had intestinal metaplasia with dysplasia or adenocarcinoma. As a result of surgical retention of the vagus nerve to maintain gastric acid secretion, the pH value in the forestomach was unchanged after the operation. The absence of bile detection explained why there was no obvious duodenal juice reflux in the forestomach. These results indicate that the simple lack of food and long-term stimulation of gastric juice might not cause the stratified squamous epithelium of intestinal metaplasia with dysplasia or adenocarcinoma. The histological structure of the forestomach and esophagus of the rat is the same: both comprise stratified squamous epithelial cells. It can be concluded that long-term stimulation by gastric juice of esophageal stratified squamous epithelial cells may only cause inflammation, mucosal damage, and mild intestinal metaplasia, but no induction of intestinal metaplasia with dysplasia or adenocarcinoma.
However, due to the small sample size of the study, the observation time was short, and there may be some limitations to the results. Nevertheless, the result provides new ideas and methods for the pathogenesis of EAC.
The incidence of esophageal adenocarcinoma (EAC) is currently rising faster than any other cancers in the Western world, although the cause of this increase is largely unknown. However, the relationship between the specific reflux components and the induction of EAC remains unclear.
Gastroesophageal reflux can cause EAC, and the mechanisms have been the subject of extensive research. The specific gastroesophageal reflux components responsible for EAC remain largely unknown. In this study, the authors demonstrated that stimulation of long-term gastric juice on esophageal stratified squamous epithelial cells may only cause inflammation, mucosal damage, and mild intestinal metaplasia, but no induction of intestinal metaplasia with dysplasia or adenocarcinoma.
Recent reports have highlighted the importance of duodenal juice in the pathogenesis of EAC. This study indicates that forestomach gastric juice exposure does not cause adenocarcinogenesis. The results of this study therefore suggest that gastric juice plays no role in the pathogenesis of EAC.
By understanding of the roles of gastric juice in the pathogenesis of EAC, this study may represent a future strategy for therapeutic intervention in the treatment of patients with EAC.
Metaplasia is the reversible replacement of one differentiated cell type with another mature differentiated cell type. Dysplasia is an expansion of immature cells, with a decrease in the number and location of mature cells. Duodenogastroesophageal reflux is esophagus exposure to gastric and duodenal juice.
The manuscript proposes interesting aspects of the development of EAC (Barrett’s esophagus and carcinoma), although contradictory to the current and past literature.
P- Reviewer Gockel I S- Editor Huang XZ L- Editor A E- Editor Li JY
| 1. | Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54:i1-i5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 964] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 4. | Lee IS, Choi SC, Shim KN, Jee SR, Huh KC, Lee JH, Lee KJ, Park HS, Lee YC, Jung HY. Prevalence of Barrett’s esophagus remains low in the Korean population: nationwide cross-sectional prospective multicenter study. Dig Dis Sci. 2010;55:1932-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Theisen J, Peters JH, Stein HJ. Experimental evidence for mutagenic potential of duodenogastric juice on Barrett’s esophagus. World J Surg. 2003;27:1018-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Fujikawa H, Saijyo T, Ito S, Ii K. [Studies of experimental model of reflux esophagitis in rats by ligature on both lower portion of duodenum and most of forestomach]. Nihon Shokakibyo Gakkai Zasshi. 1994;91:829-838. [PubMed] |
| 7. | Orel R, Vidmar G. Do acid and bile reflux into the esophagus simultaneously? Temporal relationship between duodenogastro-esophageal reflux and esophageal pH. Pediatr Int. 2007;49:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Kauer WK, Stein HJ. Emerging concepts of bile reflux in the constellation of gastroesophageal reflux disease. J Gastrointest Surg. 2010;14:S9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Chen KH, Mukaisho K, Sugihara H, Araki Y, Yamamoto G, Hattori T. High animal-fat intake changes the bile-acid composition of bile juice and enhances the development of Barrett’s esophagus and esophageal adenocarcinoma in a rat duodenal-contents reflux model. Cancer Sci. 2007;98:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Miyashita T, Ohta T, Fujimura T, Ninomiya I, Fushida S, Hattori T, Miwa K. Duodenal juice stimulates oesophageal stem cells to induce Barrett’s oesophagus and oesophageal adenocarcinoma in rats. Oncol Rep. 2006;15:1469-1475. [PubMed] |
| 11. | Miyashita T, Miwa K, Fujimura T, Ninomiya I, Fushida S, Shah FA, Harmon JW, Hattori T, Ohta T. The severity of duodeno-esophageal reflux influences the development of different histological types of esophageal cancer in a rat model. Int J Cancer. 2013;132:1496-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Freedman J, Ye W, Näslund E, Lagergren J. Association between cholecystectomy and adenocarcinoma of the esophagus. Gastroenterology. 2001;121:548-553. [PubMed] |
| 13. | Theisen J, Peters JH, Fein M, Hughes M, Hagen JA, Demeester SR, Demeester TR, Laird PW. The mutagenic potential of duodenoesophageal reflux. Ann Surg. 2005;241:63-68. [PubMed] |
| 14. | Zhang T, Zhang F, Han Y, Gu Z, Zhou Y, Cheng Q, Zhu Y, Zhang C, Wang Y. A rat surgical model of esophageal metaplasia and adenocarcinoma-induced by mixed reflux of gastric acid and duodenal contents. Dig Dis Sci. 2007;52:3202-3208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Chen H, Li X, Ge Z, Gao Y, Chen X, Cui Y. Rabeprazole combined with hydrotalcite is effective for patients with bile reflux gastritis after cholecystectomy. Can J Gastroenterol. 2010;24:197-201. [PubMed] |
| 16. | Aqeilan RI, Hagan JP, Aqeilan HA, Pichiorri F, Fong LY, Croce CM. Inactivation of the Wwox gene accelerates forestomach tumor progression in vivo. Cancer Res. 2007;67:5606-5610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Oba M, Miwa K, Fujimura T, Harada S, Sasaki S, Oyama K, Ohta T, Hattori T. A selective cyclooxygenase-2 inhibitor prevents inflammation-related squamous cell carcinogenesis of the forestomach via duodenogastric reflux in rats. Cancer. 2009;115:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Proctor DM, Gatto NM, Hong SJ, Allamneni KP. Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci. 2007;98:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Armstrong D, Sifrim D. New pharmacologic approaches in gastroesophageal reflux disease. Gastroenterol Clin North Am. 2010;39:393-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Buxbaum JL, Eloubeidi MA. Endoscopic evaluation and treatment of esophageal cance. Minerva Gastroenterol Dietol. 2009;55:455-469. [PubMed] |
| 22. | Herbella FA, Patti MG. Gastroesophageal reflux disease: From pathophysiology to treatment. World J Gastroenterol. 2010;16:3745-3749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (3)] |
| 23. | Lahiri S, Singh P, Singh S, Rasheed N, Palit G, Pant KK. Melatonin protects against experimental reflux esophagitis. J Pineal Res. 2009;46:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Grotenhuis BA, van Lanschot JJ, Dinjens WN, Wijnhoven BP. The pathogenesis of Barrett’s metaplasia and the progression to esophageal adenocarcinoma. Recent Results Cancer Res. 2010;182:39-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Miner PB. Review article: physiologic and clinical effects of proton pump inhibitors on non-acidic and acidic gastro-oesophageal reflux. Aliment Pharmacol Ther. 2006;23:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Attwood SE, Harrison LA, Preston SL, Jankowski JA. Esophageal adenocarcinoma in “mice and men”: back to basics! Am J Gastroenterol. 2008;103:2367-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Islami F, Kamangar F, Boffetta P. Use of proton pump inhibitors and risk of progression of Barrett’s esophagus to neoplastic lesions. Am J Gastroenterol. 2009;104:2646-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |