INTRODUCTION
Barrett’s esophagus (BE) is a condition where normal squamous epithelium is replaced by metaplastic intestinal-like columnar epithelium containing goblet cells. This premalignant lesion is associated with a nearly 40-fold increased risk for the development of esophageal adenocarcinoma (EAC), a cancer with poor prognosis, and a median survival of less than one year[1]. EAC is most common in the Caucasian population in the western countries. EAC incidence increased almost six fold between 1975 and 2001[2] and EAC mortality also increased more than sevenfold[2]. Currently, EAC has the fastest growing incidence rate of all cancers in the United States. Approximately 17000 patients will be diagnosed with esophageal cancer in 2012 and about 14600 patients will die of this cancer in the United States[3].
There is overwhelming evidence that BE arises as a consequence of chronic gastroesophageal reflux disease (GERD). GERD is a very common medical condition in the United States affecting 40% of the adult population at least monthly. One third of these patients have erosive esophagitis and 6%-14% of patients undergoing endoscopy for symptomatic GERD have BE[1]. This represents about 2 million people in the United States alone[4]. The rate of transformation to cancer is about 0.1%-0.2% per year[3].
Histopathologic steps in the progression of BE include: (1) metaplasia of the normal esophageal squamous epithelium to a specialized intestinal glandular epithelium; (2) low-grade dysplasia; (3) high-grade dysplasia; and (4) esophageal adenocarcinoma with invasive and metastatic potential. However, little is known, about the signaling pathways promoting the development of metaplasia and dysplasia.
CHRONIC INFLAMMATION AND CYTOKINE DYSREGULATION IN BE
Epidemiological studies and animal models demonstrate that chronic inflammation predisposes to the development of various forms of cancer including gastrointestinal malignancies[5]. In the esophagus, chronic inflammation is triggered by repeated exposure to components of refluxate such as gastric acid and bile acids. Indeed, chronic reflux is the strongest risk factor for the development of BE and EAC[6]. A major regulatory pathway linking inflammation and cancer is activation of nuclear factor κB (NF-κB) signaling. The same pathway initiates transcription of cytokines. In agreement with the inflammatory hypothesis of BE/EAC development, NF-κB is constitutively activated in BE or EAC but is not detected in esophagitis or the adjacent normal esophageal mucosa[7].
Esophageal mucosa damaged by refluxate is commonly infiltrated by inflammatory cells of different lineages. First, the damaged site is infiltrated by neutrophils and monocytes (acute inflammation) followed by lymphocytes and plasma cells primarily at the site of metaplasia (chronic inflammation)[8]. Cytokines that are produced by the inflammatory cells and by Barrett’s epithelium play a crucial role in BE carcinogenesis[9]. Furthermore, noxious compounds, such as reactive oxygen and nitrogen species, released during chronic inflammation may damage DNA and induce mutations that subsequently promote cancer development.
Interestingly, Barrett’s esophagus is characterized by a unique cytokine environment compared to erosive esophagitis. While BE is associated with Th2 cytokines, erosive esophagitis is distinguished primarily by a Th1 cytokines profile[10]. This difference in the cytokine profile does not seem to be simply a result of the development of intestinal metaplasia since the cytokine profile is completely different in the duodenum or the gastric antrum[10]. We analyzed multiple cytokines in human tissues using cytokine arrays[11]. Interleukin-6 (IL-6) levels were consistently increased in BE compared to control tissues. The expression of other cytokines, such as IL-8, was variable and inconsistent.
IL-6 AND CANCER
This review is focused on the IL-6/signal transducer and activator of transcription 3 (STAT3) pathway. IL-6 is a potent, pleiotropic Th2 cytokine that regulates immune defense response. Its release is triggered by tissue damage or infection. IL-6 acts as both a pro-inflammatory and anti-inflammatory cytokine. IL-6 plays a central role in the transition from the acute to the chronic phase of the inflammatory process[12]. Importantly, the IL-6 pathway is one of the most important mechanisms linking inflammation to cancer[13].
IL-6 overexpression is implicated in the pathogenesis of different tumors, including cancers of the ovary, prostate, breast, kidney and lung[14]. IL-6 is also associated with the development of colon cancer, predominantly colitis-associated colon cancer. Recent in vivo evidence shows that IL-6 controls tumor formation and growth in a mouse colitis-associated colon cancer[15]. These studies indicate that the ablation of IL-6 reduces tumor burden, while the elevation of IL-6 levels accelerates tumor formation. The effects of IL-6 are mediated by STAT3. As expected, STAT3 deficiency reduced tumor incidence and growth, while STAT3 hyperactivation had an opposite effect in this model[15]. These studies clearly indicate that IL-6/STAT3 signaling is crucial in the carcinogenesis that is linked to inflammation, such as colitis-associated colon cancer.
Only a few studies investigating the role of IL-6 in esophageal carcinogenesis were reported[11,16,17]. We have shown that IL-6 is secreted from BE and EAC tissues and that phosphorylated STAT3 is expressed in BE and EAC[11,16]. These studies were confirmed by Zhang et al[17]. Non-transformed and transformed human Barrett’s epithelial cell lines were used in this study. Phospho-STAT3 was expressed only by transformed Barrett’s cells, which also exhibited higher levels of IL-6 mRNA and of IL-6 and Mcl-1 proteins than non-transformed Barrett’s cells.
In a recent study, serum IL-6 was significantly increased in esophageal cancer (86%) as compared to carcinoembryonic antigen (30%) and squamous cell cancer antigen (24%)[18]. This was noted for both squamous cell carcinoma of the esophagus (87.1%, 23% and 33%, respectively) and EAC (7%, 39% and 13%, respectively). Interestingly, concentrations of IL-6 depended on distant metastases and patient’ survival[18]. Importantly, both colitis-associated colon cancer and esophageal adenocarcinoma are associated with chronic inflammation. Therefore, elevated IL-6/STAT3 signaling is one of the key pathways involved in esophageal tumorigenesis.
AUTOCRINE PRODUCTION OF IL-6 BY CANCER CELLS
One strategy used by cancer cells to upregulate growth and survival pathways is through autocrine production of growth and survival factors. IL-6 is produced by different cells, including immune cells and epithelial cells[19]. Expression of IL-6 by cancer cells suggests that IL-6 acts as an autocrine growth factor to promote tumorigenesis[20].
But why is IL-6 a crucial factor in tumorigenesis if STAT3 can be activated by other cytokines? Grivennikov et al[21] suggested that tumors choose IL-6 to constitutively activate STAT3, because immune cells together with malignant cells are capable of producing massive amounts of “start-up” IL-6 (but not other family members) required for tumor progression. Indeed, both IL-6 and the IL-6 receptor are expressed in intestinal epithelial cells and these proteins are also increased in colorectal cancers[22]. Importantly, our studies indicate that premalignant BE tissue expresses membrane-bound IL-6 receptor as well as soluble IL-6 receptor (sIL-6R) and secretes increased amounts of IL-6 as BE progresses to esophageal adenocarcinoma (unpublished data)[11,16].
STAT3 AND CANCER
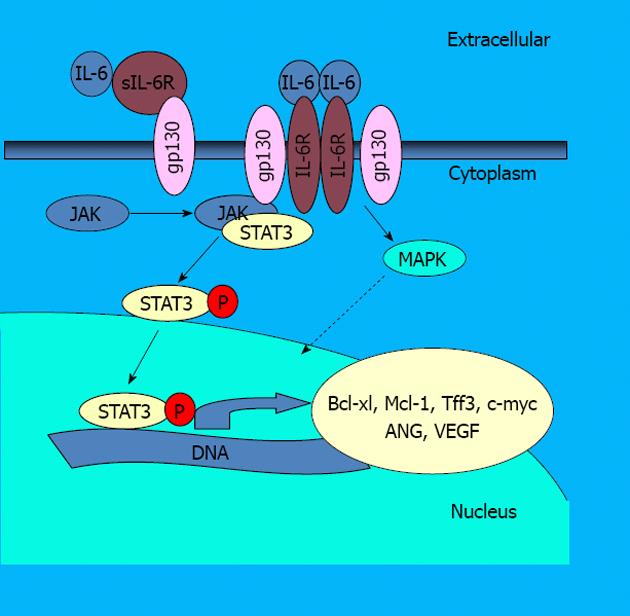
IL-6 activity is mediated through activation of at least three different pathways. First, IL-6 binds to either cognate IL-6 receptor (IL-6Ra) or sIL-6R. Followed by binding to the receptors: (1) IL-6 induces association of signal transducer gp130 and ErbB, which leads to the activation of the MAP kinase pathway and activation of transcription factor NF-IL-6; (2) IL-6 promotes activation of Phosphatidylinositol 3-kinases, a prominent kinase associated with NF-κB activation and apoptosis resistance[23]; and (3) IL-6 signaling is primarily mediated by the Janus kinase (JAK)/STAT pathway (Figure 1). In this pathway the complex of IL-6 and its receptor interacts with the membrane bound gp130[24]. This event leads to the phosphorylation of JAKs and subsequent phosphorylation of the transcription factor STAT3. Activated STAT3 then forms dimers and translocates from the cytoplasm to the nucleus. In the nucleus, STAT3 activates the transcription of specific genes by binding to consensus DNA elements.
Figure 1 Interleukin 6 signaling scheme of the interleukin 6/signal transducer and activator of transcription 3 signaling pathway.
IL-6: Interleukin 6; STAT3: Signal transducer and activator of transcription 3; sIL-6R: Soluble IL-6 receptor; IL-6R: IL-6 receptor; JAK: Janus kinase; MAPK: Mitogen-activated protein kinase; VEGF: Vascular endothelial growth factor; ANG: Angiopoietin.
There are six essential alterations to normal cell physiology, which together define a cancer cell. These include: evasion of apoptosis, self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, limitless replicative potential, tissue invasion and metastasis and sustained angiogenesis[25]. STAT3 participates in the regulation of these processes[26]. Particularly, STAT3 increases the expression of genes that are required for angiogenesis, uncontrolled proliferation and survival[27]. These include genes such as anti-apoptotic genes (Bcl-xL, Mcl1 and survivin), or genes involved in proliferation (c-MYC, cyclin D1) or angiogenesis (vascular endothelial growth factor). All these proteins are associated with tumorigenesis and they are expressed in BE or EAC[11,28-31].
In addition, STAT3 contributes to constitutive NF-κB activation in tumor cells. Recent studies show that STAT3 prolongs NF-κB nuclear retention through acetyltransferase p300-mediated RelA acetylation, thereby interfering with NF-κB nuclear export and thus inducing permanent NF-κB activation. Another important effect of STAT3 is that STAT3 negatively regulates the expression of tumor suppressor gene p53[27]. Importantly, p53 activity can be restored in cells by inhibiting STAT3 signaling[27].
STAT3 REGULATION
The activation of STAT3 is regulated by suppressors of cytokine signaling (SOCS) and protein inhibitors of activated STATs (PIASs). These proteins are often deregulated in different cancers. SOCS-3 negatively regulates activated receptor complexes by inactivating JAKSs or by blocking recruitment sites for STAT3[32]. It also target signaling complexes for ubiquitination and degradation. PIAS3 blocks the DNA-binding activity of STAT3 and inhibits STAT3-mediated gene activation[33]. Silencing of SOCS3 expression due to aberrant methylation of the gene in various cell lines and cancers was reported by He et al[34]. Inactivation of SOCS-3 is frequently observed also in dysplastic Barrett’s esophagus and EAC due to promoter hypermethylation[35]. In normal squamous epithelium and normal gastric mucosa, SOCS-3 methylation was not observed. The expression of PIAS3, another inhibitor of activated STAT3 protein, was also decreased in various cancers including prostate, colon, gastric or brain cancer[36]. However, such studies have not been performed in BE or EAC.
INCREASE IN IL-6 ASSOCIATED WITH CANCER IN MALE
The reasons for the higher prevalence of BE in males are not clear. Similarly to esophageal adenocarcinoma, hepatocellular carcinoma (HCC) is more prevalent in the male population. Recently, Naugler et al[37] identified a possible mechanism for this gender disparity in HCC. They found in a mouse model of HCC that administration of diethylnitrosamine induced an increase in serum IL-6 in males compared to females. In wild type animals the incidence of HCC was 100% in males and only 13% in females. In contrast IL-6-/- males and females exhibited a similar very low incidence of HCC and longer survival[37]. The absence of IL-6 resulted in almost complete inhibition of diethylnitrosamine-induced hepatocarcinogenesis. Their study indicated that estrogen mediated suppression of IL-6 is crucial in preventing hepatocellular carcinoma. Perhaps, a similar mechanism is involved in esophageal tumorigenesis, and that is why males are affected by this disease more often than women.
BILE ACIDS AND APOPTOSIS RESISTANCE IN ESOPHAGEAL CANCER
Avoidance of apoptosis is one of the major characteristics of cancer[25]. The normal squamous epithelium is exposed to low pH and/or hydrophobic bile acids during esophageal reflux. Although a short-term effect of bile acids is the induction of apoptosis, a long-term effect of repeated exposures to bile acids is a selection for cells resistant to apoptosis[38]. These cells have a growth advantage in the presence of agents that ordinarily induce apoptosis and they proliferate to form a field of apoptosis resistant cells[39].
Our data suggest that epithelial cells of Barrett’s tissue are resistant to apoptosis induced by bile acids[40]. These results are consistent with the reported increase in the expression of Mcl-1 and Bcl-xL in BE[40] and studies suggesting that apoptosis resistance may lead to transformation from BE to adenocarcinoma[41].
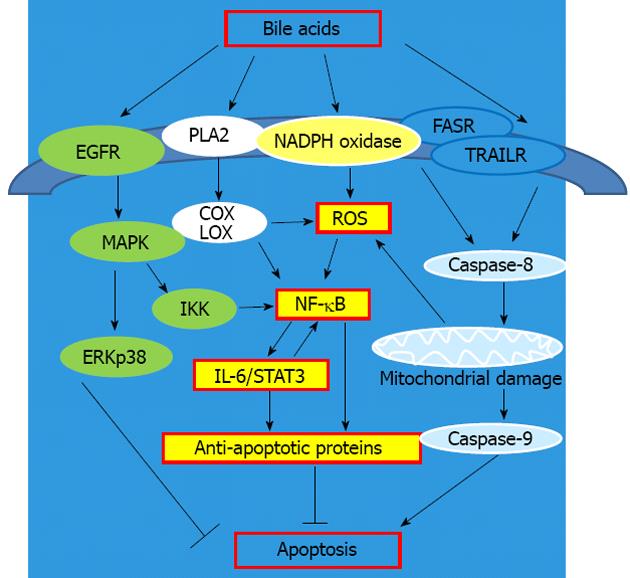
It is clear from many studies that bile acids activate both pro-survival and apoptotic pathways (Figure 2). The classic survival pathways induced by bile acids involve membrane perturbation, the activation of phospholipase A2 and the synthesis of prostaglandins and leukotrienes, catalyzed by cyclooxygenase and lipooxygenase, with reactive oxygen species (ROS) as a byproduct[42]. Bile acids also activate, in a ligand-independent manner, the epidermal growth factor receptor (EGFR) and receptors of the tumor necrosis factor superfamily (e.g., FAS, TRAIL)[43]. Activation of the EGFR pathway is generally pro-survival, whereas activation of the Fas and TRAIL pathways are pro-apoptotic.
Figure 2 Apoptosis and the signaling pathways activated by bile acids.
EGFR: Epidermal growth factor receptor; MAPK: Mitogen-activated protein kinase; IL-6: Interleukin 6; STAT3: Signal transducer and activator of transcription 3; COX: Cyclooxygenase; LOX: Lipooxygenase; IKK: IκB kinase; ROS: Reactive oxygen species; NF-κB: Nuclear factor kappa B; PLA2: Phospholipase A2; NAPDH: Nicotinamide adenine dinucleotide phosphate; ERK: Extracellular-signal-regulated kinase; FASR: FAS receptor; TRAILR: TRAIL receptor.
Hydrophobic bile acids also generate ROS by activation of nicotinamide adenine dinucleotide phosphate-oxidase, phospholipase A2, and by damaging mitochondria[44]. Our studies showed that deoxycholic acid significantly increases levels of superoxide, hydrogen peroxide and peroxynitrite[45]. Furthermore, we reported that human esophageal biopsies produce ROS after exposure to acidified medium containing bile acid cocktail[46]. It was shown that in esophageal cells ROS produced by bile acids directly activate the redox sensitive transcriptional factor NF-κB[47]. Consequently NF-κB upregulates production of different cytokines, such as IL-6, which leads to an increase in STAT3 signaling and expression of anti-apoptotic and prosurvival proteins. Indeed, a recent study showed that IL-6 and activated STAT3 were increased in transformed Barrett’s cells (transfected with H-ras and p53 siRNA)[17].
In addition, Quante et al[48] recently developed L2-IL-1b transgenic mouse model of BE/EAC. In this model human IL-1b is overexpressed in mouse esophagus and forestomach to mimic chronic esophageal inflammation. Furthermore, L2-IL-1b mice were fed 0.2% deoxycholic acid to accelerate the development of BE and EAC. Interestingly, when these transgenic mice were crossed with IL-6-/- mice no metaplastic and/or dysplastic lesions were found[48]. These studies confirm our previous results indicating the importance of IL-6 in BE/EAC development.
DISCUSSION
This review provides an evidence for a strong link between chronic inflammation, IL-6, STAT3 activation and esophageal carcinogenesis. IL-6 is a cytokine that is frequently increased in different cancers. Bile and gastric acid in the refluxate are two of the major factors involved in the pathogenesis of BE. We hypothesize that repeated exposure of esophageal tissue to bile acids leads to IL-6 upregulation, increased activation of STAT3, apoptosis resistance and cancer development. This hypothesis is consistent with the studies demonstrating that tissue biopsies from BE patients (1) secrete large amounts of IL-6; (2) are resistant to apoptosis induced by bile acids; and (3) express activated STAT3 and anti-apoptotic proteins regulated by IL-6/STAT3 signaling, Bcl-xL and Mcl-1[11,16,40].
P- Reviewers Bak YT, Cullen JJ S- Editor Huang XZ L- Editor A E- Editor Li JY