Published online Apr 7, 2013. doi: 10.3748/wjg.v19.i13.2028
Revised: November 1, 2012
Accepted: November 11, 2012
Published online: April 7, 2013
Processing time: 238 Days and 12 Hours
AIM: To evaluate the diagnostic capability of calprotectin in ascitic fluid for detecting a polymorphonuclear (PMN) cell count > 250/μL ascites.
METHODS: In this prospective observational study, a total of 130 ascites samples were analysed from 71 consecutive patients referred for paracentesis. Total and differential leukocyte cell counts were determined manually with a Neubauer chamber and gentian-violet stain. Calprotectin was measured in 1 mL ascetic fluid by enzyme-linked immunosorbent assay (ELISA) and a point-of-care (POC) lateral flow assay with the Quantum Blue® Reader (Bühlmann Laboratories). All measurements were carried out in a central laboratory by senior personnel blinded to patient history. A PMN count > 250/μL was the primary endpoint of the study. The diagnostic value of ascitic calprotectin measurement was assessed by comparing to the final diagnosis of each patient that had been adjudicated by investigators blinded to calprotectin values.
RESULTS: The PMN count was > 250/μL in 19 samples (14.6%) from 15 patients (21.1%) and varied widely among the study population (range 10-19 800/mL and 1-17 820/mL, respectively). Spontaneous bacterial peritonitis (SBP) was the final diagnosis in four patients (5.6%). All patients with PMN ≤ 250/μL had negative bacterial culture. PMN count was elevated in five patients with peritoneal carcinomatosis, three with lymphoma, one with neuroendocrine carcinoma, and two with secondary peritonitis due to abdominal perforation. PMN cell counts correlated with ascitic calprotectin values (Spearman’s rho; r = 0.457 for ELISA, r = 0.473 for POC). A considerable range of ascitic calprotectin concentrations was detected by ELISA [median 0.43 μg/mL, interquartile range (IQR) 0.23-1.23 (range 0.10-14.93)] and POC [median 0.38 μg/mL, IQR 0.38-0.56 (range 0.38-13.31)]. Ascitic calprotectin levels were higher in samples with PMN > 250/μL, by both ELISA [median (IQR) 2.48 μg/mL (1.61-3.65) vs 0.10 μg/mL (0.10-0.36), P < 0.001] and POC [2.78 μg/mL (2.05-5.37) vs 0.38 μg/mL (0.38-0.41), P < 0.001]. The area under the receiver operating characteristics curve for identifying an elevated PMN count was 0.977 (95%CI: 0.933 to 0.995) for ELISA and 0.982 (95%CI: 0.942 to 0.997) for POC (P = 0.246 vs ELISA). Using the optimal cut-off value for ELISA (0.63 μg/mL), ascitic calprotectin had 94.8% sensitivity, 89.2% specificity, positive and negative likelihood ratios of 8.76 and 0.06 respectively, positive and negative predictive values of 60.0% and 99.0% respectively, and 90.0% overall accuracy. Using the optimal cut-off value for POC (0.51 μg/mL), the respective values were 100.0%, 84.7%, 6.53, 0.00, 52.8%, 100% and 87.7%. Correlation between ELISA and POC was excellent (r = 0.873, P < 0.001). The mean ± SD of the difference was -0.11 ± 0.48 μg/mL with limits of agreement of + 0.8 μg/mL (95%CI: 0.69 to 0.98) and -1.1 μg/mL (95%CI: -1.19 to -0.91).
CONCLUSION: Ascitic calprotectin reliably predicts PMN count > 250/μL, which may prove useful in the diagnosis of SBP, especially with a readily available bedside testing device.
- Citation: Burri E, Schulte F, Muser J, Meier R, Beglinger C. Measurement of calprotectin in ascitic fluid to identify elevated polymorphonuclear cell count. World J Gastroenterol 2013; 19(13): 2028-2036
- URL: https://www.wjgnet.com/1007-9327/full/v19/i13/2028.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i13.2028
Liver cirrhosis is the clinical end-stage of different entities of chronic liver disease when patients suffer from substantial mortality and morbidity, both of which are positively correlated with disease severity[1,2]. Ascites is the most common complication, and around 60% of patients with compensated cirrhosis develop ascites within 10 years of disease onset[3]. Spontaneous bacterial peritonitis (SBP) is an important cause of morbidity and mortality in cirrhotic patients with ascites. SBP is estimated to affect 10%-30% of cirrhotic patients hospitalised with ascites, and mortality in this group approaches 30%[4,5]. Many of these patients are asymptomatic, and it is therefore recommended that all patients with ascites undergo paracentesis at the time of admission to confirm the SBP status[5]. Although SBP is less prevalent in an outpatient setting, it is reasonable to also evaluate the ascitic fluid of outpatients because of the high mortality associated with SBP.
The diagnosis of SBP is based upon the polymorphonuclear (PMN) leukocyte cell count exceeding 250/μL in ascitic fluid[6,7]. Currently, differential cell count is usually performed by a manual method using light microscopy and counting chambers. However, the diagnosis is often delayed when laboratory personnel are not readily available or in the private practice setting where specimens are sent to an offsite laboratory. This is a major drawback, as rapid diagnosis of SBP and immediate initiation of antibiotic treatment is of paramount importance. Alternative methods using automated PMN counting[8,9], reagent strips (urine dipsticks)[10-26], or ascitic lactoferrin[27] have been developed; unfortunately, their diagnostic accuracies are limited and their use is dependent upon availability of laboratory personnel and reagents/components from the commercial source. Therefore, an accurate and convenient method of rapid diagnosis of SBP remains an unmet clinical need.
Calprotectin, a calcium and zinc-binding protein, is detected almost exclusively in neutrophils[28], and its presence in body fluids is proportional to the influx of neutrophils[29-33]. However, only one study to date has investigated calprotectin levels in ascites and found higher concentrations in patients with malignant disease than in those with non-malignant disease[34]. In contrast, faecal calprotectin is a well-established marker of inflammation and is used to monitor inflammatory bowel disease[35]. A rapid bedside test has been developed to measure calprotectin in faeces; systematic comparison with the established enzyme-linked immunosorbent assay (ELISA) technique showed good correlation between the two tests’ results[36] and the rapid bedside test has been suggested as an equally valuable tool for diagnosing inflammatory bowel disease[37]. It is possible that such a rapid bedside test may be useful for measuring calprotectin in ascitic fluid to indicate PNM levels and SBP status, however the diagnostic accuracy of such a measurement in ascitic fluid is unknown.
This study was designed to test our hypothesis that calprotectin in ascitic fluid could be useful as a surrogate PMN marker for identifying SBP patients (> 250/μL PNM). To this end, we measured calprotectin in ascites of consecutive patients referred for paracentesis using a rapid bedside test and compared the results to those from the traditional ELISA.
In this prospective observational study, we recruited patients with ascites referred for paracentesis to the Department of Gastroenterology and Hepatology at the University Hospital Basel, and to the Department of Gastroenterology, Hepatology and Clinical Nutrition at the Cantonal Hospital Liestal in Switzerland. All patients with ascites were eligible for study enrolment, irrespective of the aetiology of ascites. The decision to perform paracentesis was based on clinical findings evaluated by the referring physician who was otherwise not involved in the study. Exclusion criteria were age < 18 years and recent abdominal surgery (< 3 mo). Standardised patient history, clinical symptoms, and demographic data were obtained from all participants. The study was carried out in accordance with the principles of the Declaration of Helsinki and with pre-approval from the local Ethic Committees of both study sites. All patients provided written informed consent prior to participation in any protocol-specific procedures.
The diagnostic value of ascitic calprotectin measurement was assessed in comparison to the adjudicated final diagnosis. A PMN count > 250/μL was the primary endpoint of the study.
One month after study participation, the final diagnosis (SBP) and the aetiology of ascites were independently adjudicated in a blinded fashion by two board-certified gastroenterologists not involved in the patients’ care. Their final assessment was based upon available medical records, including PMN count and the results of all diagnostic investigations, as well as the patient’s response to treatment. Current recommendations were followed[6,7]. The two physicians designated the aetiology of ascites by choosing one or more of the following diagnoses from a standardized list: liver cirrhosis (alcoholic, chronic hepatitis, non-alcoholic steatohepatitis, hemochromatosis, primary biliary cirrhosis, others to be specified), hepatocellular carcinoma, cholangiocellular carcinoma, liver metastasis, peritoneal carcinomatosis, right heart failure, nephrotic syndrome, and others to be specified. If more than one cause of ascites was identified, the leading disorder responsible for the current episode was established and recorded. Any disagreements in the final diagnosis of a given study participant were resolved by consensus with a third clinician who was considered an expert in the field and recruited to independently review and adjudicate the cases.
Paracentesis was performed under aseptic conditions with the patient in the supine position and the puncture site in the left or right lower quadrant. Prior to needle insertion, ultrasound was performed to visualise the intra-abdominal structures. No study participant suffered complications related to the abdominal puncture procedure. All samples for diagnostic testing were immediately collected at the bedside and processed by laboratory personnel without further delay. Specifically, aliquots of approximately 1mL ascites were centrifuged for 15 min at 500 ×g. The supernatant phase was transferred to a fresh tube and stored at -20 °C until analysis by ELISA or POC, which occurred within 72 h.
Blood samples were also obtained at this time. The ascites samples were used to measure total cell count, PMN count, calprotectin, albumin, total protein, glucose, and lactate dehydrogenase. In addition, two 10 mL aliquots of ascites were subjected to bacterial culture (bottle method) respectively. The serum-ascites albumin gradient (SAAG) was calculated as the difference of albumin in serum and albumin in ascites.
All laboratory analyses were performed in the Central Laboratories BL (Schönenbuch, Switzerland) by senior laboratory personnel blinded to patient history and calprotectin levels. Total and differential leukocyte cell counts were determined by the manual method using a Neubauer chamber and gentian-violet staining (Leukotic®; bioanalytic GmbH, Freiburg, Germany). The Central Laboratories BL is accredited according to ISO/IEC 17 025 and ISO 15 189 standards. For all study participants who underwent repeated paracentesis, the cytopathological analysis was performed at least once.
Ascitic calprotectin in ascites was assayed using a commercially-available ELISA (Bühlmann Laboratories AG, Schönenbuch, Switzerland) and following the manufacturer’s instructions. Briefly, 10 μL aliquots of the supernatant samples were diluted 1:50 in incubation buffer and 100 μL was applied to a microtiter plate coated with a monoclonal capture antibody highly specific for the calprotectin heterodimeric and polymeric complexes. After incubation, washing and further incubation with a detection antibody conjugated to horseradish peroxidase, the tetramethylbenzidine chromogenic substrate was added. The reaction was terminated by a stop solution and the absorbance (optical density at 450 nm) was measured by spectrophotometry. The measuring range of the test was 0.2-12 μg calprotectin/mL ascites with an intra- and interassay coefficient of 4.7% and 11.3%, respectively.
The Quantum Blue® quantitative calprotectin lateral flow assay (Bühlmann Laboratories AG) was used for the point-of-care (POC) measurement of ascitic calprotectin. The Quantum Blue® reader is currently marketed for 2500 USD ($), and the test cartridges cost 20 USD per sample and analysis. Aliquots of 60 μL 1:10 diluted ascites samples (20 μL ascites in 180 μL extraction buffer) were pipetted respectively onto the sample loading port of the test cartridge. After a 12 min incubation, the test cartridge was quantitatively read by the Quantum Blue® Reader. The measurement range of the lateral flow test was 0.38-3.8 μg calprotectin/mL ascites, with an interassay coefficient of 15.6%. Specimens with concentrations above this measurement range were further diluted with extraction buffer.
In addition, a random subgroup of samples (n = 17) was immediately measured by POC, without first performing the centrifugation step of processing. These results were compared to the results from the POC measurements obtained in the laboratory setting after processing and storage.
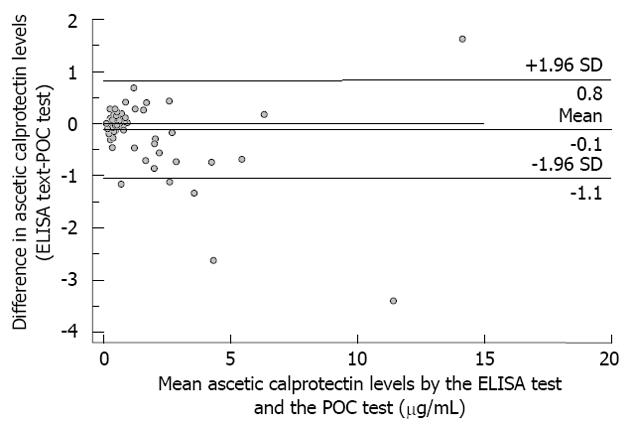
All statistical analyses were performed using the SPSS software package, version 19.0 (SPSS Inc., Chicago, IL, United States). A P-value of less than 0.05 indicated statistical significance. Intergroup comparisons were made using the Mann-Whitney U test and the χ2 test where appropriate. Correlations between numerical data were determined with the Spearman’s rank correlation coefficient. All hypothesis testing was two-tailed. The Bland-Altman plot was used to assess agreement between ELISA test results and POC test results, in which the differences between the results of the two tests for each individual patient were plotted against the corresponding mean of the two readings. The mean and SD of the differences and the limits of agreement, defined as the mean ± 2 SD of the difference (95%CI), were calculated. Analysis of the receiver operator characteristics (ROC) and calculation of the area under the curve (AUC) were used to evaluate the capability of calprotectin to identify a PMN count > 250/μL. The ROC analysis identified the cut-off points for maximal diagnostic capability. The test characteristics of sensitivity, specificity, positive and negative likelihood ratios (LR+ and LR-), and positive and negative predictive values (NPV) were determined. Overall accuracy of the test was calculated according to the following formula: [(true positive test results + true negative test results)/total population]. As this study was exploratory in design, no formal power calculations were carried out.
A total of 136 samples from 75 patients were prospectively collected from October 2010 to January 2012. Among these, 130 samples were included in the final analysis, representing 71 patients (94.7% of the total; 40 males and 31 females) with a median age of 64 years (IQR 55-71 years). Sixty-three of the patients (88.7%) had been referred for diagnostic paracentesis. Twenty-four of the patients (33.8%) underwent the procedure more than once (median 3, range 2-12).
The majority of patients (54, 76.1%) suffered from liver cirrhosis (Table 1). A total of 11 patients (15.5%) had malignant ascites, which included three ovarian, two lymphomas, two breast, one stomach, one colorectal, one pancreatic cancer, and one neuroendocrine carcinoma. Of those 11 patients, two also had liver cirrhosis. Additionally, three patients with ascites also had heart failure and five patients with ascites also had portal hypertension from metastatic liver disease (but no malignant cells were present in the ascites). No intervention-related complications occurred after paracentesis.
| Aetiology of liver cirrhosis | |
| Alcoholic | 36 (66.6) |
| Viral hepatitis | 7 (13.0) |
| Alcoholic + viral hepatitis | 5 (9.3) |
| Non alcoholic steatohepatitis | 1 (1.9) |
| Others | 5 (9.3) |
| Child-turcotte-pugh class | |
| Child A | 0 |
| Child B | 29 (53.7) |
| Child C | 25 (46.3) |
| MELD score | 12.2 (10.0-16.0) |
Total cell count and PMN cell count at presentation varied widely among the study population (range 10-19 800/mL and 1-17 820/mL, respectively). PMN count > 250/mL was detected in 19 samples (14.6%) from 15 patients (21.1%). Among the study population, SBP was the final diagnosis for four patients (5.6%) and only one of these four had positive ascitic bacterial cultures (Streptococcus pneumoniae). All bacterial cultures from patients with PMN ≤ 250/mL were negative. Additionally, PMN count was elevated in five patients with peritoneal carcinomatosis (two with ovarian cancer, and one each with gastric, colorectal and pancreatic cancer), in three patients with lymphoma, in one patient with neuroendocrine carcinoma, and in two patients with secondary peritonitis due to an abdominal perforation. All patients with SBP received antibiotic treatment and recovered well. None of the patients died. Table 2 details the findings from ascitic fluid analysis.
| Variable | PMN count > 250/μL (n = 19) | PMN count ≤250/μL (n = 111) |
| Total cell count | 1300.0 (350.0-19800.0) | 250.0 (10.0-1970.0) |
| PMN count | 553.0 (277.0-17820.0) | 21.0 (1.0-212.0) |
| Albumin, g/L | 13.0 (4.8-16.8) | 7.0 (3.0-10.0) |
| Protein, g/L | 22.0 (9.3-37.5) | 12.0 (8.0-20.0) |
| LDH, U/L | 117.0 (100.8-138.5) | 55.0 (42.0-81.0) |
| Glucose, mmol/L | 7.6 (6.2-9.7) | 7.0 (6.2-8.2) |
The ascitic calprotectin concentrations ranged considerably in the ELISA [median 0.43 μg/mL, IQR 0.23-1.23 (range 0.10-14.93)] and POC test [median 0.38 μg/mL, IQR 0.38-0.562 (range 0.38-13.31)]. However, the calprotectin values measured by the laboratory-based ELISA and the POC test correlated well with the PMN count (r = 0.476, P < 0.001 and r = 0.473, P < 0.001, respectively), and the correlation between the two tests was excellent (r = 0.873, P < 0.001). The degree of agreement between the measurements of ascitic calprotectin from the ELISA and the POC test is illustrated in Figure 1. The mean ± SD of the difference was -0.11 ± 0.48 μg/mL, with limits of agreement of +0.8 μg/mL (95%CI: 0.69 to 0.98) and -1.1 μg/mL (95%CI: -1.19 to -0.91).
Comparative analysis of the POC detection of ascitic calprotectin levels in samples measured at the bedside (unprocessed and processed after centrifugation) and in the lab (after centrifugation) showed that the calprotectin measurements correlated well. For unprocessed samples, r = 0.831 (P < 0.001), and for processed samples, r = 0.656 (P = 0.004).
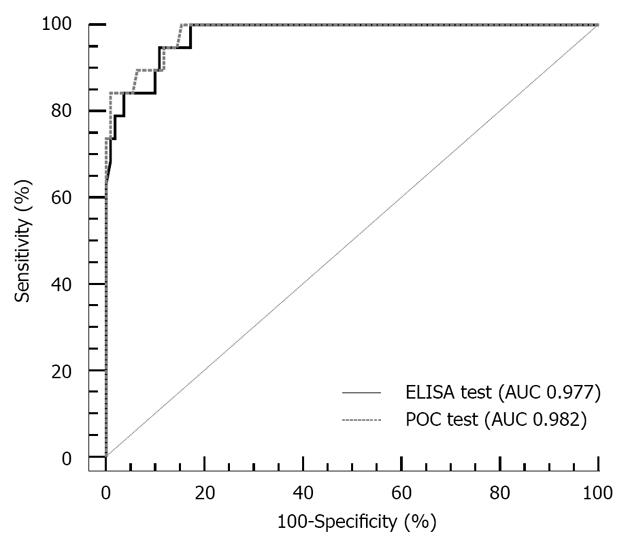
Ascitic calprotectin levels were higher in samples (n = 19) with PMN > 250/μL, both when measured by ELISA [median (IQR) 2.48 μg/mL (1.61-3.65) vs 0.10 μg/mL (0.10-0.36), P < 0.001] and the POC test [median 2.78 μg/mL (2.05-5.37) vs 0.38 μg/mL (0.38-0.41), P < 0.001] (Figure 2). Evaluation of the ascitic calprotectin measurement as a diagnostic test to identify patients with PMN count > 250/μL yielded an AUC of 0.977 (95%CI: 0.933-0.995) for the ELISA and an AUC of 0.982 (95%CI: 0.942-0.997) for the POC test. Furthermore, the two tests did not show significantly different diagnostic capacity (P = 0.246 vs ELISA) (Figure 3).
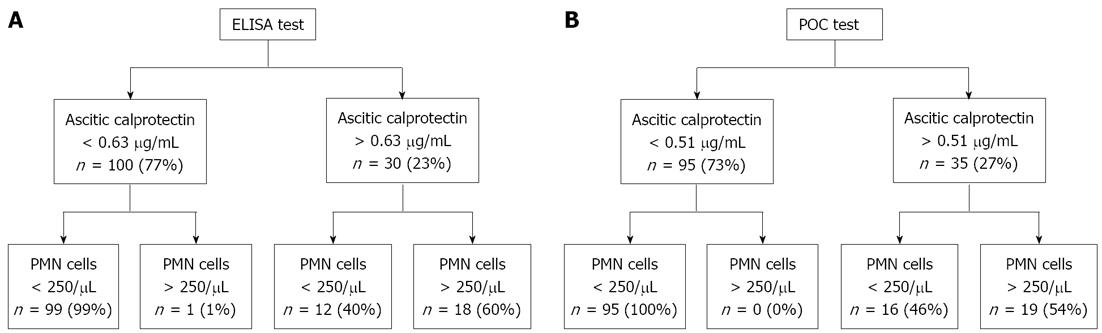
Using the optimal cut-off value from the ROC of ELISA (0.63 μg/mL), ascitic calprotectin yielded a sensitivity of 95%, a specificity of 89.2%, and an accuracy of 90.0% (Table 3). To identify all patients with PMN count > 250/μL and to obtain 100% test sensitivity, a slightly lower cut-off value (0.44 μg/mL) is necessary. However, use of this lower value is accompanied by lower specificity (82.9%) and lower LR+ (5.84).
| AUC (95%CI) | Best cut-off (μg/mL) | Sens (%) | Spec (%) | LR+ | LR- | PPV (%) | NPV (%) | Accuracy (%) | |
| ELISA | 0.977 (0.933-0.995) | 0.63 | 94.8 | 89.2 | 8.76 | 0.06 | 60.0 | 99.0 | 90.0 |
| POC | 0.982 (0.942-0.997) | 0.51 | 100 | 84.7 | 6.53 | 0.00 | 52.8 | 100 | 87.7 |
Analysis of the POC test characteristics revealed a nearly identical profile to the ELISA characteristics (Table 3). The optimal cut-off value for POC (0.51 μg/mL) yielded a sensitivity of 100% and a specificity of 84.7%, with 6.53 LR+ and 0.0 LR-. The overall accuracy of the POC test was 87.7% (Figure 4).
Patients with false positive test results had PMN counts between 3 and 212 (median 70.0, IQR 35.0-127.5) for the ELISA, and between 3 and 197 (median 45.0, IQR 16.0-100.0) for the POC test.
The ELISA and POC test had similar diagnostic capability for identifying PMN > 250/μL in the subgroup of patients with liver cirrhosis (95 samples from 54 patients; ELISA AUC 0.987, and POC test AUC 0.982). In addition, when the ascites samples were analysed according to the SAAG > 11g/L (115 samples from 62 patients), the AUCs of ascitic calprotectin were 0.983 for the ELISA and 0.988 for the POC test (data not shown).
This prospective study evaluated the diagnostic utility of measuring calprotectin in ascites to identify ascitic PMN counts > 250/μL in patients referred for paracentesis, and provides the following new information: Patients with an elevated PMN count (> 250/μL) had higher ascitic calprotectin levels than those with normal cell counts; this finding indicates that ascitic calprotectin levels correlate well and reliably with PMN count. It is clinically significant that calprotectin levels in ascitic patients can identify elevated PMN counts using both laboratory-based ELISA and bedside POC testing. Indeed, ascitic calprotectin may serve as a surrogate marker for PMN count and would be amenable to routine SBP screening, especially when measured by a bedside test.
Ascites is commonly found in patients with liver cirrhosis and may promote bacterial translocation, enhancing the risk of SBP[3]. SBP in outpatients is rare, but when it occurs it often requires hospitalisation to manage to disease course[4,5]. In our study, four of 71 patients were diagnosed with SBP (5.6%). In general, SBP symptoms are nonspecific and current guidelines recommend paracentesis be performed in all patients with ascites to rule out abdominal infection[6,7]. The diagnosis of SBP in patients with liver cirrhosis is based on a PMN count of > 250/μL in ascitic fluid, with or without positive bacterial cultures[5-7]. This cut-off is recognized as more sensitive than other criteria (PMN > 500/μL; white blood cell count > 500/μL)[38,39] for identifying SBP[40]. SBP diagnosis based solely on bacterial culture is considered unreliable, since up to 60% of patients with increased PMN count are reported as culture-negative[41,42]. In our study, all patients with culture-positive abdominal infection, including both SBP and secondary peritonitis patients, had elevated PMN counts. In the four SBP patients, the bacterial cultures were positive for only one (25.0%).
Our study measured calprotectin in ascitic fluid in 130 unselected samples from 71 consecutive patients. Ascitic calprotectin levels correlated well and reliably with PMN counts, and the samples with PMN > 250/μL also had higher ascitic calprotectin levels than the samples with PMN ≤ 250/μL. Both the ELISA and the POC test accurately measured ascitic calprotectin, and the correlation between the two tests was excellent with high sensitivity (95% and 100%, respectively) and high specificity (89% and 85%, respectively) at the optimal cut-off points (from ROC analysis). In a diagnostic test that is used to screen for a specific disease, it is preferred to test all patients at risk, especially when potentially life-threatening complications may occur. In screening tests, high sensitivity is therefore favoured over high specificity. In our study, the NPVs of calprotectin testing in ascites were excellent (99% for the ELISA and 100% for the POC test). Notably, these results suggest that no patient with elevated PMN count would have been missed by the bedside test.
In daily clinical practice, PMN count is often not readily available and clinicians frequently rely on total cell count when initiating empiric antibiotic treatment[43]. It has been suggested that a total cell count < 1000/μL (obtained from automated cell counting procedures) is unlikely to signify SBP, having a NPV of 96%[44]. In our study, using such a criteria would have misclassified five patients (26.3%) with elevated PMN counts. Moreover, the use of total cell count in combination with ascitic calprotectin measurement did not increase the diagnostic accuracy of calprotectin testing, as calculated by ROC analysis (data not shown).
To avoid diagnostic delay, it has been proposed that automated PMN counting should replace the laborious and time-consuming manual cell counting technique[8,9]. Studies have demonstrated that automated blood cell counts correlate well with manual ascitic leukocyte differential counts[45]. However, despite the potential benefit of automated cell counters in clinical practice, widespread use of this technology is limited by the cost of the sophisticated laboratory equipment and requirement for trained operators; this is a particular challenge for practitioners’ office settings and small clinics without in-house laboratories.
The use of reagent strips (urine dipsticks) for PNM counting (by colorimetric detection of leukocyte esterase activity) has also been evaluated as a rapid SBP diagnostic tool[45,46]. A number of these studies have reported sensitivities between 85% and 100% and specificities between 90% and 100%[10-25]. However, these results came from mostly single-centre studies with small numbers of SBP cases. The only large, multicentre study reported in the literature produced very different results; in particular, using 2123 paracenteses, the sensitivity was only 45% for identifying PMN > 250/μL in cirrhotic patients[26]. Although specificity was still high, it was concluded that urinary dipstick testing lacks sufficient accuracy for diagnosing SBP. The risk of false negative results seemed to be especially problematic in patients with lower PMN counts[46]. These results dampened the initial enthusiasm for reagent strips, and currently this method is not recommended for rapid diagnosis of SBP[45].
Only one study in the literature, to date, has provided data on calprotectin measurement in ascites[35]. In that study, Homann et al[34] compared ascites from patients with malignant disease to ascites from patients with non-malignant disease. Higher ascitic calprotectin levels were found in the malignant patients and shown to correlate with increased mortality in patients with decompensated liver cirrhosis. However, the authors did not investigate the diagnostic potential of ascitic calprotectin. More recently, Parsi et al[27] measured ascitic lactoferrin (an iron-binding protein also found in PMNs) in cirrhotic patients with ascites and investigated its potential for identifying SBP. The lactoferrin measurements correctly identified PMN counts > 250/μL in 22 of 218 samples (10.1%), yielding a sensitivity of 95.5% and specificity of 97.0%. However, the quantitative assay (ELISA) used in that study is not commercially available, and to date no bedside test, qualitative or quantitative, exists for lactoferrin.
The results from our current study confirm the findings reported by Parsi et al[27]. Specifically, we show that measurement of calprotectin, a leukocyte-specific protein, may serve as a surrogate marker for the PMN count in ascitic fluid. A particular strength of our study is the quantitative measurement of calprotectin by two methods, a laboratory-based ELISA and a commercially available bedside test. Rapid bedside measurement is advantageous for hospitalised patients, as it supports early antibiotic intervention and limits unnecessary treatments. It will also benefit the outpatient setting by providing on-site testing, since samples are otherwise required to be transported to an offsite laboratory. The POC test that we used can accomplish quantitative measurement of ascitic calprotectin within minutes, and this feature is expected to minimize the problems associated with diagnostic delay that clinicians currently face. Additionally, the cost of POC testing may be less than the other methods, such as contracting with the offsite laboratories.
There are several limitations to the current study that merit consideration. First, the prevalence of SBP in our study cohort was lower than expected from the literature. Second, we included all patients with ascites, irrespective of the aetiology, and it may be that our results cannot be generalised to all patients with liver cirrhosis. Third, this was an exploratory study that aimed to establish the concept of measuring a PNM-related inflammatory protein, rather than PNM cells themselves, as an indicator of elevated cell count in ascites; therefore, no formal sample size calculation was performed. Finally, our sample size was small and larger studies are needed to evaluate this test in different clinical settings and to establish a reliable cut-off for ascitic calprotectin for optimal identification of PMN counts > 250/μL.
In conclusion, we have demonstrated that measurement of calprotectin in ascitic fluid correlates well with the PMN count and reliably predicts levels > 250/μL. Additionally, we showed that an elevated PMN count could easily be measured by a POC test device which may enable a treating physician to obtain useful bedside measurements, especially those practicing in settings with limited equipment and/or technical personnel. Further studies are warranted to define a clinically useful cut-off for the diagnosis of SBP in cirrhotic patients with ascites.
We would like to express our sincere gratitude to the patients and staff of the Gastroenterology Department, especially to Eric Pflimlin and Margot Brenneisen, and to the laboratory technicians, especially Chantal Brülhardt, for their valuable efforts. In addition, we would like to thank Kathleen A Bucher for English language editing of the manuscript.
Ascites is the most common complication of patients with cirrhosis, and around 60% of patients will develop ascites within 10 years of disease commencement. Spontaneous bacterial peritonitis (SBP) is an important cause of morbidity and mortality in these patients. SBP is estimated to affect 10%-30% of hospitalised patients with ascites, and it is recommended that all patients with ascites undergo paracentesis at the time of admission to assess SBP status and initiate timely therapy.
The diagnosis of SBP is based on a polymorphonuclear leukocyte (PMN) cell count > 250/μL in the ascitic fluid. Currently, differential cell count is usually performed manually using light microscopy and counting chambers. This procedure is time consuming, and diagnosis may be further delayed when laboratory personnel are not readily available. Several other methods to diagnose SBP (automated cell counting and urine dipstick-based screening for leukocytes) have proven unreliable in clinical practice and are inferior to the manual method. In this study, authors investigated the potential of calprotectin, a neutrophilic protein and established marker of intestinal inflammation, to screen for SBP when measured in ascites.
To date, only one study in the public literature has measured calprotectin in ascites, and the conclusion was that higher concentrations of calprotectin exist in malignant disease conditions as compared to non-malignant conditions. However, diagnostic accuracy was not assessed and calprotectin was measured using a laboratory-dependent enzyme-linked immunosorbent assay (ELISA). In this study, authors have demonstrated that measurement of calprotectin in ascitic fluid correlates well with PMN count and reliably indicates PNM levels > 250/μL. Additionally, we showed that an elevated PMN count could be easily measured within minutes using a point-of-care (POC) bedside test, suggesting its potential as a rapid diagnostic approach for SBP.
The rapid diagnosis of SBP and immediate start of antibiotic treatment is of paramount importance as mortality estimates approach 30%. A particular strength of this study is the quantitative measurement of ascitic calprotectin by two methods: a laboratory-based ELISA and a commercially available POC test device. Rapid bedside measurement is advantageous for hospitalised patients, since it facilitates timely antibiotic therapy and minimizes unnecessary treatments. However, it may be especially beneficial to an outpatient setting, where samples are otherwise required to be transported to an offsite laboratory for testing. The POC test device that we used allows quantitative measurement of ascitic calprotectin within minutes and is likely to minimize the diagnostic delay that clinicians currently face.
Calprotectin, a calcium and zinc-binding protein, is found almost exclusively in neutrophils. The presence of calprotectin in body fluids is proportional to the influx of neutrophils during inflammation.
This article discusses a new method for the diagnosis of SBP. This is a well-designed and methodologically correct exploratory study.
P- Reviewers Montalto G, Desai D, Gaya DR S- Editor Wen LL L- Editor A E- Editor Xiong L
| 1. | Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2119] [Article Influence: 111.5] [Reference Citation Analysis (2)] |
| 3. | Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 709] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis--in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol. 2001;96:1232-1236. [PubMed] |
| 5. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 611] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 6. | Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 613] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1129] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 8. | Angeloni S, Nicolini G, Merli M, Nicolao F, Pinto G, Aronne T, Attili AF, Riggio O. Validation of automated blood cell counter for the determination of polymorphonuclear cell count in the ascitic fluid of cirrhotic patients with or without spontaneous bacterial peritonitis. Am J Gastroenterol. 2003;98:1844-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Cereto F, Genescà J, Segura R. Validation of automated blood cell counters for the diagnosis of spontaneous bacterial peritonitis. Am J Gastroenterol. 2004;99:1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Vanbiervliet G, Rakotoarisoa C, Filippi J, Guérin O, Calle G, Hastier P, Mariné-Barjoan E, Schneider S, Piche T, Broussard JF. Diagnostic accuracy of a rapid urine-screening test (Multistix8SG) in cirrhotic patients with spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2002;14:1257-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Castellote J, López C, Gornals J, Tremosa G, Fariña ER, Baliellas C, Domingo A, Xiol X. Rapid diagnosis of spontaneous bacterial peritonitis by use of reagent strips. Hepatology. 2003;37:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Thévenot T, Cadranel JF, Nguyen-Khac E, Tilmant L, Tiry C, Welty S, Merzoug N. Diagnosis of spontaneous bacterial peritonitis in cirrhotic patients by use of two reagent strips. Eur J Gastroenterol Hepatol. 2004;16:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Butani RC, Shaffer RT, Szyjkowski RD, Weeks BE, Speights LG, Kadakia SC. Rapid diagnosis of infected ascitic fluid using leukocyte esterase dipstick testing. Am J Gastroenterol. 2004;99:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Sapey T, Kabissa D, Fort E, Laurin C, Mendler MH. Instant diagnosis of spontaneous bacterial peritonitis using leukocyte esterase reagent strips: Nephur-Test vs. MultistixSG. Liver Int. 2005;25:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Sapey T, Mena E, Fort E, Laurin C, Kabissa D, Runyon BA, Mendler MH. Rapid diagnosis of spontaneous bacterial peritonitis with leukocyte esterase reagent strips in a European and in an American center. J Gastroenterol Hepatol. 2005;20:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Kim DY, Kim JH, Chon CY, Han KH, Ahn SH, Kim JK, Paik YH, Lee KS, Moon YM. Usefulness of urine strip test in the rapid diagnosis of spontaneous bacterial peritonitis. Liver Int. 2005;25:1197-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Sarwar S, Alam A, Izhar M, Khan AA, Butt AK, Shafqat F, Malik K, Ahmed I, Niazi AK. Bedside diagnosis of spontaneous bacterial peritonitis using reagent strips. J Coll Physicians Surg Pak. 2005;15:418-421. [PubMed] |
| 18. | Wisniewski B, Rautou PE, Al Sirafi Y, Lambare-Narcy B, Drouhin F, Constantini D, Fischer D, Labayle D, Denis J. [Diagnosis of spontaneous ascites infection in patients with cirrhosis: reagent strips]. Presse Med. 2005;34:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Campillo B, Richardet JP, Dupeyron C. Diagnostic value of two reagent strips (Multistix 8 SG and Combur 2 LN) in cirrhotic patients with spontaneous bacterial peritonitis and symptomatic bacterascites. Gastroenterol Clin Biol. 2006;30:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Rerknimitr R, Rungsangmanoon W, Kongkam P, Kullavanijaya P. Efficacy of leukocyte esterase dipstick test as a rapid test in diagnosis of spontaneous bacterial peritonitis. World J Gastroenterol. 2006;12:7183-7187. [PubMed] |
| 21. | Braga LL, Souza MH, Barbosa AM, Furtado FM, Campelo PA, Araújo Filho AH. Diagnosis of spontaneous bacterial peritonitis in cirrhotic patients in northeastern Brazil by use of rapid urine-screening test. Sao Paulo Med J. 2006;124:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Torun S, Dolar E, Yilmaz Y, Keskin M, Kiyici M, Sinirtas M, Sarandol E, Gurel S, Nak SG, Gulten M. Evaluation of leukocyte esterase and nitrite strip tests to detect spontaneous bacterial peritonitis in cirrhotic patients. World J Gastroenterol. 2007;13:6027-6030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Gaya DR, David B Lyon T, Clarke J, Jamdar S, Inverarity D, Forrest EH, John Morris A, Stanley AJ. Bedside leucocyte esterase reagent strips with spectrophotometric analysis to rapidly exclude spontaneous bacterial peritonitis: a pilot study. Eur J Gastroenterol Hepatol. 2007;19:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Nobre SR, Cabral JE, Sofia C, Leitão MC. Value of reagent strips in the rapid diagnosis of spontaneous bacterial peritonitis. Hepatogastroenterology. 2008;55:1020-1023. [PubMed] |
| 25. | Ribeiro TC, Kondo M, Amaral AC, Parise ER, Bragagnolo Júnior MA, Souza AF. Evaluation of reagent strips for ascitic fluid leukocyte determination: is it a possible alternative for spontaneous bacterial peritonitis rapid diagnosis? Braz J Infect Dis. 2007;11:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Nousbaum JB, Cadranel JF, Nahon P, Khac EN, Moreau R, Thévenot T, Silvain C, Bureau C, Nouel O, Pilette C, Paupard T, Vanbiervliet G, Oberti F, Davion T, Jouannaud V, Roche B, Bernard PH, Beaulieu S, Danne O, Thabut D, Chagneau-Derrode C, de Lédinghen V, Mathurin P, Pauwels A, Bronowicki JP, Habersetzer F, Abergel A, Audigier JC, Sapey T, Grangé JD, Tran A. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology. 2007;45:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Parsi MA, Saadeh SN, Zein NN, Davis GL, Lopez R, Boone J, Lepe MR, Guo L, Ashfaq M, Klintmalm G. Ascitic fluid lactoferrin for diagnosis of spontaneous bacterial peritonitis. Gastroenterology. 2008;135:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Soyfoo MS, Roth J, Vogl T, Pochet R, Decaux G. Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J Rheumatol. 2009;36:2190-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Frosch M, Strey A, Vogl T, Wulffraat NM, Kuis W, Sunderkötter C, Harms E, Sorg C, Roth J. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Jung SY, Park YB, Ha YJ, Lee KH, Lee SK. Serum calprotectin as a marker for disease activity and severity in adult-onset Still’s disease. J Rheumatol. 2010;37:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkötter C, Foell D, Pasparakis M, Roth J, Goebeler M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol. 2006;155:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 305] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 34. | Homann C, Christensen E, Schlichting P, Philipsen EK, Graudal NA, Garred P. Ascites fluid and plasma calprotectin concentrations in liver disease. Scand J Gastroenterol. 2003;38:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 486] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 36. | Wassell J, Wallage M, Brewer E. Evaluation of the Quantum Blue® rapid test for faecal calprotectin. Ann Clin Biochem. 2012;49:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Sydora MJ, Sydora BC, Fedorak RN. Validation of a point-of-care desk top device to quantitate fecal calprotectin and distinguish inflammatory bowel disease from irritable bowel syndrome. J Crohns Colitis. 2012;6:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Albillos A, Cuervas-Mons V, Millán I, Cantón T, Montes J, Barrios C, Garrido A, Escartín P. Ascitic fluid polymorphonuclear cell count and serum to ascites albumin gradient in the diagnosis of bacterial peritonitis. Gastroenterology. 1990;98:134-140. [PubMed] |
| 39. | Yang CY, Liaw YF, Chu CM, Sheen IS. White count, pH and lactate in ascites in the diagnosis of spontaneous bacterial peritonitis. Hepatology. 1985;5:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Wong CL, Holroyd-Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA. 2008;299:1166-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 627] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 42. | Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 43. | Gerbes AL, Sauerbruch T, Dathe K. [Method report: German S3-guideline “ascites, spontaneous bacterial peritonitis, hepatorenal syndrome”]. Z Gastroenterol. 2011;49:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Link BC, Ziske CG, Schepke M, Schmidt-Wolf IG, Sauerbruch T. Total ascitic fluid leukocyte count for reliable exclusion of spontaneous bacterial peritonitis in patients with ascites. Eur J Gastroenterol Hepatol. 2006;18:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Riggio O, Angeloni S. Ascitic fluid analysis for diagnosis and monitoring of spontaneous bacterial peritonitis. World J Gastroenterol. 2009;15:3845-3850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Nguyen-Khac E, Cadranel JF, Thevenot T, Nousbaum JB. Review article: the utility of reagent strips in the diagnosis of infected ascites in cirrhotic patients. Aliment Pharmacol Ther. 2008;28:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |