Published online Feb 21, 2012. doi: 10.3748/wjg.v18.i7.685
Revised: August 18, 2011
Accepted: August 27, 2011
Published online: February 21, 2012
AIM: To assess the significance of chromosome translocation t(11;18)(q21;q21), B-cell lymphoma 10 (BCL-10) protein and Helicobacter pylori (H. pylori) infection in gastric mucosa-associated lymphoid tissue (MALT) lymphoma in Colombia.
METHODS: Fifty cases of gastric MALT lymphoma and their respective post-treatment follow-up biopsies were examined to assess the presence of the translocation t(11;18)(q21;q21) as identified by fluorescence in situ hybridization; to detect protein expression patterns of BCL10 using immunohistochemistry; and for evaluation of tumor histology to determine the correlation of these factors and resistance to H. pylori eradication.
RESULTS: Infection with H. pylori was confirmed in all cases of gastric MALT lymphoma in association with chronic gastritis. Bacterial eradication led to tumor regression in 66% of cases. The translocation t(11;18)(q21;q21) was not present in any of these cases, nor was there evidence of tumor transformation to diffuse large B-cell lymphoma. Thirty-four percent of the patients showed resistance to tumor regression, and within this group, 7 cases, representing 14% of all those analyzed, were considered to be t(11;18)(q21;q21)-positive gastric MALT lymphomas. Protein expression of BCL10 in the nucleus was associated with the presence of translocation and treatment resistance. Cases that were considered unresponsive to therapy were histologically characterized by the presence of homogeneous tumor cells and a lack of plasmacytic differentiation. Responder cases exhibited higher cellular heterogeneity and a greater frequency of plasma cells.
CONCLUSION: Both t(11;18)(q21;q21)-positive MALT lymphoma cases and those with nuclear BCL10 expression are considered resistant to H. pylori eradication. It is suggested that chronic antigenic stimulation is not a dominant event in resistant cases.
-
Citation: Yepes S, Torres MM, Saavedra C, Andrade R. Gastric mucosa-associated lymphoid tissue lymphomas and
Helicobacter pylori infection: A Colombian perspective. World J Gastroenterol 2012; 18(7): 685-691 - URL: https://www.wjgnet.com/1007-9327/full/v18/i7/685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i7.685
Gastric B-cell lymphoma (BCL) of mucosa-associated lymphoid tissue (MALT) is an indolent B-cell non-Hodgkin lymphoma that arises from the mucosal lymphoid tissue and is normally acquired as a reaction to Helicobacter pylori (H. pylori) infection[1-3]. This disease is considered one of the best models reflecting how genetic events lead to oncogenesis, determine tumor biology, dictate clinical behavior and represent viable therapeutic targets.
Gastric MALT lymphoma pathogenesis is a multistep process initiated by infection with H. pylori, which induces genetic abnormalities and subsequent malignant transformation. Gene alterations include trisomy of chromosomes 3, 7, 12 or 18 and the disease-specific chromosome translocations t(1;14)(p22;q32), t(14;18)(q32;q21), t(11;18)(q21;q21) and t(3;14)(p13;q32) resulting in IGH-BCL10, IGH-MALT1, API2-MALT1 and IGH-FOXP1 rearrangements respectively. Notably, these events are associated with the activation of nuclear factor-κB (NF-κB)[4,5].
Various studies of gastric MALT lymphoma have shown that t(11;18)(q21;q21) is present in approximately 30% of cases. The clinical importance of the translocation is well-characterized: positive cases are usually unresponsive to H. pylori eradication, which is the generally accepted first-choice therapy[6,7]. The translocation is often present in advanced cases of the disease but rarely undergoes high-grade transformation to diffuse large B-cell lymphoma (DLBCL)[8-10].
However, complete tumor regression is known to occur in 20% of patients with t(11;18)(q21;q21)-positive disease. Therefore, all patients who have H. pylori-positive gastric MALT lymphoma should undergo eradication therapy, regardless of their t(11;18)(q21;q21) status[11]. On the other hand, new evidence has shown that translocations can be found at approximately equivalent frequencies in both gastric MALT lymphomas and gastric DLBCLs[12].
Although most patients with gastric MALT lymphoma experience an indolent clinical course[13], there is considerable individual variability in the extent of the disease response to treatment, relapse and event-free survival. Consequently, optimal management is changing, and there is a need for an improved understanding of the genetic abnormalities underlying gastric MALT lymphoma and for new genetic biomarkers that are able to guide prognosis and management.
The incidence of chromosomal translocations in gastric MALT lymphoma has been described mainly from patients in Western countries[14-17]. The prevalence of t(11;18)(q21;q21)-positive MALT lymphoma has been reported to be within a range of 15%-24% in Europe[14,15,18] but is considered relatively rare in the United States (5%)[17].
The incidence of MALT lymphoma and its genetic background has not been established and validated in Latin America; likewise, the ideal prognosis and management of the Colombian patient population is unclear given the complex genetic background of the disease.
In Colombia, the identification of MALT lymphoma resistant to H. pylori eradication therapy is not typically considered in initial diagnoses and patient classification is possible only after repeated follow-up assessments involving endoscopy and histology. This situation warrants validation and a search for biomarkers of treatment response to direct the treatment regimen.
In the present study, we attempted to evaluate the t(11;18)(q21;q21) status and BCL10 staining patterns in patients with gastric MALT lymphomas and the relationship among these factors with the response to the eradication of H. pylori in a sample of patients from Colombia.
Fifty cases of gastric MALT lymphomas were retrieved from the archives of the Department of Pathology, Fundación Santa Fe de Bogotá. All of these cases had hematoxylin and eosin-stained sections and immunohistochemistry (IHC)-marked slides available for reevaluation. Cases selected must have had previous histopathological diagnoses, available archival material and complete information about diagnosis and disease monitoring.
Diagnosis of MALT lymphoma was made on the basis of morphological and immunophenotypic examination according to the criteria described by Isaacson et al[19] and subsequently included in the Revised European American Lymphoma/World Health Organization (WHO) classification. Other factors evaluated included the accompanying epithelium (assessment of gastritis, atrophy and metaplasia) and a characterization of the tumoral infiltrate (large cells, centrocyte-like/small lymphocytes, monocytoid cells, plasma cell differentiation, residual follicles, colonization follicles and Dutcher’s bodies). Detection of H. pylori was performed by histology and/or histochemistry using a Genta stain on all follow-up biopsies.
All 50 cases analyzed were treated with triple antibiotic therapy as the first-choice measure. This consisted of a proton pump inhibitor (such as omeprazole, lansoprazole or pantoprazole) plus two antibiotics (amoxicillin and clarithromycin) administered according to the manufacturer’s recommended dose for 14 d. To confirm H. pylori eradication, a first endoscopic mucosal biopsy was conducted 2-3 mo after the cessation of antibiotic treatment, followed by endoscopy every 6 mo for at least 2 years at the discretion of the treating physician.
Surveillance of patients was performed by serial gastric biopsies to allow the histological evaluation of the tumor’s response to treatment. Histological responses were graded using the Wotherspoon histological score. This system assigns a score and diagnosis based on histological characteristics of the tissue: a score of zero represents a normal diagnosis with scattered plasma cells in the lamina propria; score of one represents chronic active gastritis with small clusters of lymphocytes in the lamina propria, a lack of lymphoid follicles and lymphoepithelial lesions; a score of two represents chronic active gastritis with florid lymphoid follicle formation, prominent lymphoid follicles with surrounding mantle zone and plasma cells, and the absence of lymphoepithelial lesions; a score of three represents a likely reactive suspicious lymphoid infiltrate with lymphoid follicles surrounded by small lymphocytes that infiltrate diffusely into the lamina propria and occasionally into the epithelium; a score of four represents a suspicious lymphoid infiltrate, likely lymphoma, and lymphoid follicles surrounded by marginal zone cells that infiltrate diffusely into the lamina propria and the epithelium in small groups; a score of five represents MALT lymphoma with the presence of dense diffuse infiltrates of marginal zone cells into the lamina propria with prominent lymphoepithelial lesions[20].
Lymphoma remission was investigated by regular endoscopic examinations, including multiple biopsies, conducted at 6-mo intervals. Histological responses were graded using the Wotherspoon histological score, considering scores 0-2 to be a complete lymphoma regression (CR), score 3 to be a partial remission and scores 4-5 to be no response (no change). A persistence score of 5, mainly characterized by the presence of lymphoepithelial lesions after antibiotic treatment, was the criterion for tumor resistance to therapy[20].
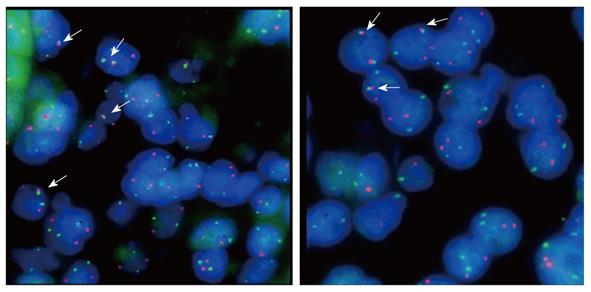
The authors performed manual microdissection of tumor tissue from each of the biopsies with initial diagnoses of gastric MALT lymphoma and subsequently organized the tissues for inclusion in a new block, generating tissue microarrays to perform fluorescent in-situ hybridization (FISH) and IHC for BCL10 expression. The FISH for t(11;18)(q21;q21) was performed using standardized protocols appropriate for paraffin-embedded tissue and the commercial Dual Color probe LSI API2-MALT1 (Vysis, Downers Grove, IL, United States).
The cut-off point for translocation-positive status was determined through the analysis of 6 control samples of hyperplastic tonsils fixed in neutral formalin and embedded in paraffin, and was defined as the arithmetic mean count of 100 nuclei plus three standard deviations obtained for each sample. Assessments were performed using an Olympus BX51 fluorescence microscope with a 100-watt bulb for each 100 cells examined.
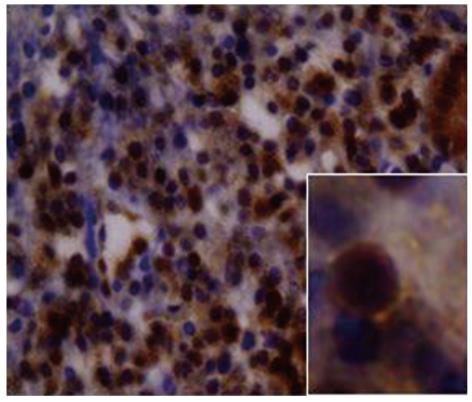
Immunohistochemical analysis using standardized procedures for CD20, CD3, CD43, BCL2, cytokeratin and kappa and lambda light chain immunoglobulin antibodies was performed to confirm the MALT lymphoma diagnosis and in follow-up biopsies when necessary. Expression of BCL10 was also determined using the immunoperoxidase technique in paraffin-embedded sections with a monoclonal mouse anti-human BCL10 antibody (Dako-Cytomation, Glosturp, Denmark). Briefly, 4-μm-thick paraffin sections were placed on silanized slides, dewaxed in xylene and hydrated through graded solutions of alcohol. Slides were then immersed in 0.01 mol/L citrate buffer (pH 6.0) and epitope retrieval was performed using a pressure cooker. The sections were then incubated with the diluted (1:40) primary antibodies and staining was performed using the EnVision system (DakoCytomation) according to the manufacturer’s recommendations. Hyperplastic tonsils that were positive for cytoplasmic expression of the protein were used in each experiment as a control. Samples were considered positive for nuclear expression of BCL10 when detected in more than 5% of the nuclei of tumor cells.
H. pylori infection was confirmed in all cases of gastric MALT lymphoma in association with chronic gastritis. Ninety percent of cases showed the presence of H. pylori in the histological analysis of early gastric biopsies; in the remaining 10%, the presence of bacteria was documented in subsequent biopsies before the initiation of the treatment and infection was scored as mild in most cases.
The patients were classified as responders or non-responders to treatment (Table 1). All patients classified as responders showed lymphoma regression, and the median time for follow-up after remission was 29 mo (range: 6-39 mo). There were 33 (66%) patients considered as responders to treatment and 17 (34%) patients considered non-responders who did not reach remission after H. pylori eradication. The median time to CR after the completion of antibiotic therapy was 3.2 mo (range: 1-18 mo). The bacteria were successfully eradicated in all patients treated with the standard triple therapy applied, including all non-responders.
| Gastric maltlymphoma | Treatmentresponse | API2-MALT1positive | API2-MALT1negative |
| A group | Responders | 0 | 33 |
| B group | Non-responders | 7 | |
| C group | Non-responders | 10 |
Non-responder cases were defined as tissues with histological persistence of gastric MALT lymphoma infiltrate despite successful H. pylori eradication as determined by histological and immunohistochemical examination. Within this group, seven cases, representing 14% of all cases analyzed, were considered positive for translocation. Cases were considered positive when more than 6% of tumor cells demonstrated the presence of fusion protein for a minimum count of 100 cells, which was the parameter set as the cut-off point for translocation-positive status for the technique. There was a statistically significant association between treatment response and the presence of the t(11;18)(q21;q21) translocation (Yales χ2 = 12.57, P < 0.05) because there were 7 patients within the group of non-responders who displayed the chromosomal arrangement of interest. Figure 1 shows the fluorescence patterns in positive and negative cases in the translocation study. When the response to treatment and the BCL10 staining pattern were analyzed, it was observed that all non-responders had nuclear expression of the BCL10 protein in a moderately nuclear pattern, as is shown in Figure 2. In addition, these cases showed evidence of translocation t(11;18)(q21;q21). However, in the responder group, BCL10 expression was confined to the cytoplasm. This finding confirms results reported elsewhere. Based on these results, the authors divided the cases into three groups (Table 1). Group A: patients who responded to the eradication and were negative for the studied translocation (66%); group B: patients who did not respond to eradication and were negative for t(11;18)(q21;q21) (20%); and group C: cases that did not respond to eradication of H. pylori and were positive for t(11;18)(q21;q21) (14%).
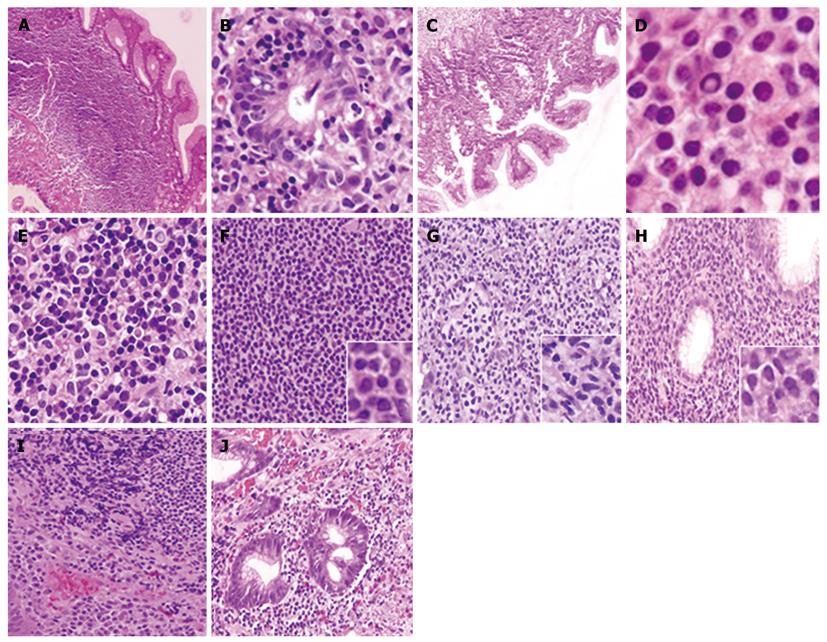
The results of the comparisons between groups A and B (responders vs non-responders with fusion), show statistically significant differences in plasma cell differentiation variables with a higher percentage in group A. These groups also differed in the nuclear expression of BCL10 protein, which was observed more frequently in group B. The other variables showed no significant associations. Comparisons between groups A and C (responders vs non-responders without fusion) showed statistically significant differences in the presence of centrocytes/small lymphocytes, which appeared at a higher rate in group C, and nuclear expression of BCL10, which also occurred at a higher percentage in group C. Other variables did not show any significant associations. Comparisons between groups B and C did not show any significant associations with the variables assessed (Table 2). Characteristic examples of the morphology described above are presented in Figure 3.
| Features | Group | P value | ||||
| A | B | C | A vs B | A vs C | B vs C | |
| n (%) | 33 (66) | 10 (20) | 7 (14) | |||
| Large cells | ||||||
| Positive | 8 (23.2) | 0 | 0 | > 0.05 | > 0.05 | NA |
| Negative | 25 (75.8) | 10 (100) | 7 (100) | |||
| Centrocyte-like/small lymphocytes | ||||||
| Positive | 16 (48.5) | 6 (60) | 7 (100) | > 0.05 | < 0.05a | > 0.05 |
| Negative | 17 (51.5) | 4 (40) | 0 | |||
| Monocytoid cells | ||||||
| Positive | 8 (24.2) | 3 (30) | 0 | > 0.05 | > 0.05 | > 0.05 |
| Negative | 25 (75.8) | 7 (70) | 7 (100) | |||
| Plasma cell differentiation | ||||||
| Positive | 18 (54.5) | 1(10) | 1 (14.3) | < 0.05a | > 0.05 | > 0.05 |
| Negative | 15 (45.5) | 9 (90) | 6 (85.7) | |||
| Residual follicles | ||||||
| Positive | 5 (15.2) | 2 (20) | 0 | > 0.05 | > 0.05 | > 0.05 |
| Negative | 28 (84.8) | 8 (80) | 7 (100) | |||
| Colonization follicles | ||||||
| Positive | 6 (18.2) | 3 (30) | 1 (14.3) | > 0.05 | > 0.05 | > 0.05 |
| Negative | 27 (81.8) | 7 (70) | 6 (85.7) | |||
| Dutcher bodies | ||||||
| Positive | 6 (18.2) | 0 | 0 | > 0.05 | > 0.05 | NA |
| Negative | 27 (81.8) | 10 (100) | 7 (100) | |||
| B-cell lymphoma 10 | ||||||
| Positive | 0 | 10 (100) | 7 (100) | < 0.05a | < 0.05a | NA |
| Negative | 32 (100) | 0 | 0 | |||
Known predictive factors for assessing the outcome of MALT lymphoma include the evaluation of transformation to a high-grade B-cell lymphoma, clinical stage of the disease and molecular markers of response to treatment. It is now known that the translocation t(11;18)(q21;q21) occurs frequently in gastric MALT lymphomas in advanced stages of the disease showing resistance to treatment. However, new evidence has recently demonstrated that tumor regression might also be achieved in patients with t(11;18)(q21;q21) after H. pylori eradication[11]. In addition, it has been recognized that the presence of t(11;18)(q21;q21) in gastric MALT lymphomas does not exclude progression to DLBCL[4,12]. Further research is necessary to understand the molecular mechanisms involved in these latter situations.
Complete clinical staging for this study could not be obtained for all patients because cases studied were referred for pathological evaluation from different institutions. Also, this work was focused on the histological characteristics, response to bacterial eradication treatment and presence of molecular markers such as t(11;18)(q21;q21) and BCL10 protein expression. Outside of these factors, there are other variables that may also help predict the course of disease.
These results have demonstrated that BCL10 nuclear expression and the determination of the t(11;18)(q21;q21) translocation involved in neoplastic signaling processes mediated by NF-κB are two useful markers for predicting the independent status of H. pylori in gastric MALT lymphomas; its presence, therefore, allows for the identification of patients who will not respond to bacterial eradication therapy as a means of promoting tumor regression. The resistance of the tumor to therapy observed in translocation-positive cases is explained by the fact that the fusion protein generates a bypass of the NF-κB signaling pathway thereby allowing constitutive activation of the transcription factor, self-generating growth and independent antigenic stimulation. On the other hand, the association of BCL10 with tumor resistance can be explained by the presence of nuclear export signals of the gene MALT1 that allows the export of BCL10 from the nucleus to the cytoplasm. Nakagawa et al[21] argued that IAP2 usually mediates BCL10 degradation through its ubiquitin ligase activity and inhibits antigen-receptor-mediated signaling. This normal function is absent in the fusion protein, resulting in the stability of BCL10 in lymphomas with t(11;18)(q21;q21) and the activation of the NF-κB signal transduction pathway. Consequently, the translocation generates a gain-of-function activity in MALT1 and a loss of API2 function.
Thirty-four percent of patients treated with antibiotic therapy in the sample group showed resistance to tumor regression; within this group, there were cases in which the fusion signal was detected by FISH, a finding that may explain the mechanism of autonomous growth and independence of antigenic stimulation as previously discussed. This study therefore confirms that patients with extranodal marginal zone lymphoma carrying t(11;18)(q21;q21) do not respond to treatment for H. pylori as a therapeutic measure and should be treated by other methods, such as radiotherapy and chemotherapy. These results also endorse the recommendation that every patient with a diagnosis of gastric MALT lymphoma should be evaluated for the presence of the aforementioned translocation by molecular methods.
Treatment resistance in cases that do not have the t(11;18)(q21;q21) translocation could possibly be due to the carrying of other molecular aberrations, such as t(1;14)(p22;q32). However, such cases would have shown evidence of a strong nuclear BCL10 staining pattern and/or genetic evidence of the translocation.
Patients in whom the aforementioned translocations are not detected frequently have trisomy of chromosomes 3, 7, 12 or 18; these cases are still considered dependent on antigenic stimulation. Additionally, it is known that the trisomy of these chromosomes is seen in cases where transformation to high-grade tumors is present after the inactivation of the P53 gene[21]. t(11;18)(q21;q21) and different aneuploidies, such as trisomy of chromosome 3, 7, 12 or 18, are usually considered mutually exclusive events, suggesting that at least two different routes lead to the development of the lymphoma. In patients with aneuploidy events, the oncogenesis mechanism is unclear; it is proposed that cell growth may be induced as a result of high copy numbers of genes involved in proliferation. Several genes located on chromosome 3 have been suggested as possibly responsible for these changes, including BCL6 and FOXP1[21].
The authors found that cases classified as non-res-ponders to therapy and positive for translocation are histologically characterized by the presence of more homogeneous tumor cells. In most cases, these cells were predominantly centrocyte-like/small lymphocytes, and this cell homogeneity could possibly result from a process of clonal selection. Additionally, in cases not responsive to therapy, fewer tumor cells appeared to differentiate into plasma cells, suggesting a decrease in the immune response by memory cells of the marginal zone. This is unlike the prominent cellular heterogeneity seen in responder patients, although this result has not been extensively studied and would require larger sample sizes to avoid possible statistical bias.
We applied Wotherspoon’s criteria for histological evaluation of response to treatment given the current widespread use of this system adopted by the WHO in 2001 for the histological differential diagnosis of gastric MALT lymphoma and extensive use by pathologists worldwide for the evaluation of therapeutic response. However, there are other useful systems proposed for the evaluation of therapeutic response to treatment, such as the Groupe d’Etude des Lymphomes de l’Adult (GELA) system that can be used for the same objective[22]. There are no comparative studies that convincingly prove the superiority of any system, even though the GELA system has the advantage of assessment of stromal changes in the lamina propria[23]. The GELA system may become a useful tool if its reproducibility can be confirmed by further testing in a large series[24]. Therefore, the utilization of the WHO/Wotherspoon score accompanied by the assessment of stromal changes for the post-treatment evaluation in MALT lymphoma is an interesting topic for future studies.
The authors thank Dr. Margarita Baldión for choosing tumor material, Dr. Adriana Plata for her assistance in the FISH procedure and Yubeli Abello for her technical collaboration.
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is caused by infection with Helicobacter pylori (H. pylori), which induces genetic abnormalities and malignant transformations. H. pylori eradication therapy leads to complete lymphoma regression in some patients in the early stages of the disease.
There is considerable individual variability in the extent of the disease, response to treatment, relapse and event-free survival. Consequently, optimal management should be established and there is a need for improved understanding of the genetic abnormalities underlying gastric MALT lymphoma.
This is the first study in Colombia concerning the assessment and significance of the gastric MALT lymphoma-associated t(11;18)(q21;q21) chromosomal translocation and its relationship with H. pylori infection in a country with a high incidence of cancer associated with virulent H. pylori strains, a significant cause of mortality and a major public health problem.
This study confirms, in a previously unexplored population, the importance of molecular markers in the selection of different treatment regimens for individual patients to increase the probability of survival.
Gastric MALT lymphoma is a low-grade malignant lymphoma of the stomach originating from B cells and is associated with chronic infection by H. pylori, a gram-negative bacterium strongly linked to the development of inflammation and stomach cancer. The disease carries chromosomal aberrations such as t(11;18)(q21;q21), useful for the evaluation of therapeutic response.
It was shown that the presence of t(11;18)(q21;q21) and nuclear expression of BCL10 are associated with the treatment resistance. These findings have been documented in patients of other ethnic origins and are thus confirmatory; the results are of relevance to disease management in patients.
Peer reviewers: Hong-Xiang Liu, PhD, Department of Pathology, Division of Molecular Histopathology, University of Cambridge, PO Box 231, Level 3, Lab Block, Addenbrooke’s Hospital, Hills Road, Cambridge CB2 2QQ, United Kingdom; Yusuf Bayraktar, Professor, Department of Gastroenterology, School of Medicine, Hacettepe University, Ankara 06100, Turkey; Bernabe Matias Quesada, MD, Department of Surgery, Hospital Cosme Argerich, Talcahuano 944 9A, Buenos Aires 1013, Argentina
S- Editor Lv S L- Editor Logan S E- Editor Li JY
| 1. | Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1293] [Cited by in RCA: 1201] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 2. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1229] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 3. | Eck M, Schmausser B, Haas R, Greiner A, Czub S, Müller-Hermelink HK. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 138] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Sagaert X, Van Cutsem E, De Hertogh G, Geboes K, Tousseyn T. Gastric MALT lymphoma: a model of chronic inflammation-induced tumor development. Nat Rev Gastroenterol Hepatol. 2010;7:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1869] [Cited by in RCA: 1939] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 6. | Zullo A, Hassan C, Andriani A, Cristofari F, De Francesco V, Ierardi E, Tomao S, Morini S, Vaira D. Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: a pooled data analysis. Am J Gastroenterol. 2009;104:1932-1937; quiz 1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Zucca E, Dreyling M. Gastric marginal zone lymphoma of MALT type: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19 Suppl 2:ii70-ii71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, Leblond V, Speight P, Goodlad J, Lavergne-Slove A. Variable frequencies of t(11; 18)(q21; q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Alpen B, Neubauer A, Dierlamm J, Marynen P, Thiede C, Bayerdörfer E, Stolte M. Translocation t(11; 18) absent in early gastric marginal zone B-cell lymphoma of MALT type responding to eradication of Helicobacter pylori infection. Blood. 2000;95:4014-4015. [PubMed] |
| 10. | Sugiyama T, Asaka M, Nakamura T, Nakamura S, Yonezumi S, Seto M. API2-MALT1 chimeric transcript is a predictive marker for the responsiveness of H. pylori eradication treatment in low-grade gastric MALT lymphoma. Gastroenterology. 2001;120:1884-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Toracchio S, Ota H, de Jong D, Wotherspoon A, Rugge M, Graham DY, Samani A, El-Zimaity HM. Translocation t(11; 18)(q21; q21) in gastric B-cell lymphomas. Cancer Sci. 2009;100:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Isaacson PG, Wotherspoon AC, Diss T, Pan LX. Follicular colonization in B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Surg Pathol. 1991;15:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 150] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Streubel B, Simonitsch-Klupp I, Müllauer L, Lamprecht A, Huber D, Siebert R, Stolte M, Trautinger F, Lukas J, Püspök A. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Ye H, Gong L, Liu H, Hamoudi RA, Shirali S, Ho L, Chott A, Streubel B, Siebert R, Gesk S. MALT lymphoma with t(14; 18)(q32; q21)/IGH-MALT1 is characterized by strong cytoplasmic MALT1 and BCL10 expression. J Pathol. 2005;205:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Sagaert X, de Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J, Wlodarska I, De Wolf-Peeters C. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:2490-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Remstein ED, Dogan A, Einerson RR, Paternoster SF, Fink SR, Law M, Dewald GW, Kurtin PJ. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006;30:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Wündisch T, Thiede C, Morgner A, Dempfle A, Günther A, Liu H, Ye H, Du MQ, Kim TD, Bayerdörffer E. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23:8018-8024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Isaacson PG, Muller-Hermelink HK, Piris MA. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press 2001; 157-160. |
| 20. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1386] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 21. | Nakagawa M, Seto M, Hosokawa Y. Molecular pathogenesis of MALT lymphoma: two signaling pathways underlying the antiapoptotic effect of API2-MALT1 fusion protein. Leukemia. 2006;20:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Copie-Bergman C, Gaulard P, Lavergne-Slove A, Brousse N, Fléjou JF, Dordonne K, de Mascarel A, Wotherspoon AC. Proposal for a new histological grading system for post-treatment evaluation of gastric MALT lymphoma. Gut. 2003;52:1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Shiozawa E, Norose T, Kaneko K, Yamochi-Onizuka T, Takimoto M, Imawari M, Ota H. Clinicopathological comparison of the World Health Organization/Wotherspoon score to the Groupe d'Etude des Lymphomes de l'Adult grade for the post-treatment evaluation of gastric mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. 2009;24:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Copie-Bergman C, Wotherspoon A. MALT lymphoma pathology, initial diagnosis, and posttreatment evaluation. Extranodal Lymphomas Pathology and Management. London: Informa Health Care 2008; 114-123. |