Published online Apr 21, 2013. doi: 10.3748/wjg.v19.i15.2319
Revised: December 19, 2012
Accepted: March 6, 2013
Published online: April 21, 2013
AIM: To investigate the effect of biliary drainage on inducible nitric oxide synthase (iNOS), CD14 and TGR5 expression in rats with obstructive jaundice (OJ).
METHODS: Male adult Sprague-Dawley rats were randomly assigned to four groups: OJ, sham operation (SH), internal biliary drainage (ID) and external biliary drainage (ED). Rat models were successfully established by two operations and succumbed for extraction of Kupffer cells (KCs) and liver tissue collection on the 8th and 15th day. KCs were isolated by in situ hepatic perfusion and digested with collagen IV, density gradient centrifuged by percoll reagent and purified by cell culture attachment. The isolated KCs were cultured with the endotoxin lipopolysaccharide (LPS) with and without the addition of ursodeoxycholic acid (UDCA). The expression of iNOS, CD14 and bile acid receptor-TGR5 protein in rat liver tissues was determined by immunohistochemistry. The expression of iNOS and CD14 messenger RNA (mRNA) on the isolated KCs was detected by reverse transcription polymerase chain reaction (PCR) and the TGR5 mRNA level in KCs was measured by real-time quantitative PCR.
RESULTS: The iNOS protein was markedly expressed in the liver of OJ rats, but rare expressed in SH rats. After relief of OJ, the iNOS expression was decidedly suppressed in the ID group (ID vs OJ, P < 0.01), but obviously increased in rats of ED (ED vs OJ, P = 0.004). When interfered only with LPS, the expression of iNOS mRNA by KCs was increased in the OJ group compared with the SH group (P = 0.004). After relief of biliary obstruction, the iNOS mRNA expression showed slight changes in the ED group (ED vs OJ, P = 0.71), but dropped in the ID group (ID vs OJ, P = 0.001). Compared with the simple intervention with LPS, the expressions of iNOS mRNA were significantly inhibited in all four groups after interfered with both LPS and UDCA (P < 0.01, respectively). After bile duct ligation, the CD14 protein expression in rat liver was significantly strengthened (OJ vs SH, P < 0.01), but the CD14 mRNA level by KCs was not up-regulated (OJ vs SH, P = 0.822). After relieving the OJ, the expression of CD14 protein was reduced in the ID group (ID vs OJ, P < 0.01), but not reduced in ED group (ED vs OJ, P = 0.591). And then the CD14 mRNA expression was aggravated by ED (ED vs OJ, P < 0.01), but was not significantly different between the ID group and the SH and OJ groups (ID vs SH, P = 0.944; ID vs OJ, P = 0.513, respectively). The expression of TGR5 protein and mRNA increased significantly in OJ rats (OJ vs SH, P = 0.001, respectively). After relief of OJ, ID could reduce the expression of TGR5 protein and mRNA to the levels of SH group (ID vs SH, P = 0.22 and P = 0.354, respectively), but ED could not (ED vs SH, P = 0.001, respectively).
CONCLUSION: ID could be attributed to the regulatory function of activation of KCs and release of inflammatory mediators.
Core tip: To date, there are still controversies over whether and how to perform preoperative biliary drainage in patients with malignant or benign obstructive jaundice (OJ), even though the complication-related mortality rate for OJ patients was high after surgery. Internal biliary drainage could reverse the raised expression of inducible nitric oxide synthase and CD14 both in protein and messenger RNA levels in obstructive jaundice rat models, but external drainage could not. The mechanism of internal biliary drainage superior to external drainage in relief of obstructive jaundice might be attributed to the regulatory function of activation of Kupffer cells and release of inflammatory mediators.
- Citation: Wang ZK, Xiao JG, Huang XF, Gong YC, Li W. Effect of biliary drainage on inducible nitric oxide synthase, CD14 and TGR5 expression in obstructive jaundice rats. World J Gastroenterol 2013; 19(15): 2319-2330
- URL: https://www.wjgnet.com/1007-9327/full/v19/i15/2319.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i15.2319
To date, there are still controversies over whether and how to perform preoperative biliary drainage in patients with malignant or benign obstructive jaundice (OJ), even though the complication-related mortality rate for OJ patients was high after surgery. One of the controversies is the necessity of relieving biliary obstruction before surgery[1-4]. Some investigators suggested that preoperative biliary drainage should not be routinely performed in OJ patients planned for surgery due to the complications associated with the procedure itself, which might outweigh the potential benefit of it[1,2]. On the contrary, other investigators confirmed the effect of preoperative biliary drainage in reducing the postoperative morbidity and mortality, complications of infection and hospital stay in patients with OJ[3,4]. The second debate is which is the appropriate drainage method, internal biliary drainage (ID) or external biliary drainage (ED)[5-8]? Some studies suggested that ID (e.g., biliary stent endoprosthesis) could recover the enterohepatic circulation of bile acid, improve the intestinal barrier function and reduce endotoxin-related complications more obviously than ED, and therefore, ID may contribute to the early recovery and improve the patients’ life quality compared with ED[5,6]. On the contrary, some systematic reviews reported that ED (e.g., percutaneous transhepatic biliary drainage, endoscopic nasobiliary drainage and T-tube drainage) was superior to ID, because ID could increase the risk of retrograde cholangitis and had lower diagnostic value than ED[7]. Moreover, ED is better than ID concerning the recovery of cellular immunity and liver inflammation in the short term after relief from biliary obstruction[8].
Kupffer cells (KCs) as the resident liver macrophages, constitute a vital component of the reticuloendothelial system, and KCs are critically involved in the pathogenesis of OJ by acting as antigen presenting cells and producing many endotoxin-related inflammatory mediators. So we carried out a series of experimental studies based on the immune function of KC in OJ rat model to address those questions. Our previous experimental studies found that KC from rats with OJ could produce large amounts of endotoxin-mediated nitric oxide and ID was superior to ED in reversing the distorted nitric oxide due to the regulation of inducible nitric oxide synthase (iNOS), messenger RNA (mRNA)[9,10]. ID could reverse the serum levels of endotoxin and proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), but ED could not[10]. Although our earlier studies demonstrated the necessity of relieving obstruction preoperatively and the advantages of ID in relief of OJ in contrast with ED in animal models, but the mechanism is still unclear.
Lipopolysaccharide (LPS, endotoxin) as an abundant component of the cell wall of gram-negative bacteria, could provoke a generalized pro- inflammatory response and stimulate the production of pro-inflammatory mediators through the activation of KC in patients with OJ[11]. Recent investigations showed that CD14, one of the most important LPS receptors, could play an important role in the activation of KC and LPS-mediated liver injury[12,13]. We proposed a hypothesis that CD14 as a receptor of endotoxin might play an important role in the immune suppression in OJ, and related to the effects of biliary drainage.
Bile acids could successfully reduced endotoxin related complications following surgery in patients with OJ, and the immunomodulatory function of bile acids is obtaining more attention[14,15]. Recently, the plasma membrane bound, G-protein coupled bile acid receptor TGR5 (Gpbar-1, M-Bar) has been first described by two separate research groups[16,17]. TGR5 is highly expressed in CD14-positive monocytes/macrophages[16]. Studies from Keitel et al[18] demonstrated that TGR5 was localized in the plasma membrane of isolated KC, and bile acids could alter macrophage function by affecting phagocytic activity and inhibiting LPS-induced cytokines expression in KC via TGR5-cAMP dependent pathways. The immunoreactivity of TGR5 in KC was increased in rat livers following bile duct ligation, suggesting that TGR5 may play a protective role in OJ preventing excessive cytokine production, thereby reducing liver injury.
This study aims to detect the protein expression of iNOS, CD14 and TGR5 in liver and the mRNA expression in isolated KCs, and to investigate the immunomodulatory effect of ursodeoxycholic acid (UDCA) in terms of the expression of iNOS mRNA after relief of OJ by ID and ED in rats, in order to further explore the mechanism whether ID is superior to ED in relief of OJ.
Two hundred and forty adult male Sprague-Dawley rats weighing 270-350 g were used in the study. All animals were purchased from the Laboratory Animal Center of Academy of Military Medical Sciences [License: SCXK (Jun) 2007-004] and housed in the Experimental Animal Service Center of the Chinese People’s Liberation Army General Hospital. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee of the General Hospital of the Chinese People’s Liberation Army. Rats were fed with a standard diet of commercial rat chow and tap water ad libitum. The experiment was approved by the Animal Research Ethics Committee of the General Hospital of the Chinese People’s Liberation Army.
Animal models were induced using a modified method in our previous study[19]. In brief, rats were randomly assigned to four groups: OJ, sham operation (SH), ID and ED groups. All procedures were performed under anesthesia with 1.5% isoflurane and 98.5% oxygen using a delivery and scavenging system designed in our laboratory[19]. Rats were subjected to laparotomy twice every seven days. OJ was induced by common bile duct ligation and SH was produced by separating bile duct locally but not dividing. ID was performed by implanting a drainage tube between the dilated end of common bile duct and duodenum, while ED was performed by exteriorizing a drainage tube at the nape of the rat. The rat models were succumbed for extraction of KC and liver tissue collection on the 8th and 15th day.
The caudate lobe of the liver was removed and the blood vessels were tied off for facilitating the isolation of KC in site perfusion. Liver tissues were fixed immediately in 10% neutral buffered formalin and paraffin-embedded blocks were made. Serial sections 5 μm thick were stained with hematoxylin and eosin for evaluation of portal inflammation, hepatocellular necrosis and inflammatory cell infiltration. Sections were examined under a light microscope (CMS800, Olympus, Tokyo, Japan).
The immunohistochemical staining was performed on sections of liver samples. Briefly, 5 μm paraffin sections were deparaffinized with xylene and rehydrated in a gradient of ethanol solutions. Antigen retrieval was carried out by microwave heating the sections for 20 min in citric acid buffer, and then cooling for 15 min at room temperature (RT). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide at RT for 10 min. Nonspecific binding was blocked by incubating the sections for 10-15 min in the normal goat serum (5%, 100-150 μL). The sections were incubated overnight at 4 °C with the anti-iNOS antibody (rabbit polyclonal antibody against iNOS; Santa Cruz, CA, United States) at a dilution of 1:200, the anti-CD14 antibody (rabbit polyclonal antibody against CD14, BA0719, Boster Biotechnology, Wuhan, China) at a dilution of 1:200 and the anti-TGR5 antibody (rabbit polyclonal antibody against TGR5, SC-98888, Santa Cruz, CA, United States) at a dilution of 1:100, respectively. Following several rinses in phosphate buffered solution, the sections were incubated with the biotinylated goat anti-rabbit immunoglobulin G antibody (Zhongshan Jinqiao Biotechnology, Beijing, China) for 30 min at 37 °C. Finally, the sections were colored with DAB at RT for 1-15 min, counterstained with hematoxylin for 30 s, dehydrated through gradient ethanol, cleared in xylene and then mounted with permount. Images were obtained using a light microscope (CMS800; Olympus, Tokyo, Japan). Immunoreactivity of iNOS, CD14 and TGR5 in rat liver was morphometrically identified by the Image Pro Plus 6.0 image analysis software system (Media Cybernetics, MD, United States).
KCs were isolated and purified as previously described by a combination of Percoll gradient centrifuging and traditional attachment method[9]. Briefly, non-parenchymal liver cells were dispersed by retrograde in situ collagenase perfusion from the inferior vena cava to the portal vein by Leffert’s solution with collagenase IV (0.2 mg/mL; Sigma, NY, United States). KCs were purified by centrifugation through the two bands of Percoll gradients, and suspended in RPMI-1640 culture media (Gibco, Carlsbad, CA, United States) containing 1% penicillin/streptomycin and 10% heat-inactivated fetal bovine serum. Moreover, KCs were inoculated into the cell culture dish (Corning, NY, United States) in a humidified incubator with 5% CO2 and 95% air at 37 °C. After 3 h cell culture, the non-adherent cells were washed away with a warm Hanks balanced salt solution, and then KCs attached to the bottom of the dish were used for TGR5 mRNA measurement immediately, cultured continuously for 18 h with LPS at a final concentration of 10 ng/mL for CD14 mRNA detection, and cultured with LPS (10 ng/mL) and LPS (10 ng/mL) + UDCA (0.1 mmol/L) respectively for iNOS mRNA detection[20,21]. Viability of KCs was assessed with trypan blue exclusion test and purity was confirmed by peroxidase staining[9].
The iNOS mRNA expression was measured as described in our previous experiment[10]. Briefly, after the isolated KCs were cultured with LPS (10 ng/mL) or LPS (10 ng/mL) + UDCA (0.1 mmol/L) in vitro for 18 h, the expression levels of iNOS mRNA by KCs in these four groups were detected by reverse transcription polymerase chain reaction (RT-PCR).
Semiquantitative analysis of CD14 mRNA by KC was detected by RT-PCR after the isolated KCs were cultured with endotoxin (10 ng/mL) in vitro for 18 h. Total RNA was extracted by TRIzol reagent from Invitrogen and 1μg RNA was primed with oligo (dT) using a reverse transcriptase kit from Promega according to the manufacturer’s instructions. The sequences of CD14 primer were derived from the published CD14 gene sequences: CD14 mRNA-sense: 5’-CTCAACCTAGAGCCGTTTCT-3’, CD14 mRNA-antisense: 5’-CAGGA TTGTCAGACAGGTCT-3’; β-actin-sense: 5’-ATCATGTTGAGACCTTCAACA -3’, β-actin-antisense: 5’-CATCTCTTGCTCGAAGTCCA-3’[11]. Two μL cDNA production from RT-PCR reaction system was amplified in an automated thermocycler from Eppendorf. The conditions for amplification were as follows: pre-denaturation for 5 min at 94 °C for 1 cycle; denaturation for 1 min at 94 °C, annealing for 1 min at 58 °C and extension for 1 min at 72 °C for a total of 35 cycles of PCR, followed by a final extension for 7 min at 72 °C for 1 cycle. The PCR products were electrophoresed in 1.5% agarose gels containing ethidium bromide and reviewed under the ultraviolet light with the gel documentation system (UVP, Coldspring, Wilmington, DE, United States). Band intensity of each sample was determined using Glow Discharge Spectroscopy image analysis software (Coldspring, Wilmington, DE, United States).
Quantitative analysis of TGR5 mRNA was performed by RTQ-PCR. Total RNA from KCs was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. The quality and quantity of RNA were assessed using 1% agarose gel electrophoresis and spectrophotometric analysis of 260/280 ratios, and then RNA was stored at -70 °C prior to analysis. RNA was reversely transcribed with oligo (dT) primer using a reverse transcriptase kit (Promega, Madison, WI, United States). The resulting cDNA was detected using SYBR Green I dye (Qiagen GmbH, Hilden, Germany) and amplified using the BIOER Linegene-3320 system (Hangzhou Bioer Technology, China). Thermocycling conditions for RTQ-PCR were 1 cycle at 95 °C for 2 min and 45 cycles at 95 °C for 20 s, 60 °C for 25 s and 72 °C for 30 s. The primers were designed by Primer Premier 5.0 and Oligo 6.0 based on GeneBank and β-actin was used as an internal reference gene to normalize the transcript levels. The primer sequences were as follows: TGR5-sense: 5’-CCTGGACCGCCACTTACG-3’, TGR5-antisense: 5’-CCCTGTGAGTAGCCCAGCTAGT-3’; β-actin-sense: 5’-C CCATCTATGAGGGTTACGC-3’, β-actin-antisense: 5’-TTTAATGTCACG CACGATTTC-3’. The relative mRNA levels of TGR5 were measured according to the 2-ΔΔCT method.
Continuous data were expressed as mean ± SD. The one-way analysis of variance, Student Newman Keuls-q test or nonparametric test of K independent sample were used for the continuous data. A P value less than 0.05 was considered statistically significant. All statistical analyses of the experimental data were performed with SPSS 17.0 software (Chicago, IL, United States).
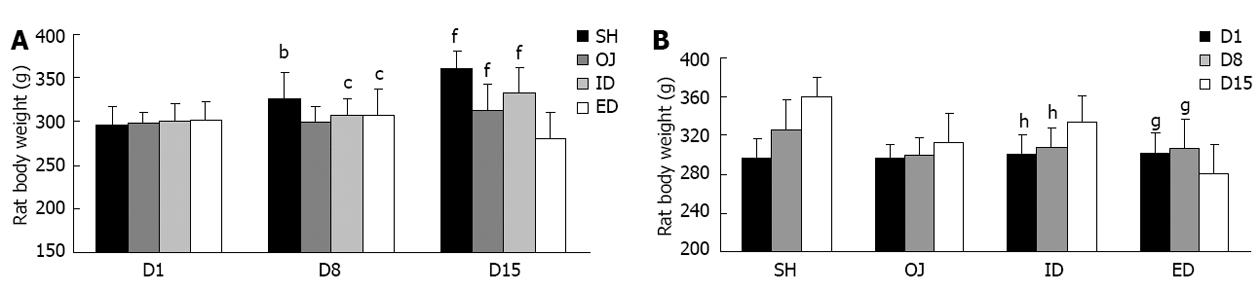
After bile duct ligation, the main cause of rat death was biliary leakage. During and after the biliary drainage procedures, hemorrhage and dehydration were the main death reasons. Finally, 126 rats were enrolled into this study and divided into four groups: OJ (n = 32), SH (n = 30), ID (n = 35) and ED (n = 29) groups. Rats were kept under veterinary care and body weights were measured on the 1st, 8th and 15th day before laparotomy (Table 1). After bile duct ligation, skin stained yellow and lethargy were observed in OJ rat models. OJ rats ate markedly less food and there was a slowly increasing trend of body weight between the 1st, 8th and 15th day (P = 0.155). On the contrary, weight gain was more significant in SH rats compared with that in OJ rats both on the 8th and 15th day (P < 0.01). After relief of OJ, the appetite of ID rats recovered and the body weight of ID rats on the 15th day was significantly higher than that on the 8th day (P < 0.01). On the contrary, weight loss was observed in ED rats and the body weight on day 15 was significantly lower than that on the 1st or 8th day (P < 0.05) (Figure 1). Moreover, the bile from ED could stimulate the neck wound and cause local skin inflammation.
| Day | Body weight | |||
| OJ (n = 32) | SH (n = 30) | ID (n = 35) | ED (n = 29) | |
| D1 | 297.65 ± 13.95 | 296.90 ± 20.04 | 300.60 ± 19.29 | 301.90 ± 20.65 |
| D8 | 299.70 ± 17.95 | 326.10 ± 30.16 | 307.60 ± 19.38 | 306.90 ± 29.89 |
| D15 | 312.10 ± 30.11 | 360.20 ± 20.27 | 333.65 ± 28.12 | 281.15 ± 29.99 |
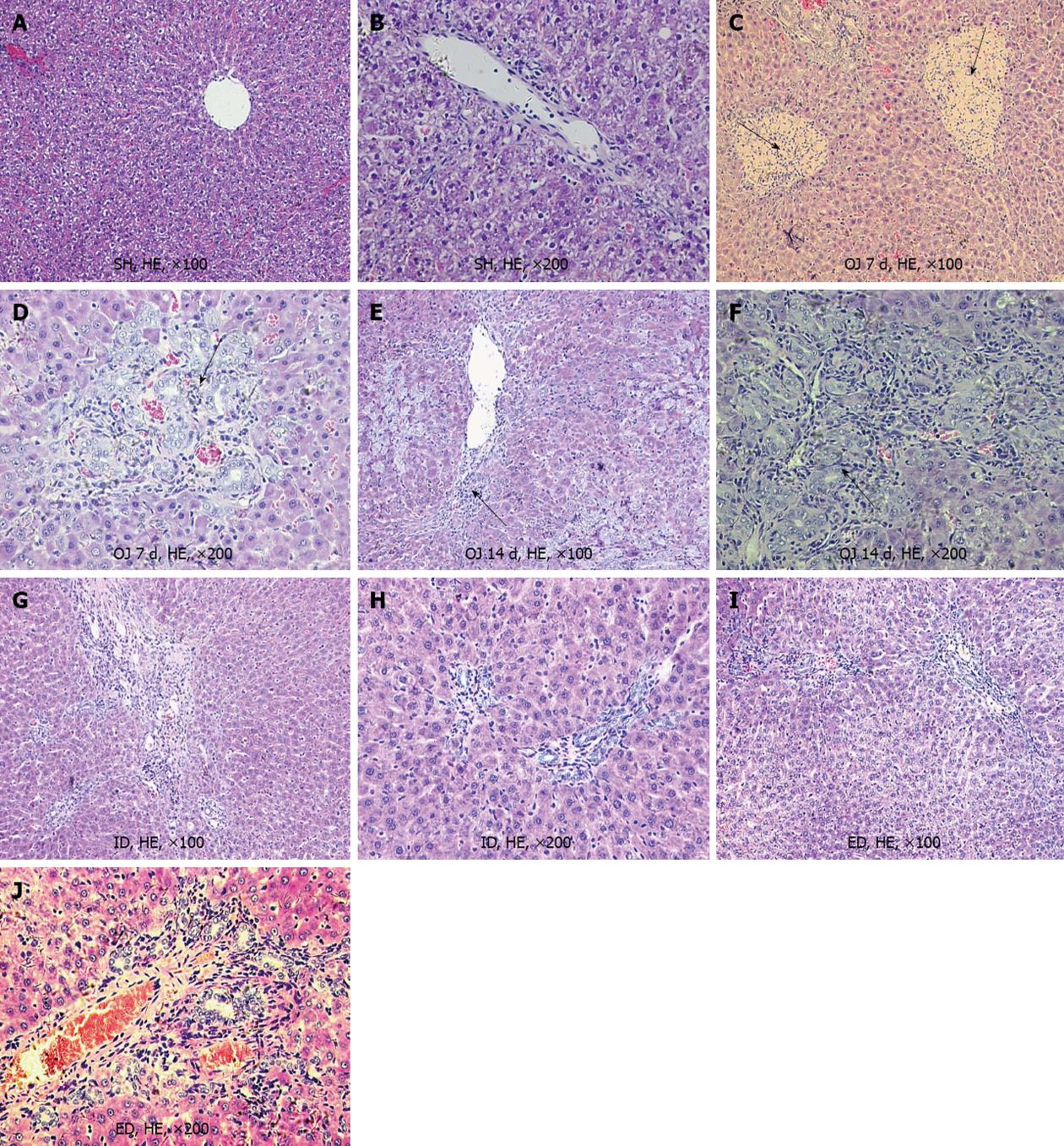
After bile duct ligation for 7 d, the liver of OJ rats showed hepatocellular degeneration and mild bile duct proliferation with acute inflammatory cell infiltration in the portal and periportal areas. Focal necrosis and mild fibrosis were present in the cholestatic liver, but liver cirrhosis did not occur in OJ rats on the 8th day. However, following bile duct ligation for 14 d, the liver showed moderate to severe bile duct proliferation and fibrous expansion of the portal tracts with severe fibrosis and signs of early cirrhosis in the liver (Figure 2). After sham operation for 7 d, histologic study revealed a normal liver lobular architecture and no pathological changes in livers from SH rats. After 7 d of biliary drainage by ID and ED, the liver displayed normal morphological features of hepatocytes and preserved lobular architecture with only mild bile duct proliferation. Furthermore, the progression of fibrosis and cirrhosis stopped and reversal of the progression of cirrhosis and inflammation was observed (Figure 2).
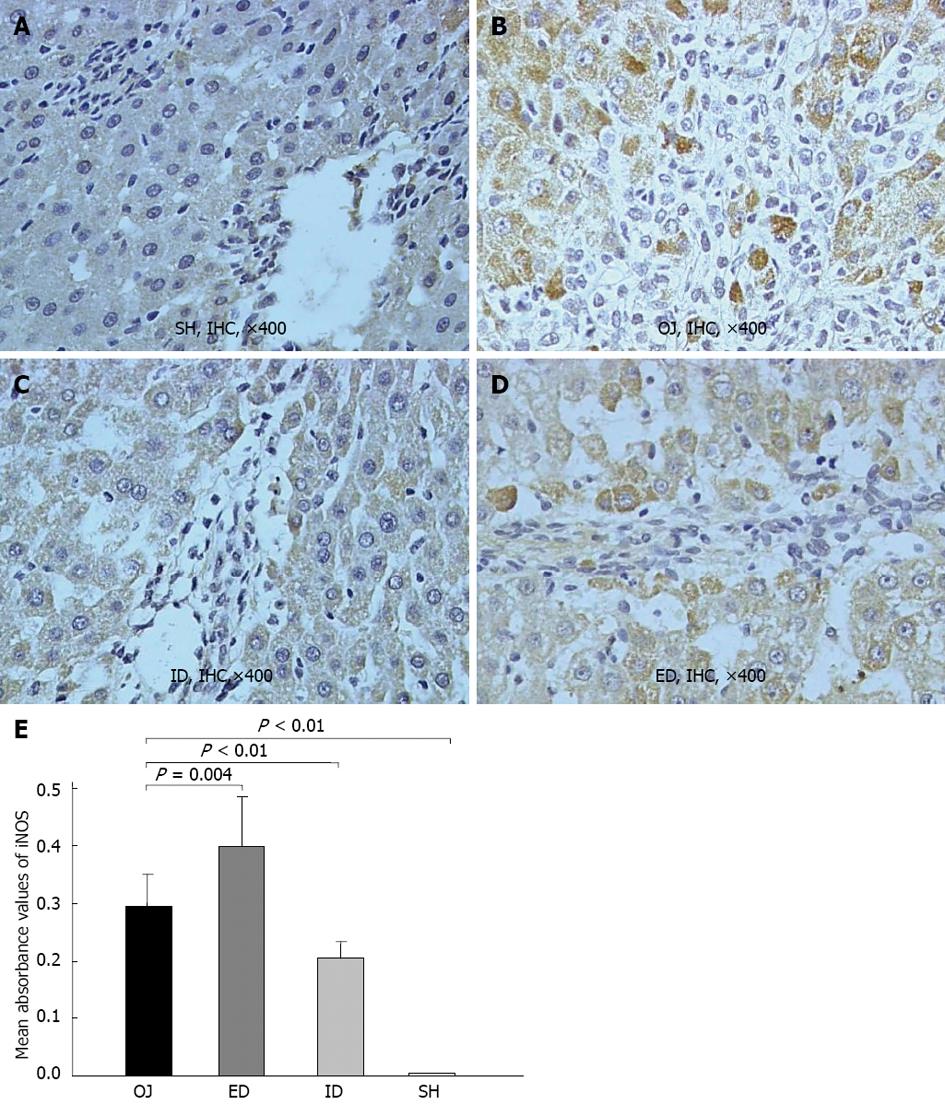
There was rare expression of iNOS protein in SH rats, but the liver of OJ rats markedly expressed iNOS protein in terms of the mean optical density (0.296 ± 0.055). After relief of OJ by ID, the expression of iNOS was noticeably suppressed (0.204 ± 0.029) when compared with the higher expression observed in OJ rat models (ID vs OJ, P < 0.01). However, the expression of liver iNOS obviously increased in rats of ED (0.399 ± 0.086) (ED vs OJ, P = 0.004) (Figure 3).
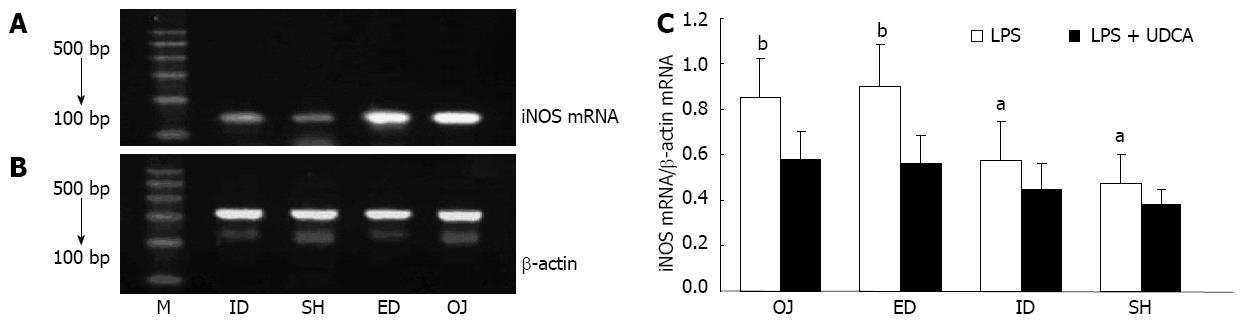
When interfered only with LPS, the expression of iNOS mRNA by KC was stronger in the OJ group (0.58 ± 0.13) than in SH group (0.38 ± 0.07) (OJ vs SH, P = 0.004). After relief of biliary obstruction, iNOS mRNA expression showed slight changes in the ED group (0.59 ± 0.12) (ED vs OJ, P = 0.71), but dropped in the ID group (0.45 ± 0.12) as compared with ED and OJ groups (ID vs ED, P = 0.004; ID vs OJ, P = 0.001). When interfered with LPS and UDCA, inhibited iNOS mRNA expressions by KC were seen in all four groups (Figure 4).
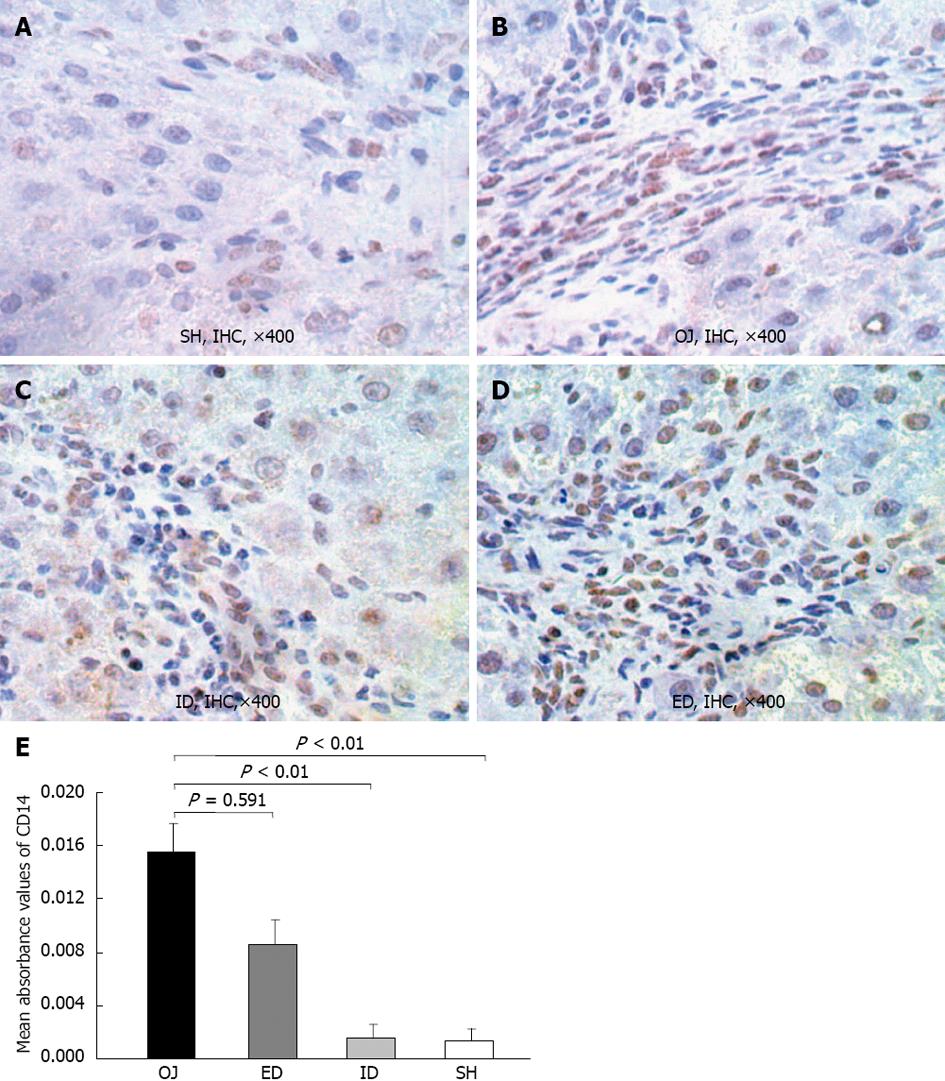
The immunoreactivity of CD14 protein was mainly detected in the membrane of KCs on the edge of liver sinusoid and portal areas. Moreover, in some sinusoidal liver endothelial cells and hepatic stellate cells, the expression of CD14 was also detected, and even a small quantity of positive expression was located on the surface of hepatocytes. Slight intrahepatic expression of CD14 was observed in SH rats (0.0014 ± 0.0008), but after bile duct ligation, the expression of CD14 protein in OJ rats was significantly stronger (0.0156 ± 0.0021) (OJ vs SH, P < 0.01). The expression of CD 14 protein in liver tissues reduced in ID rats (0.0015 ± 0.001) compared with OJ rats (ID vs OJ, P < 0.01), but not reduced in ED rats (0.0086 ± 0.0019) (ED vs OJ, P = 0.591) (Figure 5).
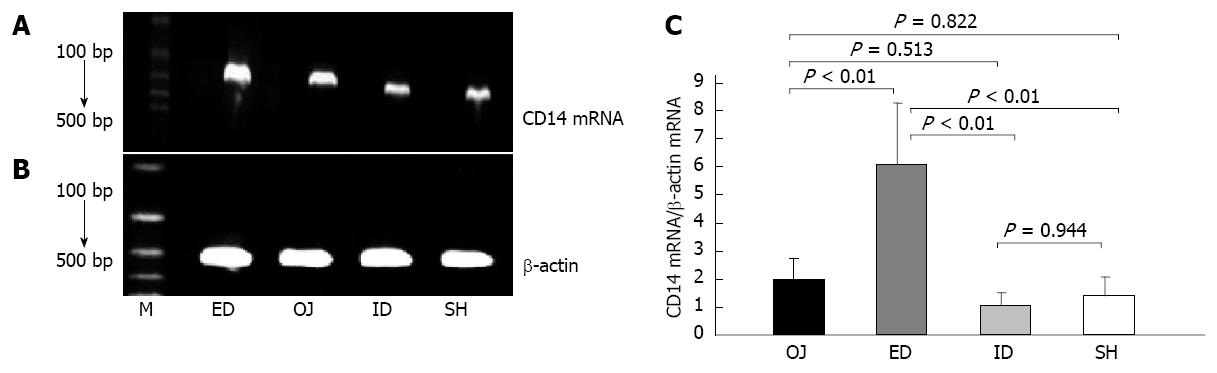
Under the stimulation of LPS, the expression of CD14 mRNA by KC was not strengthened in OJ group (1.998 ± 0.74) compared with that in SH group (1.388 ± 0.683) (OJ vs SH, P = 0.822). After relieving the OJ, the expression of CD14 mRNA was aggravated by ED (6.104 ± 2.171) (ED vs OJ, SH and ID, P < 0.01, respectively), but the expression of CD14 mRNA in ID group (1.018 ± 0.489) was not significantly different compared with that in SH and OJ groups (ID vs SH, P = 0.944; ID vs OJ, P = 0.513, respectively) (Figure 6).
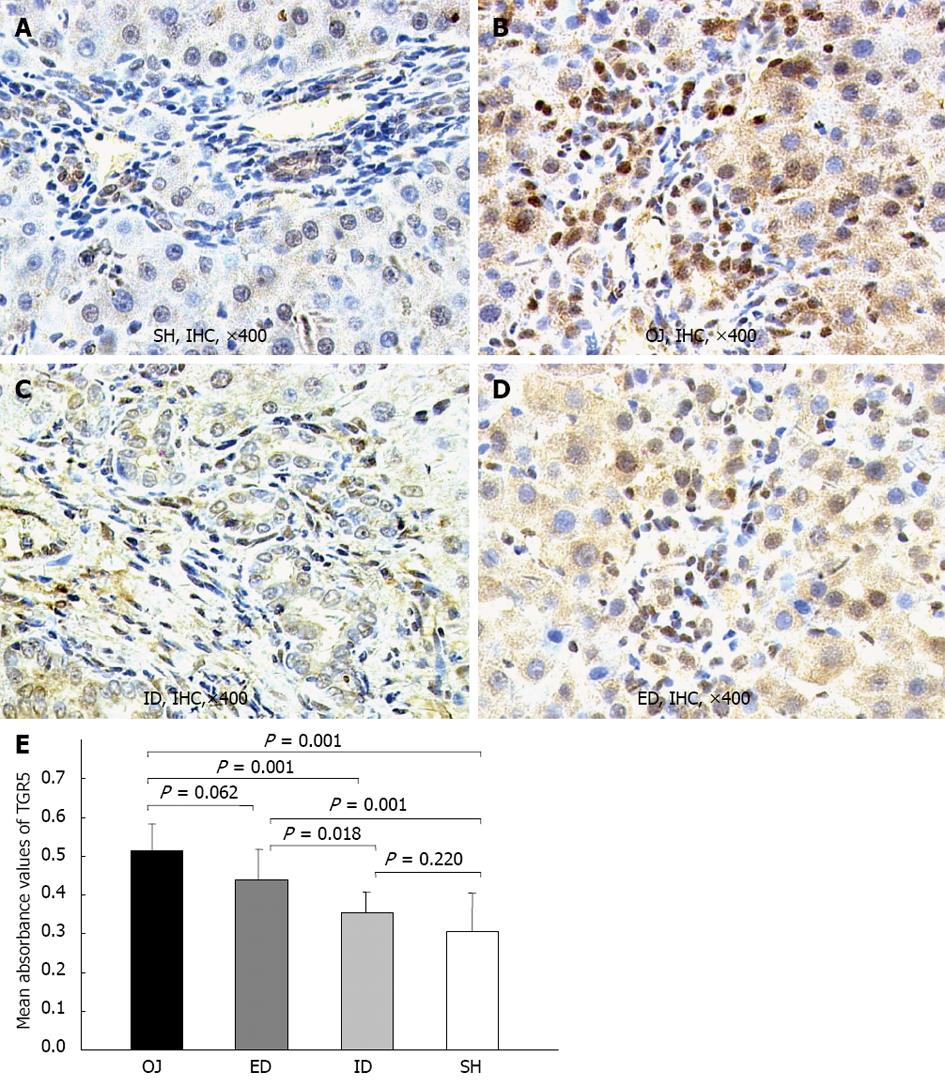
TGR5 was mainly located in the plasma membrane of KCs, and in the cell wall of some sinusoidal endothelial cells and biliary epithelial cells. The expression of TGR5 protein in rat liver was significantly stronger in OJ group (0.513 ± 0.07) than in SH group (0.305 ± 0.01) (P = 0.001). ID could substantially down-regulate the expression level of TGR5 protein (0.356 ± 0.051) (ID vs OJ, P = 0.001) and there was no difference between ID and SH groups (ID vs SH, P = 0.22). On the contrary, the expression of TGR5 protein could not be inhibited by ED (0.439 ± 0.078) (ED vs OJ, P = 0.062) (Figure 7).
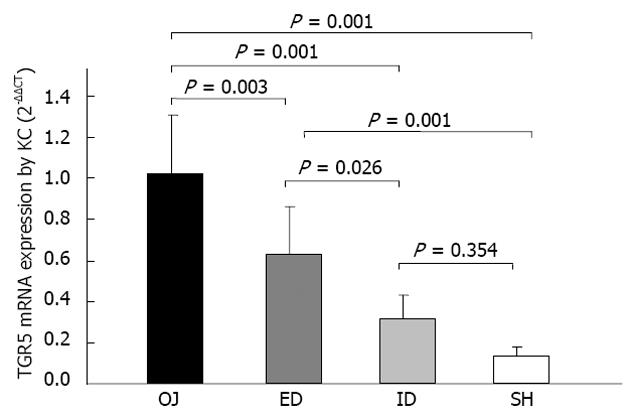
The expression of TGR5 mRNA by KCs was considerably stronger in OJ group (1.024 ± 0.325) (2-ΔΔCT) than in SH group (0.133 ± 0.045) (P = 0.001). There was no significant difference between ID group (0.320 ± 0.115) and SH group (P = 0.354). It was significantly stronger in ED group (0.632 ± 0.233) than in SH group (P = 0.001), and there was significant difference between ED and OJ group (P = 0.003) (Figure 8).
Patients with malignant or benign OJ carry an increased risk of postoperative complications and a high mortality rate. Whether preoperative biliary drainage is still necessary for OJ patients planned for surgery is questioned by many experts[1,4]. Another debate focuses on whether ID is superior to ED in terms of reducing the postoperative mortality and some associated complications[5,6]. During the past decades, many clinical studies have been carried out to address the two controversies, but it is difficult to reach a consensus. So we have performed a series of experimental studies based on the immune function of KCs to present some preliminary perspectives about these questions[9,10,19].
In the present study, we observed that the expression of iNOS protein was markedly enhanced 14 d after bile duct ligation, but rarely expressed in SH rat models, which was in agreement with the results observed in other previous studies[22,23]. Moreover, we found that ID could suppress the expression of iNOS while the expression of iNOS protein was promoted by ED. Our earlier studies have confirmed that KCs from rats with OJ produced large amounts of endotoxin-mediated NO[9]. ID was better than ED in reversing the distorted NO production by KCs based on the activities of iNOS mRNA under the stimulation of LPS[10]. Altogether, these findings underlined that the production of NO in OJ rats could be induced by iNOS in both protein and mRNA levels, and ID was superior to ED in depressing the expression of iNOS.
The major pathogenic role of LPS in the progression of OJ has been supported in previous studies[24,25]. LPS as a substantial component of the outer membrane of gram-negative bacteria, could stimulate the production of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and other mediators (e.g., ROS, NO, iNOS) via CD14/toll-like receptor pathway[23,26,27]. CD14 as one of the most important LPS recognition receptors is responsible for the activation of KCs by pathophysiological concentrations of LPS[12,13]. The data presented here show that the CD14 protein expression in rat liver was substantially up-regulated in the OJ group compared with the SH group. After relieving the OJ by ID, the expression of CD14 protein in liver tissues was significantly reduced, but could not by ED. We investigated whether the induction of CD14 protein expression was correlated with the CD14 mRNA level, and found that the expression of CD14 mRNA by KC was not strengthened on OJ rats compared with SH rats, because the rat models receiving bile duct ligation for 14 d could develop severe fibrosis and cirrhosis in the liver, which influenced the isolation of KCs and the following analysis for CD14 mRNA. ID could decrease the expression of CD14 mRNA in rats with OJ and regulated the sensibility of CD14 gene to endotoxin, but ED enhanced the expression of CD14 mRNA. In addition, our previous studies have already found that the levels of serum endotoxin, TNF-α and IL-6, the production of NO and the expression of iNOS by KCs were increased in OJ rats, and ID could entirely reverse the changes, but external drainage could not[9,10]. Taken together, our results indicated that the LPS receptor-CD14 on the membrane of KCs could play an important role in the immune suppression in OJ, and related to the effects of biliary drainage.
The essential difference between ID and ED may be associated with the re-establishment of enterohepatic circulation of bile acids. Although ED could partially recover damaged liver function, it could not set up normal enterohepatic circulation[19]. A large amount of bile lost through ED resulting in malabsorption of fat, loss of some immune substances as well as imbalance of water and electrolytes. Several studies have confirmed the immunomodulatory function of bile acids, for instance, oral or intravenous administration of UDCA could reduce endotoxin-related complications in OJ[14,15,28]. The anti-inflammatory effect of UDCA is attributed to the inhibition of the production of endotoxin induced pro-inflammatory mediators[20,21,29,30]. In the present study, we found that the expression level of iNOS mRNA by isolated KCs was lower under the stimulation with LPS + UDCA compared with simple stimulation of LPS among the four groups. The consequence confirmed that UDCA had the suppressive effect on the endotoxin.
Recently, the bile acid receptor-TGR5 as a member of the G protein coupled receptors localized at the plasma membrane and internalized into the cytoplasm in response to its activation, has been identified as the first cell surface receptor for bile acids by two different groups respectively[16,17]. TGR5 is highly expressed in CD14 positive monocytes and macrophages[16-18]. KC as the CD14-positive and liver resident macrophages, has a higher expression level of TGR5 mRNA compared with other white blood cells[17]. Several studies have reported that bile acids could inhibit LPS-induced pro-inflammatory cytokine expression in KC via TGR5-dependent pathway[17,18]. The present study found that the immunoreactivity of TGR5 was mainly detected in the plasma membrane of KC and SEC, and also localized in some intracellular compartments, but merely in hepatocytes. There was slight expression of TGR5 in SH rats, but stronger expression in OJ rats. Keitel et al[31] have confirmed that TGR5 staining was not strong in SEC of OJ rats, so the high expression level of TGR5 in OJ rats was specific for KC. After relief of OJ, the TGR5-immunoreactivity could be reversed by ID, but not by ED. We also found that the induction of TGR5 protein expression was correlated with the expression level of TGR5 mRNA. Bile duct ligation could result in higher expression of TGR5 mRNA compared with sham operation. Likewise, ID could down-regulate the expression of TGR5 mRNA, but ED could not. Keitel et al[18] demonstrated the direct link between the protein and gene expression levels of TGR5 and the gene expression of pro-inflammatory cytokines by KCs. Based on our results, the variation of TGR5 was in accordance with the changes of serum endotoxin and pro-inflammatory cytokines among these four groups[9,10]. Altogether, activation of TGR5 in KC could prevent excessive cytokine production, thereby alleviating liver injury, which indicated that the ID was superior to ED in relief of OJ, and the mechanism may be based on the regulation process of TGR5 in both protein and gene levels.
Besides the dysfunction of liver KC leading to the diminished clearance of endotoxin and the production of large amounts of pro-inflammatory cytokines, lack of bile acids in intestine through bile duct ligation could result in the disruption of the epithelial barrier, translocation of bacteria and endotoxin across the mucosa into lymph nodes and remote organ systems, and sometimes could cause lethal endotoxemia[32]. Inagaki et al[33] have confirmed that the farnesoid X receptor (FXR), a nuclear receptor for bile acids, could induce genes involved in neuroprotection and inhibited bacterial overgrowth and mucosal injury in ileum caused by bile duct ligation. Bile acid receptors such as FXR and TGR5, mainly exerting in the lipid and cholesterol metabolism, are increasingly recognized as one of the new frontiers of immunology. These receptors expressed in liver and gut might play an important role in the reaction to inflammation in enterohepatic tissues. Our study demonstrated that ID was superior to ED in relief of OJ, which might be based on the regulation process of TGR5 by KCs, further investigations derived from intestinal macrophages are necessary to elucidate the immunoregulatory role of bile acid receptor.
In conclusion, we found that internal biliary drainage could reverse the raised expression of iNOS and CD14 in both protein and mRNA levels in obstructive jaundice rat models, but external drainage could not. In addition, UDCA could protect KCs from the endotoxin of LPS related to the down-regulation of iNOS mRNA expression. The up-regulation of TGR5 in protein and gene levels in obstructive jaundice could play a protective role in alleviating the inflammatory reaction. The mechanism of internal biliary drainage superior to external drainage in relief of obstructive jaundice might be attributed to the regulatory function of activation of KCs and release of inflammatory mediators.
To date, there are still some controversies over whether and how to perform preoperative biliary drainage in patients with malignant or benign obstructive jaundice (OJ), even though the complication-related mortality rate is high for OJ patients followed by surgery.
During the past decades, many clinical studies have been performed to address the two controversies, but it is difficult to reach a consensus. The authors performed a series of experimental studies based on the immune function of Kupffer cell (KC) in an attempt to offer some preliminary perspectives about these questions.
The authors found that internal biliary drainage could reverse the raised expression of inducible nitric oxide synthase (iNOS) and CD14 in both protein and messenger RNA levels in obstructive jaundice rat models, but external drainage could not. The mechanism of internal biliary drainage superior to external drainage in relief of obstructive jaundice might be attributed to the regulatory function of activation of KC and release of inflammatory mediators.
This is an experimental study concerning superiority of internal biliary drainage to external biliary drainage. Internal biliary drainage could reverse the high expression of iNOS, CD14, and TGR5 in rats with obstructive jaundice, but external drainage could not. The mechanism might be attributed to the regulatory function of activation of KCs and release of inflammatory mediators. The manuscript itself is interesting and includes some new insights of biliary drainage.
P- Reviewers Endo I, Kumar A S- Editor Gou SX L- Editor Ma JY E- Editor Li JY
| 1. | van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 693] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 2. | Eshuis WJ, van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, Kuipers EJ, Coene PP, Kubben FJ, Gerritsen JJ, Greve JW. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Cho HC, Lee JK, Lee KH, Lee KT, Paik S, Choo SW, Do YS, Choo IW. Are endoscopic or percutaneous biliary drainage effective for obstructive jaundice caused by hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2011;23:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | van der Gaag NA, Kloek JJ, de Castro SM, Busch OR, van Gulik TM, Gouma DJ. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009;13:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Kawakami H, Kuwatani M, Onodera M, Haba S, Eto K, Ehira N, Yamato H, Kudo T, Tanaka E, Hirano S. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011;46:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Mok KT, Wang BW, Chang HC, Lin SL. External biliary drainage plus bile acid feeding is not equal to internal drainage in preserving the cellular immunity following prolonged obstructive jaundice. Dig Dis Sci. 2001;46:1864-1870. [PubMed] |
| 7. | Kamiya S, Nagino M, Kanazawa H, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y. The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity, and microflora. Ann Surg. 2004;239:510-517. [PubMed] |
| 8. | Mizuguchi K, Ajiki T, Onoyama H, Tomita M, Kuroda Y. Short-term effects of external and internal biliary drainage on liver and cellular immunity in experimental obstructive jaundice. J Hepatobiliary Pancreat Surg. 2004;11:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Li W, Chan AC, Lau JY, Lee DW, Ng EK, Sung JJ, Chung SC. Superoxide and nitric oxide production by Kupffer cells in rats with obstructive jaundice: effect of internal and external drainage. J Gastroenterol Hepatol. 2004;19:160-165. [PubMed] |
| 10. | Meng Y, Gong YC, Dou Y, Li W. Changes of serum cytokines and expression of inducible nitric oxide synthase mRNA by Kupffer cells after relief from obstructive jaundice in rats. J Gastroenterol Hepatol. 2009;24:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923-927. [PubMed] |
| 12. | Su GL, Goyert SM, Fan MH, Aminlari A, Gong KQ, Klein RD, Myc A, Alarcon WH, Steinstraesser L, Remick DG. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest Liver Physiol. 2002;283:G640-G645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903-914. [PubMed] |
| 14. | Cahill CJ. Prevention of postoperative renal failure in patients with obstructive jaundice--the role of bile salts. Br J Surg. 1983;70:590-595. [PubMed] |
| 15. | Pain JA, Bailey ME. Prevention of endotoxaemia in obstructive jaundice--a comparative study of bile salts. HPB Surg. 1988;1:21-27. [PubMed] |
| 16. | Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435-9440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1227] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 17. | Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298:714-719. [PubMed] |
| 18. | Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 19. | Li W, Chung SC. An improved rat model of obstructive jaundice and its reversal by internal and external drainage. J Surg Res. 2001;101:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Invernizzi P, Salzman AL, Szabó C, Ueta I, O’Connor M, Setchell KD. Ursodeoxycholate inhibits induction of NOS in human intestinal epithelial cells and in vivo. Am J Physiol. 1997;273:G131-G138. [PubMed] |
| 21. | Ma J, Nakajima T, Iida H, Iwasawa K, Terasawa K, Oonuma H, Jo T, Morita T, Imuta H, Suzuki Ji. Inhibitory effects of ursodeoxycholic acid on the induction of nitric oxide synthase in vascular smooth muscle cells. Eur J Pharmacol. 2003;464:79-86. [PubMed] |
| 22. | Arriero MM, López-Farré A, Fryeiro O, Rodríguez-Feo JA, Velasco S, García-Durán M, Fortes J, De La Pinta JC, Muñoz LE, Celdrán A. Expression of inducible nitric oxide synthase in the liver of bile duct-ligated Wistar rats with modulation by lymphomononuclear cells. Surgery. 2001;129:255-266. [PubMed] |
| 23. | Cağlikülekci M, Pata C, Apa DD, Dirlik M, Tamer L, Yaylak F, Kanik A, Aydin S. The effect of N-acetylcysteine (NAC) on liver and renal tissue inducible nitric oxide synthase (iNOS) and tissue lipid peroxidation in obstructive jaundice stimulated by lipopolysaccharide (LPS). Pharmacol Res. 2004;49:227-238. [PubMed] |
| 24. | Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. 2005;241:159-167. [PubMed] |
| 25. | Yorganci K, Baykal A, Kologlu M, Saribaş Z, Hascelik G, Sayek I. Endotoxin challenge causes a proinflammatory state in obstructive jaundice. J Invest Surg. 2004;17:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Bemelmans MH, Gouma DJ, Greve JW, Buurman WA. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992;15:1132-1136. [PubMed] |
| 27. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2272] [Cited by in RCA: 2240] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 28. | Hori Y, Ohyanagi H. Protective effect of the intravenous administration of ursodeoxycholic acid against endotoxemia in rats with obstructive jaundice. Surg Today. 1997;27:140-144. [PubMed] |
| 29. | Greve JW, Gouma DJ, Buurman WA. Bile acids inhibit endotoxin-induced release of tumor necrosis factor by monocytes: an in vitro study. Hepatology. 1989;10:454-458. [PubMed] |
| 30. | Yoshikawa M, Tsujii T, Matsumura K, Yamao J, Matsumura Y, Kubo R, Fukui H, Ishizaka S. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16:358-364. [PubMed] |
| 31. | Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, Häussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Ding JW, Andersson R, Soltesz V, Willén R, Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 1993;25:11-19. [PubMed] |
| 33. | Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920-3925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 892] [Article Influence: 46.9] [Reference Citation Analysis (0)] |