Published online Apr 7, 2013. doi: 10.3748/wjg.v19.i13.2065
Revised: January 25, 2013
Accepted: February 5, 2013
Published online: April 7, 2013
Processing time: 168 Days and 1.2 Hours
AIM: To elucidate the mechanisms of mesenteric vasodilation in portal hypertension (PHT), with a focus on endothelin signaling.
METHODS: PHT was induced in rats by common bile duct ligation (CBDL). Portal pressure (PP) was measured directly via catheters placed in the portal vein tract. The level of endothelin-1 (ET-1) in the mesenteric circulation was determined by radioimmunoassay, and the expression of the endothelin A receptor (ETAR) and endothelin B receptor (ETBR) was assessed by immunofluorescence and Western blot. Additionally, expression of G protein coupled kinase-2 (GRK2) and β-arrestin 2, which influence endothelin receptor sensitivity, were also studied by Western blot.
RESULTS: PP of CBDL rats increased significantly (11.89 ± 1.38 mmHg vs 16.34 ± 1.63 mmHg). ET-1 expression decreased in the mesenteric circulation 2 and 4 wk after CBDL. ET-1 levels in the systemic circulation of CBDL rats were increased at 2 wk and decreased at 4 wk. There was no change in ETAR expression in response to CBDL; however, increased expression of ETBR in the endothelial cells of mesenteric arterioles and capillaries was observed. In sham-operated rats, ETBR was mainly expressed in the CD31+ endothelial cells of the arterioles. With development of PHT, in addition to the endothelial cells, ETBR expression was noticeably detectable in the SMA+ smooth muscle cells of arterioles and in the CD31+ capillaries. Following CBDL, increased expression of GRK2 was also found in mesenteric tissue, though there was no change in the level of β-arrestin 2.
CONCLUSION: Decreased levels of ET-1 and increased ETBR expression in the mesenteric circulation following CBDL in rats may underlie mesenteric vasodilation in individuals with PHT. Mechanistically, increased GRK2 expression may lead to desensitization of ETAR, as well as other vasoconstrictors, promoting this vasodilatory effect.
Core tip: Portal hypertension (PHT) is a life-threatening condition which frequently develops in patients with liver cirrhosis, and has limited treatment options. For many years, endothelin-1 (ET-1) has received considerable interest in the area of liver cirrhosis for its potential contribution to PHT. The aim of the present study was to directly examine the expression of ET-1 and its receptors in the mesentery of rats with PHT, and to clarify how the ET-1 signaling system changed with the development of PHT.
- Citation: Du QH, Han L, Jiang JJ, Li PT, Wang XY, Jia X. Increased endothelin receptor B and G protein coupled kinase-2 in the mesentery of portal hypertensive rats. World J Gastroenterol 2013; 19(13): 2065-2072
- URL: https://www.wjgnet.com/1007-9327/full/v19/i13/2065.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i13.2065
Portal hypertension (PHT) is one of the most significant complications associated with liver cirrhosis, which can give rise to many other severe and often lethal conditions, such as bleeding esophageal varices. Increased resistance to portal blood flow is the primary factor leading to PHT and is aggravated by a hyperdynamic, vasodilated, splanchnic circulation[1]. Though the pathophysiology of PHT is becoming better understood, excepting β-receptor blockers, there is no effective treatment approach for PHT. One reason to explain this shortfall is that the mechanism of splanchnic vasodilation is unclear. The organs involved in splanchnic hyperdynamic circulation are those whose blood flows into the portal vein, including the intestine, mesentery, colon, spleen and stomach. Previous studies have indicated that vasodilation of the mesenteric vascular bed plays the greatest role in PHT, by increasing portal inflow[2]. In previous work from our laboratory, we also observed vasodilation of the mesenteric vascular bed; thus in the present study we focused on this tissue to try to explain splanchnic vascular dilation and possibly to identify new therapeutic targets for treating PHT.
Splanchnic vasodilation is associated with the imbalance of vasoactive mediators[3,4] and hyporeactivity to vasoconstrictors[5]. Endothelin-1 (ET-1) is a potent endothelium derived vasoactive peptide. For many years, ET-1 has received considerable interest in the area of liver cirrhosis for its potential contribution to PHT[6-9]. This has led to many studies being focused upon the effect of ET-1 and its receptors in the liver; however, there are only a few studies examining the possible mechanism of the endothelin signaling system in hyperdynamic circulation. It has been established that the divergent effect of ET-1 on blood vessels depends on the different expression of endothelin receptors on smooth muscle and endothelial cells[10]. There are two known types of ET-1 receptor: the endothelin A receptor (ETAR) and the endothelin B receptor (ETBR)[11]. ETBR has two recognized subtypes: ETB1 and ETB2[10-13]. ETAR and ETB2 are predominantly expressed in vascular smooth muscle cells, whereas ETB1 is characteristic of endothelial cells. ET-1 binds to ETAR and ETB2 to induce vasoconstriction, while ET-1 binds to ETB1 to cause vascular relaxation[14]. Both mixed ETAR-ETBR antagonists and selective ETBR antagonists have been proven to decrease portal pressure (PP) and increase mean arterial pressure (MAP), while ETAR antagonists have been shown to have no effect on MAP[14,15]. Moreover, selective ETBR inhibition in vivo significantly ameliorated hepatopulmonary syndrome (HPS)[16-18], which is also known to be caused by dilation of the microcirculation, similar to PHT[18]. Based on this literature, we speculate that ETBR may play a primary role in the hyperdynamic circulation associated with PHT.
In addition to the localization and expression level of ET-1, its signaling is known to be affected by other regulators. For example, it has been observed that in some instances of high splanchnic ET-1 expression, the vascular bed of this tissue was still dilated, leading the authors to speculate that the sensitivity of the ET-1 receptor(s) was decreased[19]. Endothelin receptors may be desensitized by phosphorylation through G-protein-coupled receptor kinases (GRKs) and binding of β-arrestin 2[20]. So far, seven kinds of GRKs have been cloned[21]. GRK2 is the most likely of the GRKs to initiate human human endothelin A and B receptor desensitization[22]. Endothelin signalling in arterial smooth muscle is tightly regulated by GRK2[23]. As such, in this study we also focused on two known regulators, GRK2 and β-arrestin 2. The current literature has indicated a possible role for ET-1 signaling in splanchnic vasodilation, though there is a lack of experimental data to support this hypothesis. The aim of the present study was to directly examine the expression of ET-1 and its receptors in the mesentery of rats with PHT, and to clarify how the ET-1 signaling system changed with the development of PHT.
Male Sprague-Dawley rats (approximately 250 g; Vital River Laboratory Animal Technology Co. Ltd., Beijing, China) underwent sham surgery or common bile duct ligation (CBDL). In brief, the common bile ducts of rats were exposed after median laparotomy and ligated twice. In each animal, the segment between the 2 ligations was resected, and the animal’s abdomen was sutured closed. Sham-operated rats served as controls. In these rats, the common bile duct was exposed, but no ligation or resection was performed. Seven animals were used in each group. The study was approved by the local committee for animal studies.
In brief, after 2 wk or 4 wk following CBDL, a PE-50 catheter was inserted into the portal vein to measure portal blood pressure. After stable recordings of portal venous pressure were obtained, blood was drawn from the superior mesenteric artery (SMA) for further analysis. The level of ET-1 was measured in the mesenteric circulation using a commercial RIA kit (PLA Institute of RIA, Beijing, China) according to the manufacturer’s protocol.
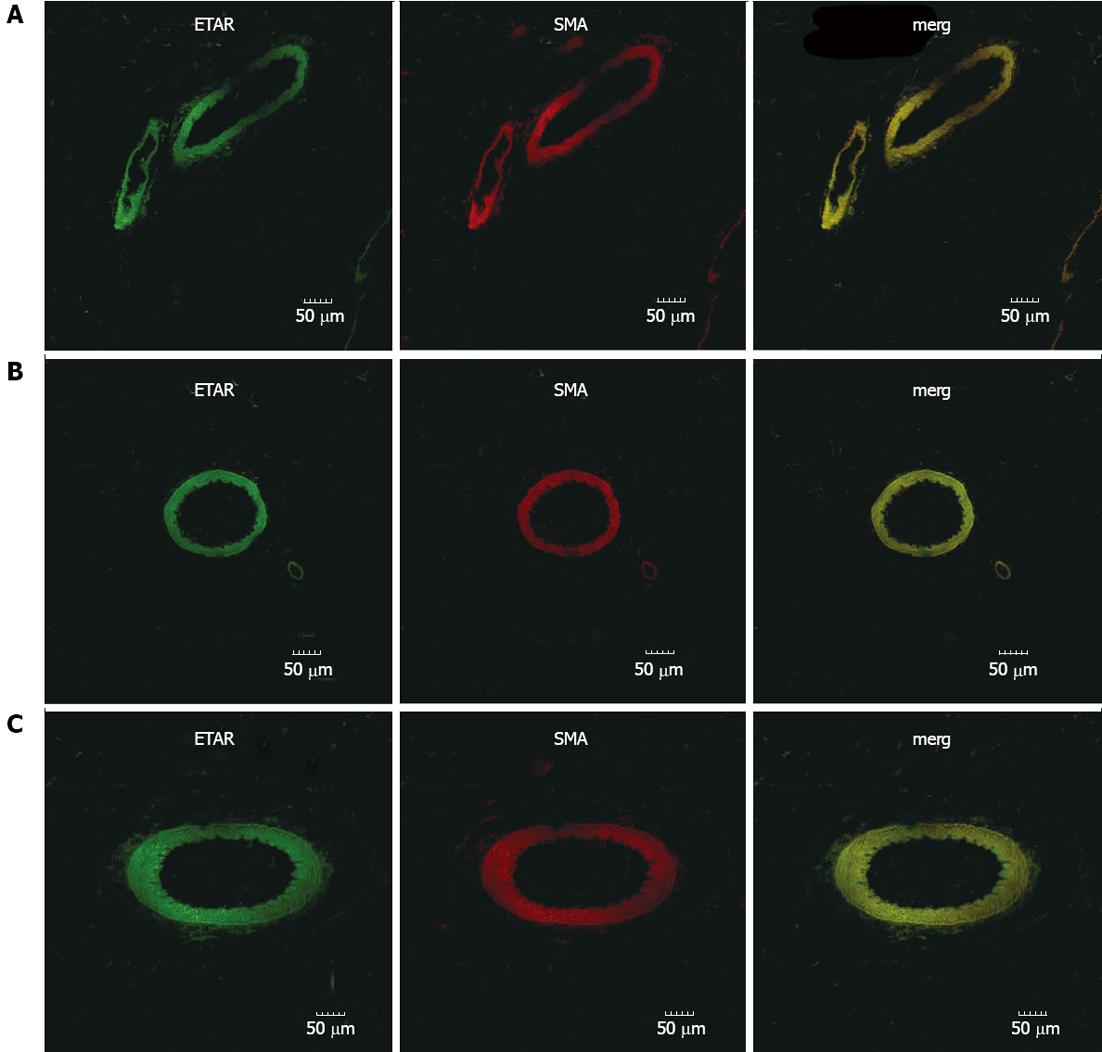
For immunofluorescence double staining of ETAR and ETBR, mesentery tissues were harvested at 2 and 4 wk and fixed in 4% neutral paraffin. After antigen retrieval, all sections were incubated with PBS containing 1% bovine serum albumin (block buffer) for 60 min in a wet chamber at room temperature. Then, for ETAR, sections were incubated with primary anti-ETAR (polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution) and anti-smooth muscle actin SMA (Santa Cruz Biotechnology, Santa Cruz, CA, United States; 1:200 dilution); for ETBR, slides were incubated with primary anti-ETBR (polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA, United States; 1:300 dilution) and anti-CD31 (polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA, United States; 1:100 dilution) antibodies at 4 °C overnight. Then all sections were washed with PBS. For the ETAR, the slides were incubated with goat anti-rabbit/mouse secondary antibodies at the same time. For the ETBR, the sections were incubated with rabbit anti-sheep and goat anti-rabbit antibodies at the same time (Invitrogen, San Diego, CA, United States) for 1 h at room temperature. Subsequently, sections were washed with phosphate-buffered saline (PBS). Control sections were incubated with secondary antibody in the absence of primary antibody. The results were analyzed using confocal laser scanning microscope.
For Western blot analysis, samples of rat mesentery were homogenized in radio immunoprecipitation assay (RIPA) lysis buffer containing 50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 5 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L sodium orthovanadate, 20 mmol/L pepstatin A, 20 mmol/L leupeptin and 1 mmol/L phenylmethanesulfonyl fluoride. The protein content of the cleared homogenates was assessed with bicinchoninic acid assay kit (Applygen, Beijing, China). After boiling with SDS sample buffer (Applygen, Beijing, China), 50 μg of protein per lane of each sample was subjected to SDS-polyacrylamide gel electrophoresis (10% gels for GRK2, 12.5% gels for ETAR, ETBR and β-arrestin 2). After blotting on polyvinylidene difluoride membrane (Millipore, Bedford, MA, United States), the membranes were probed with primary antibodies diluted in TBS containing blocking protein and 0.1% Tween, and left to incubate overnight at 4 °C. The following primary antibodies in the indicated dilutions were used: mouse anti-GRK2, 1:500 (Abcam); rabbit anti-ETAR/ETBR and mouse β-arrestin 2, 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA, United States). Thereafter, the membranes were washed and incubated with appropriate peroxidase-coupled secondary antibodies diluted 1:5000 in TBS containing blocking protein and 0.1% Tween for 45 min (goat anti-rabbit or goat anti-mouse; Jackson, West Grove, PA, United States). Detection was performed with enhanced chemiluminescence (Applygen, Beijing, China), and films were developed using Kodak film. Densitometric quantification was performed using Phoretix 1D gel image analysis software for free.
All data are presented as the mean ± SD; statistical comparisons were performed using one way analysis of variance. Physiological and biochemical findings represent averages of seven rats. Results of molecular assays represent averages of samples from at least five rats in each group. P values < 0.05 were considered statistically significant.
PP was measured in sham and experimental rats 2 and 4 wk after CBDL; PP of CBDL rats increased significantly (16.34 ± 1.63 mmHg vs 11.89 ± 1.38 mmHg for sham-operated animals). The increase in PP in CBDL rats was statistically significant compared with sham-operated rats at the 2 and 4 wk timepoint (P < 0.005).
The concentration of ET-1 was measured in the mesenteric and systemic circulation 2 and 4 wk after CBDL. At 2 and 4 wk, ET-1 levels in the mesenteric circulation of CBDL rats were 92.09 ± 13.26 pg/mL and 100.35 ± 16.36 pg/mL, which were significantly lower than that in sham-operated rats. Furthermore, in the systemic circulation, compared with sham-operated rats, ET-1 levels of CBDL rats were also significantly increased at 2 wk (142.77 ± 27.67 pg/mL); however, systemic ET-1 levels were decreased at 4 wk (88.62 ± 15.40 pg/mL).
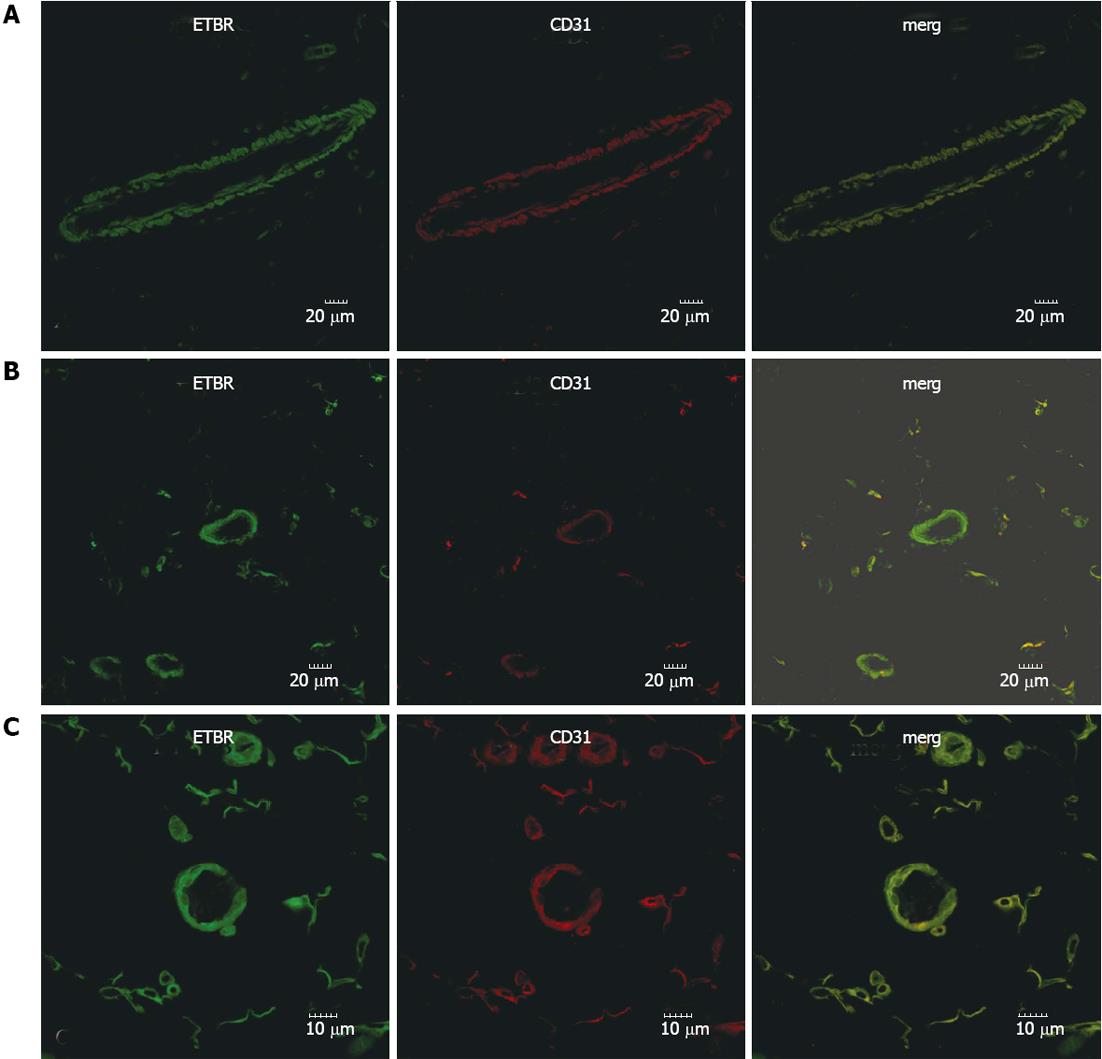
The expression pattern of ETAR and ETBR in mesenteric tissues was determined by immunofluorescence (Figures 1 and 2). From these pictures, the expression of ETAR was observed on SMA+ smooth muscle cells (Figure 1). In sham-operated rats, ETBR was mainly expressed in CD31+ endothelial cells of the vasculature, though the microcirculation also had weak immunostaining (Figure 2A). Our data indicates that with PHT development, in addition to endothelial cells, ETBR expression was noticeably detectable in the CD31+ capillaries (Figure 2B and C). We also noted increased vasodilation in the mesentery and formation of hyperdynamic circulation in CBDL rats, which was associated with increased angiogenesis (Figure 2B and C).
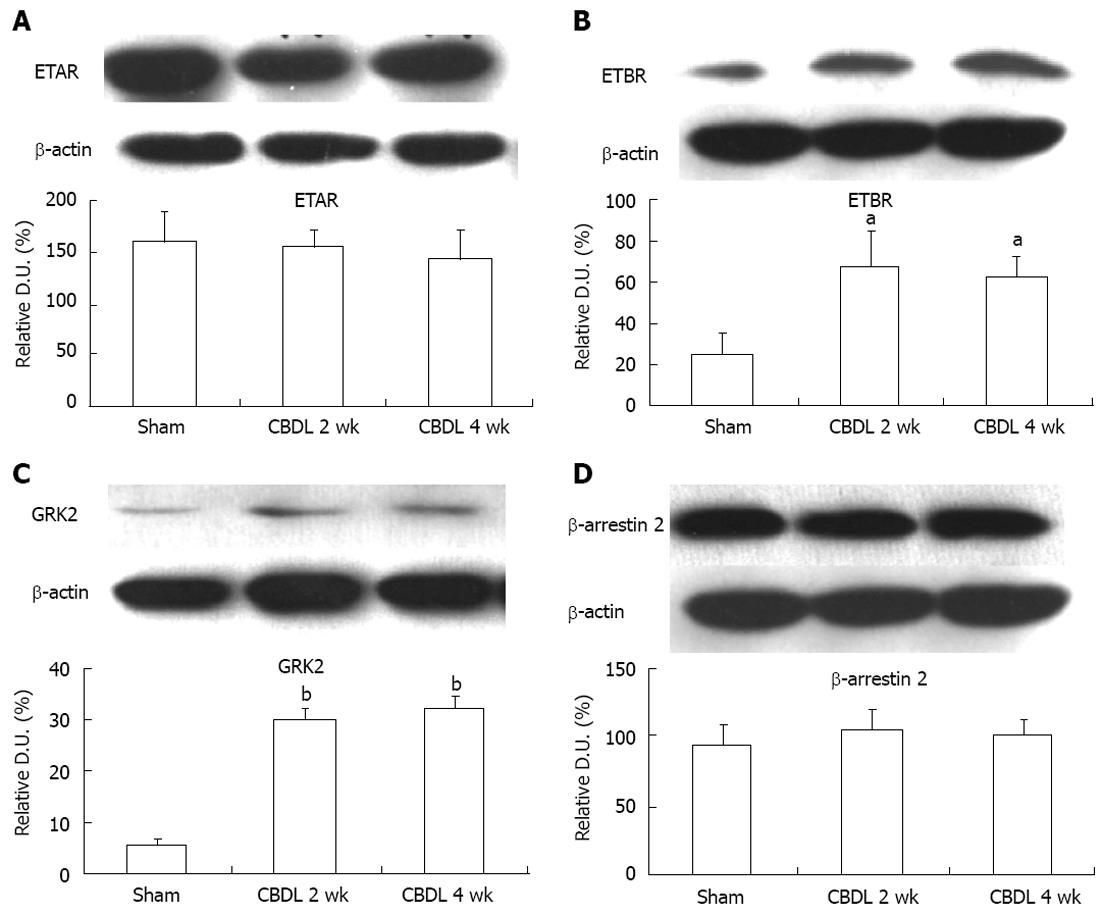
To confirm our immunofluorescence results, we investigated ETAR and ETBR expression by Western blot; we also used this method to assess GRK2 and β-arrestin 2 expression, as it relates to the sensitivity of the ET-1 receptors during the development of a hyperdynamic circulation (Figure 3). In agreement with our immunostaining, we found no statistically significant difference in ETAR expression between sham-operated and CBDL rats at the 2 and 4 wk timepoints (Figure 3A). ETBR was significantly upregulated in CBDL rats after 2 wk (P < 0.01) and 4 wk (P < 0.01), as compared to sham-operated rats (Figure 3B). Similar to previous research, which has shown that GRK2 levels are increased in the aortas of CBDL rats[24], we also observed an upregulation of GRK2 protein levels in the mesentery of CBDL rats at both timepoints, which was statistically significant (P < 0.005; Figure 3C). We also observed no significant change in the protein expression level of β-arrestin 2 between sham-operated and CBDL rats after 2 or 4 wk (P > 0.05; Figure 3D).
In accordance with Ohm’s law, PP depends on intrahepatic resistance and portal inflow. In cases of cirrhosis, both intrahepatic resistance and splanchnic blood flow are increased. The initiating factor is an increase in intrahepatic vascular resistance, whereas the increase in splanchnic blood flow is a secondary phenomenon that maintains or worsens the increased PP and gives rise to the hyperdynamic systemic state[25]. In terms of the relevant literature[16,17], it has been speculated that the ET-1 signaling system may play an important role in the hyperdynamic circulation, even though there is no direct experimental evidence. Moreover, there are also articles about endothelin receptor antagonism treatment in humans with PHT[26,27]. In this regard, this is the first in vivo study examining the expression of the ET-1 signaling system in the mesentery of PHT rats.
We have found that the concentration of ET-1 in the mesenteric circulation decreases as the liver becomes fibrotic. We also found that ETBR expression, but not ETAR expression, increased in vascular smooth muscle cells and in the microcirculation of mesentery tissue, which may mean decreased vasoconstriction and increased vasodilatation induced by local ET-1 in the mesentery of PHT rats. Furthermore, the expression of GRK2 increased significantly in CBDL rats, which may imply that desensitization of ETAR and other vasoconstrictor receptors also promotes splanchnic vasodilatation.
The ET-1 signaling system is associated with vascular dysfunction at three levels, changes in: ET-1 concentration, ET-1 receptor expression and sensitivity, and the ET-1 signaling transduction pathway. Thus, the ability to modulate ET-1 signaling at either of these levels may provide ways to improve splanchnic vascular dysfunction. Previous studies have shown that ET-1 was upregulated in the liver tissue and systemic circulation of patients[9], but the expression level of ET-1 in the splanchnic circulation is not consistent. The observed decrease in ET-1 in the splanchnic circulation 2 and 4 wk after CBDL leads us to speculate that one reason for mesenteric vascular bed dilation in this model is a local decrease in ET-1.
ET-1 must bind to its receptor to modulate vascular tone, so variations in receptor subtype expression and quantity affect the action of ET-1 on blood vessels. Using an animal model of HPS, which is also applicable to PHT, it was found that the extensive dilation of the pulmonary microcirculation was related to a selective increase in ETBR expression[16,17]. PHT and HPS can both be induced by CBDL in rats, and dilation of the microcirculation exists in both syndromes, raising the question whether ET-1 signaling through ETBR plays the same role in the hyperdynamic circulation as it does in HPS? Further research will be required to address this important question and establish if this hypothesis is correct. In the present study, we observed that ETAR expression in the mesentery was not different between sham-operated and CBDL rats. Interestingly however, ETBR expression was obviously increased by CBDL. Our immunofluorescence data indicates that, under normal physiological conditions, ETBR is primarily expressed in the endothelial cells of the vasculature, whereas the microcirculation also has weak positive staining. With the development of liver fibrosis and liver cirrhosis, ETBR was not only expressed in endothelial cells but was also strongly observed in the microcirculation. Given that the primary function of the microcirculation is to accumulate blood, we interpreted from our data that in the mesentery of CBDL rats, signaling through ETBR mediates vasodilation via release of nitrogen oxide from endothelial cells, leading to an increased blood volume. It is possible that ETBR expression in the smooth muscle cells is a compensatory mechanism to facilitate contraction of blood vessels and oppose the overdilation. Analysis of ETBR by Western blot confirmed the increase in expression following CBDL. Though we observed immune-positive staining for both ETAR and ETBR in fat tissue, this would not affect our Western blot data because the fat tissue in mesentery cannot be dissolved by the RIPA lysis buffer and was discarded during homogenization.
ET-1 binds to its Gq protein-coupled receptor to send an extracellular signal into the cell, an event which is limited by the sensitivity of the receptor. Under normal conditions, the receptor is desensitized to prevent excessive stimulation by vasoactive substances. Available experimental evidence demonstrates that augmentation of receptor sensitivity, by factors like norepinephrine, vasopressin, ET-1 or angiotensin, impairs the transduction of vasoconstrictor signals and promotes dilation of the splanchnic vascular bed[24]. It is known that GRKs and arrestins are key participants in the canonical pathways leading to phosphorylation-dependent or independent G protein-coupled receptor desensitization and endocytosis[28]. GRK2 is one member of the GRK family and has been shown to be able to specifically phosphorylate human ETAR and ETBR[20]. Our findings demonstrate that the increased PP following CBDL resulted in an upregulation of mesenteric GRK2 expression, but not β-arrestin 2. GRK2 and β-arrestin 2 may modulate ETAR and ETBR desensitization through the following mechanisms: (1) phosphorylation of ETAR by GRK2 promotes the receptor binding to β-arrestin 2, which blocks the activation of the G proteins and leads to rapid homologous desensitization; and (2) independent of phosphorylation, GRK2 interacts with G alpha(q) directly, which results in uncoupling of ET-1 receptor and its associated G proteins, thus impairing the ET-1 signal transduction pathway[29]. Increased GRK2 expression in the mesentery of PHT rats not only results in desensitization of ETAR, but also the receptors of norepinephrine and angiotensin, which indicates that such vasoactive substances are unable to mediate vasoconstriction, regardless of ligand concentration or the level of receptor expression.
In summary, PHT induced by CBDL in rats was associated with decreased levels of ET-1 in the mesenteric circulation, and increased mesenteric expression of ETBR and GRK2. We conclude that these changes underlie mesenteric vasodilation in an animal model of PHT, and are also applicable to patients with this condition. We interpret our data to indicate that the ET-1 signaling pathway is an important factor in the development of splanchnic vasodilation associated with PHT. These findings have major therapeutic implications not only for individuals with liver disease, but also for other diseases with vascular dysfunction.
The authors wish to thank Jin-Cai Hou, Hong-Tao Lei, Jun Wang for excellent technical assistance.
Portal hypertension (PHT) is a life-threatening condition which frequently develops in patients with liver cirrhosis, and has limited treated options. During PHT, vasodilation results in increased blood flow into the mesenteric circulation, thereby increasing flow into the portal circulation, which can worsen PHT. For many years, endothelin-1 (ET-1) has received considerable interest in the area of liver cirrhosis for its potential contribution to PHT. The aim of the present study was to directly examine the expression of ET-1 and its receptors in the mesentery of rats with PHT, and to clarify how the ET-1 signaling system changed with the development of PHT.
PHT can give rise to many other severe and often lethal conditions, such as bleeding esophageal varices. Increased resistance to portal blood flow is the primary factor leading to PHT and is aggravated by a hyperdynamic, vasodilated, splanchnic circulation. Though the pathophysiology of PHT is becoming better understood, excepting β-receptor blockers, there is no effective treatment approach for PHT. One reason to explain this phenomenon is that the mechanism of splanchnic vasodilation is unclear. So in this study, the authors choose mesentery tissue of hypertensive rats to research the mechanisms of vasodilation based on ET-1 and its receptors.
The current literature has indicated a possible role for ET-1 signaling in splanchnic vasodilation, though there is a lack of experimental data to support this hypothesis. This is the first in vivo study examining the expression of the ET-1 signaling system in the mesentery of PHT rats.
These findings have major therapeutic implications not only for individuals with liver disease, but also for other diseases with vascular dysfunction.
PHT is the main complication of cirrhosis and is defined as a hepatic venous pressure gradient (HVPG) of more than 5 mmHg. Clinically significant PHT is defined as HVPG of 10 mmHg or more; Hyperdynamic circulation: The hyperdynamic circulatory state of PHT is characterized by splanchnic and peripheral vasodilation, increased plasma volume and increased cardiac output.
In this paper, the authors show that expression of G protein coupled kinase-2 is downregulated while expression of the endothelin B receptor is increased in the mesentery after common bile duct ligation, a model for PHT. These findings are interesting and might represent some mechanisms underlying vasodilatation in the mesentery.
P- Reviewers Heijnen CJ, Lund AK S- Editor Song XX L- Editor O'Neill M E- Editor Xiong L
| 1. | Rössle M. Hyperdynamic circulation and portal hypertension: chicken or egg? Gut. 2011;60:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Angus PW. Role of endothelin in systemic and portal resistance in cirrhosis. Gut. 2006;55:1230-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Braillon A, Lee SS, Girod C, Peignoux-Martinot M, Valla D, Lebrec D. Role of portasystemic shunts in the hyperkinetic circulation of the portal hypertensive rat. J Lab Clin Med. 1986;108:543-548. [PubMed] |
| 4. | Pateron D, Tazi KA, Sogni P, Heller J, Chagneau C, Poirel O, Philippe M, Moreau R, Lebrec D. Role of aortic nitric oxide synthase 3 (eNOS) in the systemic vasodilation of portal hypertension. Gastroenterology. 2000;119:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Blendis L, Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol Ther. 2001;89:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Rockey DC, Fouassier L, Chung JJ, Carayon A, Vallee P, Rey C, Housset C. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Rockey DC. Vascular mediators in the injured liver. Hepatology. 2003;37:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Tièche S, De Gottardi A, Kappeler A, Shaw S, Sägesser H, Zimmermann A, Reichen J. Overexpression of endothelin-1 in bile duct ligated rats: correlation with activation of hepatic stellate cells and portal pressure. J Hepatol. 2001;34:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Degertekin B, Ozenirler S, Elbeg S, Akyol G. The serum endothelin-1 level in steatosis and NASH, and its relation with severity of liver fibrosis. Dig Dis Sci. 2007;52:2622-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Mallat A, Lotersztajn S. Multiple hepatic functions of endothelin-1: physiopathological relevance. J Hepatol. 1996;25:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Sakurai T, Yanagisawa M, Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992;13:103-108. [PubMed] |
| 12. | Clozel M, Gray GA, Breu V, Löffler BM, Osterwalder R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun. 1992;186:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 305] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Battistini B, D’Orléans-Juste P, Sirois P. Endothelins: circulating plasma levels and presence in other biologic fluids. Lab Invest. 1993;68:600-628. [PubMed] |
| 14. | Leivas A, Jiménez W, Lamas S, Bosch-Marcé M, Oriola J, Clària J, Arroyo V, Rivera F, Rodés J. Endothelin 1 does not play a major role in the homeostasis of arterial pressure in cirrhotic rats with ascites. Gastroenterology. 1995;108:1842-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Helmy A, Newby DE, Jalan R, Hayes PC, Webb DJ. Enhanced vasodilatation to endothelin antagonism in patients with compensated cirrhosis and the role of nitric oxide. Gut. 2003;52:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Luo B, Liu L, Tang L, Zhang J, Stockard CR, Grizzle WE, Fallon MB. Increased pulmonary vascular endothelin B receptor expression and responsiveness to endothelin-1 in cirrhotic and portal hypertensive rats: a potential mechanism in experimental hepatopulmonary syndrome. J Hepatol. 2003;38:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Tang L, Luo B, Patel RP, Ling Y, Zhang J, Fallon MB. Modulation of pulmonary endothelial endothelin B receptor expression and signaling: implications for experimental hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1467-L1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Ling Y, Zhang J, Luo B, Song D, Liu L, Tang L, Stockard CR, Grizzle WE, Ku DD, Fallon MB. The role of endothelin-1 and the endothelin B receptor in the pathogenesis of hepatopulmonary syndrome in the rat. Hepatology. 2004;39:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Vashist Y, Semela D, Dufour JF. Hyperdynamic circulation in liver cirrhosis: desensitization of vasoconstrictive receptors by G protein-coupled receptor kinases. Med Hypotheses. 2004;62:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 21. | Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734-17743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 157] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res. 2010;85:424-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201-17210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 696] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 24. | Hennenberg M, Trebicka J, Biecker E, Schepke M, Sauerbruch T, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology. 2007;45:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Cichoz-Lach H, Celiński K, Słomka M, Kasztelan-Szczerbińska B. Pathophysiology of portal hypertension. J Physiol Pharmacol. 2008;59 Suppl 2:231-238. [PubMed] |
| 26. | Lebrec D, Bosch J, Jalan R, Dudley FJ, Jessic R, Moreau R, Garcia-Pagan JC, Mookerjee RP, Chiossi E, Van Giersbergen PL. Hemodynamics and pharmacokinetics of tezosentan, a dual endothelin receptor antagonist, in patients with cirrhosis. Eur J Clin Pharmacol. 2012;68:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Tripathi D, Therapondos G, Ferguson JW, Newby DE, Webb DJ, Hayes PC. Endothelin-1 contributes to maintenance of systemic but not portal haemodynamics in patients with early cirrhosis: a randomised controlled trial. Gut. 2006;55:1290-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 524] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 29. | Ribas C, Penela P, Murga C, Salcedo A, García-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |