Published online Feb 21, 2012. doi: 10.3748/wjg.v18.i7.679
Revised: June 9, 2011
Accepted: June 16, 2011
Published online: February 21, 2012
AIM: To examine how high-mobility group box 1 (HMGB1) regulates hepatocyte apoptosis and, furthermore, to determine whether glycyrrhizin (GL), a known HMGB1 inhibitor, prevents HMGB1-induced hepatocyte apoptosis.
METHODS: A human hepatocellular carcinoma cell line stably transfected with a bile acid transporter (Huh-BAT cells), were used in this study. Apoptosis was quantified using 4’,6-diamidino-2-phenylindole dihydrochloride staining and the APO Percentage apoptosis assay, and its signaling cascades were explored by immunoblot analysis. Kinase signaling was evaluated by immunoblotting and by using selective inhibitors. It is also tried to identify hepatocyte apoptosis affected by the HMGB1 inhibitor, GL.
RESULTS: HMGB1 increased cellular apoptosis in Huh-BAT cells. HMGB1 led to increased cytochrome c release from mitochondria into the cytosol, and induced the cleavage of procaspase 3. However, it did not affect the activation of caspase 8. HMGB1-induced caspase 3 activation was significantly attenuated by the p38 inhibitor SB203580. GL significantly attenuated HMGB1-induced hepatocyte apoptosis. GL also prevented HMGB1-induced cytochrome c release and p38 activation in Huh-BAT cells.
CONCLUSION: The present study demonstrated that HMGB1 promoted hepatocyte apoptosis through a p38-dependent mitochondrial pathway. In addition, GL had an anti-apoptotic effect on HMGB1-treated hepatocytes.
- Citation: Gwak GY, Moon TG, Lee DH, Yoo BC. Glycyrrhizin attenuates HMGB1-induced hepatocyte apoptosis by inhibiting the p38-dependent mitochondrial pathway. World J Gastroenterol 2012; 18(7): 679-684
- URL: https://www.wjgnet.com/1007-9327/full/v18/i7/679.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i7.679
High-mobility group box 1 (HMGB1) is an evolutionarily conserved protein present in the nucleus of almost all eukaryotic cells, where it is involved in DNA replication, repair and transcription[1-3]. This molecule is known to be released by cells undergoing necrosis as well as being secreted by activated macrophages. While early studies of HMGB1 demonstrated its role as a late mediator of sepsis[4], HMGB1 has been more recently implicated as a putative danger signal involved in the pathogenesis of a variety of non-infectious inflammatory conditions including autoimmunity, cancer, trauma, and hemo-rrhagic shock, and ischemia-reperfusion injury (IRI)[5-11]. So far, HMGB1 has been studied in a number of organs including liver, lung, breast and prostate[7-9,11].
In the liver, the importance of HMGB1 signaling has been largely identified in cases of IRI, during which tissue levels of HMGB1 were elevated following reperfusion and neutralizing antibodies against HMGB1 ameliorated the damage resulting from IRI in a toll-like receptor (TLR)4-dependent manner[11]. The pathogenetic role of HMGB1 in liver disease was also clarified by studying the inflammatory response to viral infection[12]. Following hepatocyte death by hepatitis B virus-specific cytotoxic T lymphocytes in a mouse model of hepatitis, HMGB1 directs the intrahepatic recruitment of neutrophils and all other non-antigen specific inflammatory cells (natural killer cells, T cells, B cells, monocytes, macrophages and dendritic cells).
Apoptosis, a stereotyped morphologic form of cell death, is an event that contributes to liver injury in a wide range of acute and chronic liver diseases[13]. However, it is not clear whether HMGB1 contributes to apoptotic cell death in the liver. Furthermore, the regulatory mechanism of HMGB1 in hepatocyte apoptosis remains largely undefined.
Glycyrrhizin (GL) is a major active constituent of licorice root that is commonly used in Asia to treat patients with chronic hepatitis[14-16]. This compound has been associated with numerous pharmacologic effects, including anti-inflammatory, anti-viral, anti-tumor, and hepatoprotective activities[17]. Recently, GL was recognized by Sitia et al[18] as an HMGB1 inhibitor, which binds directly to both HMG boxes in HMGB1.
Thus, the aim of this study was to provide in vitro evidence and a potential theoretical basis for HMGB1 regulation of hepatocyte apoptosis in order to further elucidate the molecular mechanism of HMGB1 involvement in various pathologic conditions that can affect the liver. Furthermore, we attempted to determine whether GL attenuates HMGB1-induced hepatocyte apoptosis and, if so, to identify the signaling cascades responsible for this modulation.
Several human hepatoma cell lines were chosen for this study: Huh-7 cells stably transfected with a bile acid transporter[19] derived from a well-differentiated hepato-cellular carcinoma (HCC)[20] (Huh-BAT), HepG2 and SNU-475 cells derived from a poorly differentiated HCC[21]. All cells were cultured in Dulbecco’s Modified Eagle medium supplemented with 10% fetal bovine serum, 100 000 U/L penicillin and 100 mg/L streptomycin. In all experiments, cells were serum-starved for 12 h in order to avoid the effects of serum-induced signaling.
HMGB1 (human, recombinant expressed in E. coli) was synthesized by Sigma-Aldrich, Inc. (St. Louis, MO, United States) at a purity of > 90%. The MAPK inhibitors [SB203580 for p38 mitogen activated protein kinase (MAPK), U0126 for p42/44 MAPK or extracellular signal-regulated kinase, and SP600125 for c-Jun N-terminal kinase (JNK) and GL] were also obtained from Sigma-Aldrich, Inc.
Cells were washed twice with phosphate-buffered saline, and mitochondrial and cytosolic extracts were isolated using a mitochondria/cytosol fractionation kit (BioVision, Inc., Mountain View, CA, United States) according to the manufacturer’s instruction.
Huh-BAT cells were lysed for 20 min on ice with lysis buffer (50 mol/L Tris-HCl, pH 7.4; 1% Nonidet P-40; 0.25% sodium deoxycholate; 150 mol/L NaCl; 1 mol/L EDTA; 1 mol/L PMSF; 1 μg/mL aprotinin, leupeptin, pepstatin; 1 mol/L Na3VO4; and 1 mol/L NaF) and centrifuged at 14 000 ×g for 10 min at 4 °C. Proteins in the lysates were resolved by 10% or 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis, transferred to PVDF membranes, and probed using the following primary antibodies: mouse anti-caspase 8 (1:500 dilution) from Cell Signaling Technology (Danvers, MA, United States); rabbit anti-caspase 3 (1:1000 dilution) from Cell Signaling Technology; rabbit anti-ACTIVE® p42/p44 (1:2000 dilution), anti-ACTIVE® p38 (1:1000 dilution), and anti-ACTIVE® JNK (1:1000 dilution) specific for the phosphorylated forms of p42/p44 MAPK, p38 MAPK, and JNK, respectively, from Cell Signaling Technology; mouse anti-cytochrome c (1:500 dilution) from BD Pharmingen (San Jose, CA, United States), and goat anti-actin (1:1000 dilution) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, United States). Twenty μg of protein was used for each well in Western blotting. Primary antibody binding was detected with appropriate peroxidase-conjugated secondary antibodies (Biosource International, Camarillo, CA). Bound antibodies were visualized using a chemiluminescent substrate (ECL; Amersham, Arlington Heights, IL, United States) and the blots were exposed to Kodak X-OMAT film.
The signals in the Western blotting were quantified by densitometric scanning and normalized by using the intensity of corresponding protein band relative to the actin band.
Quantitative detection of apoptotic cells was performed using two different methods: the nuclear binding dye DAPI and fluorescence microscopy, and the APO Percentage apoptosis assay kit (Biocolor Ltd., Belfast, Northern Ireland). For the APO Percentage apoptosis assay, the cells were seeded at 104 cells per well in a 96-well plate and processed according to the manufacturer’s instructions.
All data were from at least three independent experiments using cells from a minimum of three separate isolations, and are expressed as the mean ± SD. Differences between the groups were compared using a two-tailed Student's t tests or the Mann-Whitney U test as appropriate. P values of < 0.05 were considered to be statistically significant.
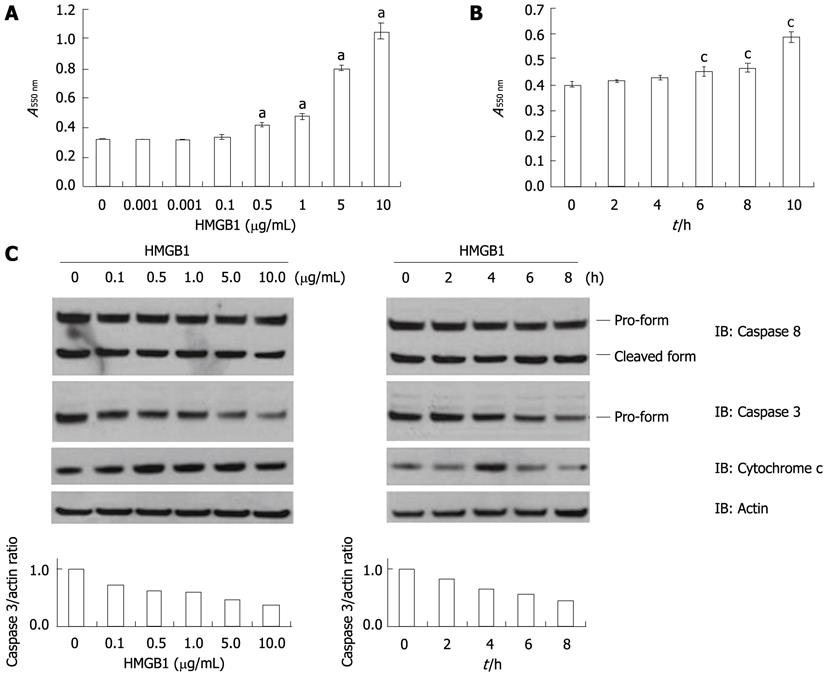
HMGB1 significantly increased cellular apoptosis in Huh-BAT cells in a dose- and time-dependent manner (Figure 1A and B). We repeated the same experiments in the other two hepatoma cell lines (HepG2 and SNU-475 cells) and observed the same effects (data not shown). We next identified the pro-apoptotic signaling pathways induced by HMGB1 treatment. HMGB1 increased cytochrome c release from mitochondria into cytosol and induced the cleavage of procaspase 3. However, it did not affect the activation of caspase 8, an initiator caspase downstream of death receptor activation (Figure 1C).
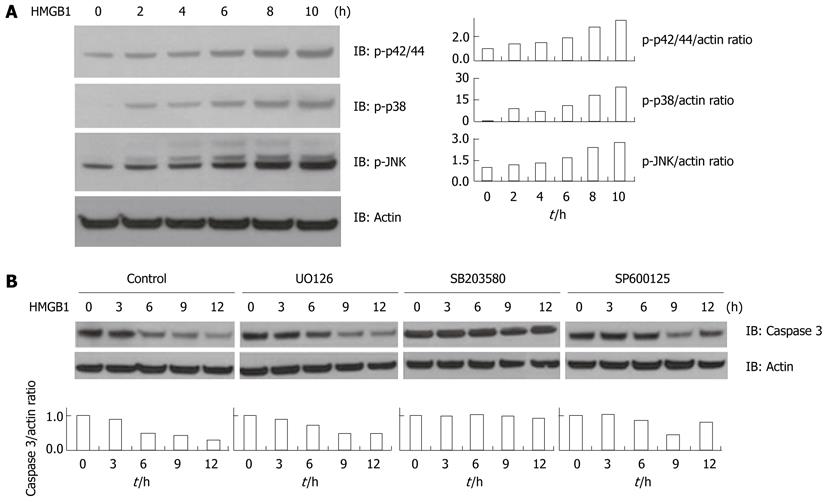
Since the MAPK family signaling cascades regulate apoptotic pathways, we next evaluated whether HMGB1 modulates MAPK activation. HMGB1 induced the activation of MAPKs such as p42/44, p38 MAPK, and JNK in Huh-BAT cells (Figure 2A). In order to explore the role of individual MAPKs in apoptotic signaling, the cells were then treated with HMGB1 either in the presence or absence of various inhibitors: U0126 for p42/44, SB203580 for p38, and SP600125 for JNK. When the cells were treated with the p38 inhibitor, HMGB1-induced caspase 3 activation was significantly attenuated whereas treatment with inhibitors of p42/44 or JNK did not affect caspase 3 cleavage (Figure 2B).
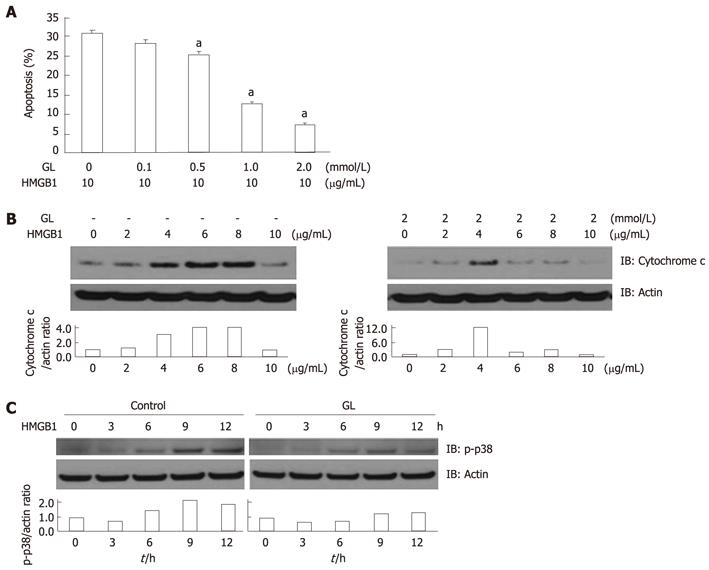
Pretreatment with GL significantly attenuated HMGB1-induced hepatocyte apoptosis in a dose-dependent manner (Figure 3A). GL also attenuated cytochrome c release from the mitochondria into cytosol (Figure 3B). Finally, pretreatment with GL decreased HMGB1-induced p38 activation in Huh-BAT cells (Figure 3C). Taken together, all of the findings from our study indicate that HMGB1 induces hepatocyte apoptosis through a p38-dependent mitochondrial pathway which was inhibited by GL.
In virtually all human liver diseases, hepatocytes undergo cell death by apoptosis[13]. Thus, therapeutic modulation of apoptosis has the potential to alter the course of human liver disease. Apoptosis may occur via two fundamental pathways: (1) the death receptor or extrinsic pathway; and (2) the mitochondrial or intrinsic pathway. Caspases, representing the family of cysteine proteases, play a critical role in both pathways. Both pathways can either directly or indirectly converge to activate the “effector caspase”, namely caspase-3, which induces DNA fragmentation and other morphological changes characteristic of apoptotic cell death[22]. In the present study, HMGB1 activated caspase 3 without affecting caspase 8, an initiator caspase downstream of death receptor activation. These findings suggest that the mitochondrial pathway is responsible for HMGB1-induced hepatocyte apoptosis, which was further supported by the findings that HMGB1 increased cytochrome c release from mitochondria into the cytosol.
MAPKs, which include p42/44, p38, and JNK, are involved in pro-apoptotic signal transduction as well as cell growth and differentiation[23]. It has been previously shown that the p38 MAPK cascade promotes either cell death or cell survival[23,24] depending on the cell type and the kinase isoforms activated by various stress stimuli[25]. There is abundant evidence that p38 participates in cellular apoptosis[26,27] with one mechanism being the modulation of Bcl-2 protein family members to maintain an apoptotic checkpoint for mitochondrial dysfunction and cytochrome c release[28,29]. Likewise, in the present study HMGB1-induced hepatocyte apoptosis occurred through a mitochondrial pathway which was p38-dependent.
GL, a triterpene glycoside extracted from licorice root (Glycyrrhiza glabra), consists of one molecule of 18b-glycyrrhetinic acid and two molecules of glucuronic acid having the structure 18-b-glycyrrhetinic acid-3-O-b-D-glucuronopyranosyl-(1/2)-b-D-glucuronide. It is known that this molecule has a variety of hepato-protective properties in terms of anti-inflammatory, antiviral, and anti-tumor effects[17]. In an animal model of acute liver injury induced by carbon tetrachloride, GL reduced the serum tumor necrosis factor-alpha (TNF-α) level and alleviates acute liver injury[30]. Moreover, GL has anti-inflammatory effects on lipopolysaccharide (LPS)-induced acute liver injury through inhibition of TNF-α release[31]. Recently, Ikeda et al[32] reported that GL reduced the number of TUNEL-positive cells in cases of acute hepatitis induced by LPS/D-galactosamine (GalN)-treatment. However, in a mouse model treated with LPS/D-GalN, anti-apoptotic effects of GL were found to be caspase-independent, and probably achieved through the prevention of an interleukin-18-mediated inflammatory response. In the present study, we demonstrated that GL attenuated HMGB1-induced hepatocyte apoptosis by blocking the p38-dependent mitochondrial pathway. Therefore, it is likely that the hepato-protective effects of GL are attributed to various mechanisms.
In summary, the present study demonstrated that HMGB1 participated in hepatocyte apoptosis through a p38-dependent mitochondrial pathway. In addition, GL had an anti-apoptotic effect on hepatocytes treated with HMGB1. Therefore, HMGB1 inhibitors, including GL, might be therapeutically efficacious in treating HMGB1-mediated liver injury such as viral hepatitis, hepatic ischemia-reperfusion injury and sepsis-related liver injury.
High mobility group box 1 (HMGB1) is an evolutionarily conserved protein present in the nucleus of almost all eukaryotic cells, where it is involved in DNA replication, repair and transcription. Glycyrrhizin (GL) is a major active constituent of licorice root that is commonly used in Asia to treat patients with chronic hepatitis. This compound has been associated with numerous pharmacologic effects, including anti-inflammatory, anti-viral, anti-tumor, and hepatoprotective activities. Recently, GL was recognized as an HMGB1 inhibitor, which binds directly to both HMG boxes in HMGB1.
The authors provide in vitro evidence and a potential theoretical basis for HMGB1 regulation of hepatocyte apoptosis in order to further elucidate the molecular mechanism of HMGB1 involvement in various pathologic conditions that can affect the liver. Furthermore, they attempted to determine whether GL attenuates HMGB1-induced hepatocyte apoptosis and, if so, to identify the signaling cascades responsible for this modulation.
Present study demonstrated that HMGB1 participated in hepatocyte apoptosis through a p38-dependent mitochondrial pathway. In addition, GL had an anti-apoptotic effect on hepatocytes treated with HMGB1.
HMGB1 inhibitors, including GL, might be therapeutically efficacious in treating HMGB1-mediated liver injury such as viral hepatitis, hepatic ischemia-reperfusion injury and sepsis-related liver injury.
This is an interesting study where the authors show that HMGB1 induces hepatocyte apoptosis which is mediated by p38. In addition, glycyrrhizin was shown to inhibit HMGB1-induced apoptosis as well as activation of p38 in the cultured hepatocyte cell line. The study is well conducted and the manuscript is well written.
Peer reviewers: Filip Braet, Associate Professor, Australian Key Centre for Microscopy and Microanalysis, Madsen Building (F09), The University of Sydney, Sydney NSW 2006, Australia; Richard A Rippe, Dr., Department of Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7038, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Li JY
| 1. | Bianchi ME, Beltrame M. Flexing DNA: HMG-box proteins and their partners. Am J Hum Genet. 1998;63:1573-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Bustin M, Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucleic Acid Res Mol Biol. 1996;54:35-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 577] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 3. | Müller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337-4340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 351] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2697] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 5. | Wittemann B, Neuer G, Michels H, Truckenbrodt H, Bautz FA. Autoantibodies to nonhistone chromosomal proteins HMG-1 and HMG-2 in sera of patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1990;33:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furie R. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002;46:2598-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Baldassarre G, Battista S, Belletti B, Thakur S, Pentimalli F, Trapasso F, Fedele M, Pierantoni G, Croce CM, Fusco A. Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma. Mol Cell Biol. 2003;23:2225-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Bussemakers MJ, van de Ven WJ, Debruyne FM, Schalken JA. Identification of high mobility group protein I(Y) as potential progression marker for prostate cancer by differential hybridization analysis. Cancer Res. 1991;51:606-611. [PubMed] |
| 9. | Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958-L965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538-R1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 845] [Cited by in RCA: 938] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 12. | Wang H, Ward MF, Fan XG, Sama AE, Li W. Potential role of high mobility group box 1 in viral infectious diseases. Viral Immunol. 2006;19:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Yoon JH, Gores GJ. Death receptor-mediated apoptosis and the liver. J Hepatol. 2002;37:400-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Yamamura Y, Kotaki H, Tanaka N, Aikawa T, Sawada Y, Iga T. The pharmacokinetics of glycyrrhizin and its restorative effect on hepatic function in patients with chronic hepatitis and in chronically carbon-tetrachloride-intoxicated rats. Biopharm Drug Dispos. 1997;18:717-725. [PubMed] |
| 15. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494-1500. [PubMed] |
| 16. | van Rossum TG, Vulto AG, Hop WC, Schalm SW. Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:2432-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Sato H, Goto W, Yamamura J, Kurokawa M, Kageyama S, Takahara T, Watanabe A, Shiraki K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Sitia G, Iannacone M, Müller S, Bianchi ME, Guidotti LG. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leukoc Biol. 2007;81:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Higuchi H, Bronk SF, Takikawa Y, Werneburg N, Takimoto R, El-Deiry W, Gores GJ. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J Biol Chem. 2001;276:38610-38618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858-3863. [PubMed] |
| 21. | Park JG, Lee JH, Kang MS, Park KJ, Jeon YM, Lee HJ, Kwon HS, Park HS, Yeo KS, Lee KU. Characterization of cell lines established from human hepatocellular carcinoma. Int J Cancer. 1995;62:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Jänicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357-9360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1546] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 23. | Liu G, Zhang Y, Bode AM, Ma WY, Dong Z. Phosphorylation of 4E-BP1 is mediated by the p38/MSK1 pathway in response to UVB irradiation. J Biol Chem. 2002;277:8810-8816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002;99:10054-10059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 254] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Giafis N, Katsoulidis E, Sassano A, Tallman MS, Higgins LS, Nebreda AR, Davis RJ, Platanias LC. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res. 2006;66:6763-6771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1154] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 27. | Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1249] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 28. | Benn SC, Woolf CJ. Adult neuron survival strategies--slamming on the brakes. Nat Rev Neurosci. 2004;5:686-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, Youle RJ, Morrison RS. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol. 2000;150:335-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 319] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Lee CH, Park SW, Kim YS, Kang SS, Kim JA, Lee SH, Lee SM. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol Pharm Bull. 2007;30:1898-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Tang B, Qiao H, Meng F, Sun X. Glycyrrhizin attenuates endotoxin- induced acute liver injury after partial hepatectomy in rats. Braz J Med Biol Res. 2007;40:1637-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Ikeda T, Abe K, Kuroda N, Kida Y, Inoue H, Wake K, Morito M, Sato T. The inhibition of apoptosis by glycyrrhizin in hepatic injury induced by injection of lipopolysaccharide / D-galactosamine in mice. Arch Histol Cytol. 2008;71:163-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |