Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.2005
Revised: December 27, 2012
Accepted: January 11, 2013
Published online: March 28, 2013
Processing time: 113 Days and 22.2 Hours
Gastric gastrointestinal stromal tumor (GIST), esophageal squamous cell carcinoma and gastric cardia adenocarcinoma are distinct neoplasms originating from different cell layers; therefore, simultaneous development of such carcinomas is relatively rare. Auxiliary examinations revealed coexistence of esophageal and gastric cardia carcinoma with lymph node metastasis in a 77-year-old man. Intraoperatively, an extraluminal tumor (about 6.0 cm × 5.0 cm × 6.0 cm) at the posterior wall of the gastric body, a tumor (about 2.5 cm × 2.0 cm) in the lower esophagus, and an infiltrative and stenosing tumor (about 1.0 cm × 2.0 cm) in the gastric cardia were detected. Wedge resection for extraluminal gastric tumor, radical esophagectomy for lower esophageal tumor, and cardiac resection with gastroesophageal (supra-aortic arch anastomoses) were performed. Postoperative histological examination showed synchronous occurrence of gastric GIST, esophageal squamous cell carcinoma, and gastric cardia adenocarcinoma. Furthermore, immunohistochemistry indicated strong staining for c-Kit/CD117, Dog-1, Ki-67 and smooth muscle, while expression of S-100 and CD34 was negative.
- Citation: Zhou Y, Wu XD, Shi Q, Jia J. Coexistence of gastrointestinal stromal tumor, esophageal and gastric cardia carcinomas. World J Gastroenterol 2013; 19(12): 2005-2008
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/2005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.2005
Recently, cases of synchronous development of a gastrointestinal stromal tumor (GIST) and another neoplasm with different incidence, etiology, evolution and prognosis have been reported more frequently[1-3]. Although squamous cell carcinoma and adenocarcinoma constitute the most common type of esophageal and gastric cardia tumor, respectively, simultaneous development of a GIST is relatively rare. Here, we report a case of synchronous occurrence of gastric GIST, esophageal squamous cell carcinoma, and gastric cardia adenocarcinoma.
A 77-year-old man presented with dysphagia for 2 mo. Upper gastrointestinal endoscopy was performed, which showed a tumor arising from the lower esophagus and extending into the lumen, and an ulcerated tumor located in the cardia, just below the gastroesophageal junction.
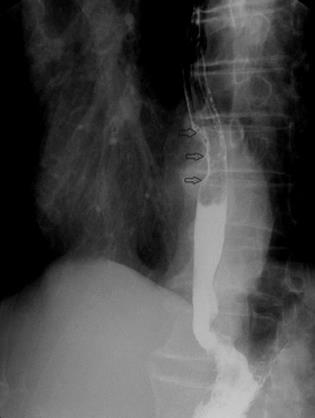
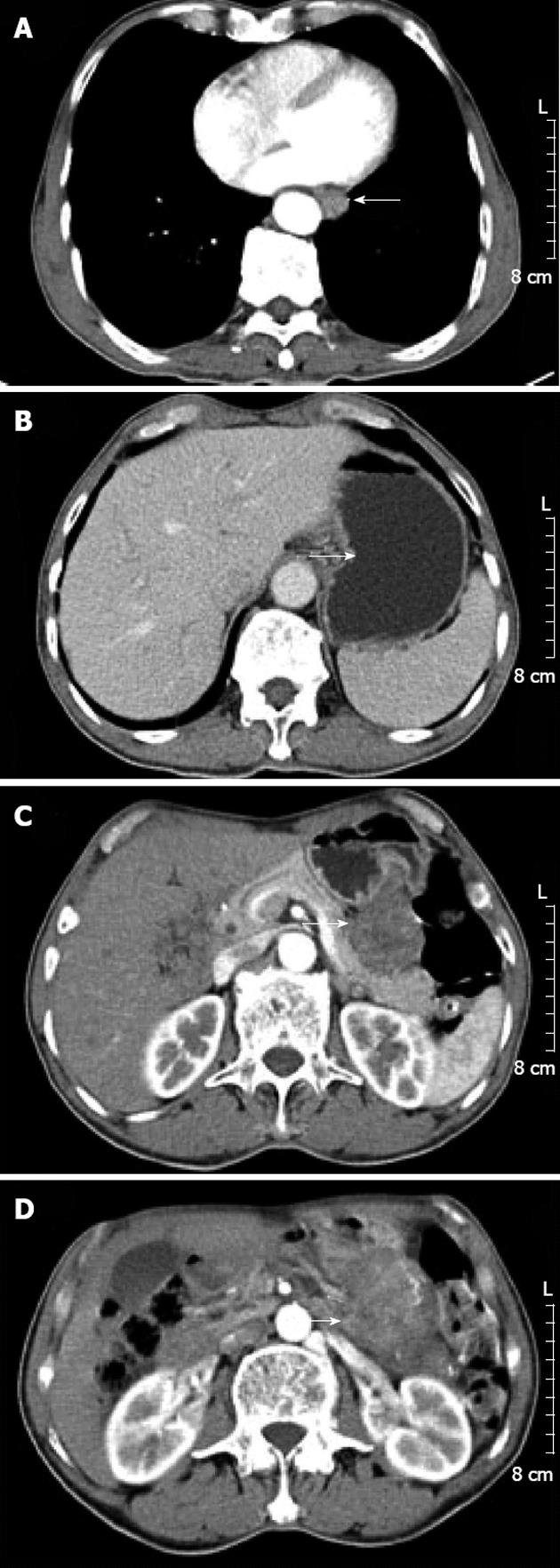
He had no relevant past history or family history. Clinical examination did not find any palpable abdominal mass. Laboratory examination was normal. Esophagography showed a filling defect in the anterior wall of the lower esophagus (Figure 1). Computed tomography (CT) showed circumferential thickening of the lower esophageal wall with loss of the lumen. Scanning at a lower level displayed focal thickening of the gastric cardia wall with marked enhancement. Furthermore, scans obtained at lower levels displayed a large, heterogeneous, round mass close to the greater curvature of the stomach. The patient was diagnosed presumptively with synchronous esophageal and gastric cardia carcinoma with lymph node metastasis (Figure 2).
Intraoperatively, an extraluminal tumor (about 6.0 cm × 5.0 cm × 6.0 cm) at the posterior wall of the gastric body, a tumor (about 2.5 cm × 2.0 cm) in the lower esophagus, and an infiltrative and stenosing tumor (about 1.0 cm × 2.0 cm) in the gastric cardia was detected. Wedge resection for extraluminal gastric tumor, radical esophagectomy for lower esophageal tumor, and cardiac resection with gastroesophageal (supra-aortic arch anastomoses) were performed.
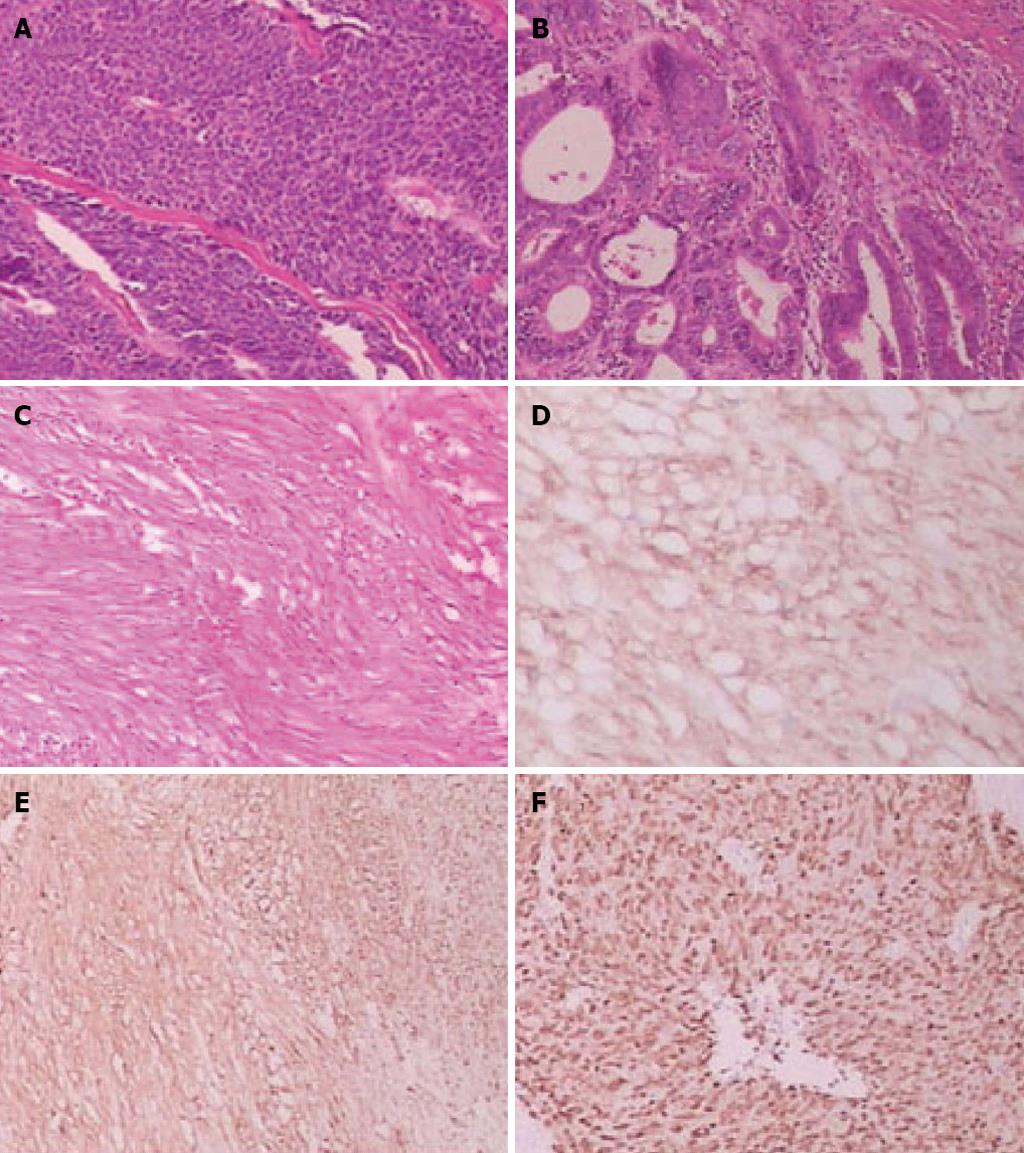
On histopathological examination, the gastric cardia tumor was a mid-differentiated gastric adenocarcinoma (pT1bN0M0), and the lower esophageal tumor was a low-mid-differentiated squamous cell carcinoma (pT3N0M0) (Figure 3). There was no vascular invasion and no lymph node metastasis.
Further histopathological examination of the extraluminal gastric tumor revealed GIST of the high-risk category, which showed a high mitotic index (> 10 mitoses/50 high-power fields). Immunohistochemistry indicated strong staining for c-Kit/CD117, Dog-1, Ki-67 and smooth muscle, while expression of S-100 and CD34 was negative (Figure 3). The patient was diagnosed with high-grade gastric GIST due to large tumor size (> 5 cm) and unfavorable histopathological features (high mitotic index and strong positivity for Ki-67).
GISTs are rare, accounting for only 0.1%-3% of all gastrointestinal malignancies. Primary GISTs arise most commonly in the stomach (50%-70%), followed by the small intestine (25%-35%), colon and rectum (5%-10%) and esophagus (< 5%)[4]. These tumors are considered to originate from interstitial cells of Cajal or their precursors, because both strongly express the c-Kit protein (CD117; a type III tyrosine kinase receptor encoded by the c-Kit proto-oncogene)[5].
Radical surgery is the main treatment in primary resectable GISTs. Recurrence, metastatic disease or unresectable tumors could be treated with imatinib (a small-molecule tyrosine-kinase inhibitor)[6].
Adenocarcinoma of the stomach ranks as the second most common cancer worldwide, which comprises 80% of all stomach cancers. Squamous cell carcinoma mainly occurs in the mid to lower esophagus, and is not commonly accompanied by other cancerous lesions. Suzuki et al[7] have reported that most lesions are in the stomach (59.6%), followed by the colon and rectum (12.3%). Various hypotheses, such as gene mutation, expression of metallothioneins, neighboring tissues being influenced by the same carcinogens, have been proposed regarding the simultaneous development of GIST and other cancers[8,9]. However, at present, no data are available to support such hypotheses. Furthermore, simultaneous occurrence of gastric GIST, esophageal squamous cell carcinoma, and gastric adenocarcinoma has not often been reported in the literature. Simple coincidence could be the most reasonable explanation.
For patients with primary GIST, surgical resection is the only chance for cure. Resection can usually be accomplished with only wedge resection of the stomach or segmental resection of the small bowel for small GISTs, whereas extensive surgery is occasionally required for larger or poorly positioned GISTs[6].
The only curative treatment for esophageal or gastric cardia cancer is surgical resection. After esophagectomy, digestive tract reconstruction can be accomplished using the remaining stomach, depending on the location of the gastric tumor. The colon or jejunum is the frequent choice for esophageal substitution. In our opinion, digestive tract reconstruction with the remaining stomach should be a reasonable choice for old people (age > 75 years). Although the GIST is large, only wedge resection of the stomach was performed to keep enough stomach for digestive tract reconstruction.
In our case, we considered gastric adenocarcinomas to be an early stage gastric cancer and esophageal squamous cell carcinoma to be a middle stage esophageal cancer. Meantime, the patient was diagnosed with high-grade gastric GIST due to large tumor size (> 5 cm) and unfavorable histopathological features (high mitotic index and strong positivity for Ki-67). Therefore, we suggested that the patient should undergo chemoradiation therapy and adjuvant imatinib treatment. However, the patient refused. A postoperative CT scan performed 3 mo later showed no evidence of tumor recurrence. The patient needs a long follow-up period.
P- Reviewer Peparini N S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L
| 1. | Theodosopoulos T, Dellaportas D, Psychogiou V, Gennatas K, Kondi-Pafiti A, Gkiokas G, Papaconstantinou I, Polymeneas G. Synchronous gastric adenocarcinoma and gastrointestinal stromal tumor (GIST) of the stomach: a case report. World J Surg Oncol. 2011;9:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Yamamoto D, Hamada Y, Tsubota Y, Kawakami K, Yamamoto C, Yamamoto M. Simultaneous development of adenocarcinoma and gastrointestinal stromal tumor (GIST) in the stomach: case report. World J Surg Oncol. 2012;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Firat Ö, Çalişkan C, Karaca C, Sezak M, Özütemız Ö, Ersın S, Güler A. Coexistence of gastric cancer and gastrointestinal stromal tumor: report of two cases. Turk J Gastroenterol. 2010;21:302-304. [PubMed] |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1176] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | Feng F, Liu XH, Xie Q, Liu WQ, Bai CG, Ma DL. Expression and mutation of c-kit gene in gastrointestinal stromal tumors. World J Gastroenterol. 2003;9:2548-2551. [PubMed] |
| 6. | Chaudhry UI, DeMatteo RP. Advances in the surgical management of gastrointestinal stromal tumor. Adv Surg. 2011;45:197-209. [PubMed] |
| 7. | Suzuki S, Nishimaki T, Suzuki T, Kanda T, Nakagawa S, Hatakeyama K. Outcomes of simultaneous resection of synchronous esophageal and extraesophageal carcinomas. J Am Coll Surg. 2002;195:23-29. [PubMed] |
| 8. | Rauf F, Ahmad Z, Muzzafar S, Hussaini AS. Synchronous occurrence of gastrointestinal stromal tumor and gastric adenocarcinoma: a case report. J Pak Med Assoc. 2006;56:184-186. [PubMed] |
| 9. | Nemes C, Rogojan L, Surdea-Blaga T, Seicean A, Dumitrascu DL, Ciuce C. Gastrointestinal stromal tumor (GIST) associated with synchronous colon adenocarcinoma - a case report. J Gastrointestin Liver Dis. 2012;21:101-103. [PubMed] |