Published online Feb 21, 2012. doi: 10.3748/wjg.v18.i7.654
Revised: April 19, 2011
Accepted: April 26, 2011
Published online: February 21, 2012
AIM: To evaluate the hepatoprotective roles of (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione (SKLB010) against carbon tetrachloride (CCl4)-induced acute and chronic liver injury and its underlying mechanisms of action.
METHODS: In the first experiment, rats were weighed and randomly divided into 5 groups (five rats in each group) to assess the protective effect of SKLB010 on acute liver injury. For induction of acute injury, rats were administered a single intraperitoneal injection of 2 mL/kg of 50% (v/v) CCl4 dissolved in olive oil (1:1). Group 1 was untreated and served as the control group; group 2 received CCl4 for induction of liver injury and served as the model group. In groups 3, 4 and 5, rats receiving CCl4 were also treated with SKLB010 at doses of 25, 50 and 100 mg/kg, respectively. Blood samples were collected at 6, 12 and 24 h after CCl4 intoxication to determine the serum activity of alanine amino transferase. Tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) were determined using enzyme-linked immunosorbent assay. At 24 h after CCl4 injection,liver fibrogenesis was evaluated by hematoxylin-eosin (HE) staining and immunohistochemical analyses. Cytokine transcript levels of TNF-α, IL-1β and inducible nitric oxide synthase in the liver tissues of rats were measured using a reverse transcriptase reverse transcription-polymerase chain reaction technique. In the second experiment, rats were randomly divided into 2 groups (15 rats in each group), and liver injury in the CCl4-administered groups was induced by a single intraperitoneal injection of 2 mL/kg of 50% (v/v) CCl4 dissolved in olive oil (1:1). The SKLB010-treated groups received oral 100 mg/kg SKLB010 before CCl4 administration. Five rats in each group were sacrificed at 2 h, 6 h, 12 h after CCl4 intoxication and small fortions of livers were rapidly frozen for extraction of total RNA, hepatic proteins and glutathione (GSH) assays. In the hepatic fibrosis model group, rats were randomly divided into 2 groups (5 rats each group). Rats were injected intraperitoneally with a mixture of CCl4 (1 mL/kg body weight) and olive oil [1:1 (v/v)] twice a week for 4 wk. In the SKLB010-treated groups, SKLB010 (100 mg/kg) was given once daily by oral gavage for 4 wk after CCl4 administration. The rats were sacrificed one week after the last injection and the livers from each group were harvested and fixed in 10% formalin for HE and immunohistochemical staining.
RESULTS: In this rat acute liver injury model, oral administration of SKLB010 blocked liver tissue injury by down-regulating the serum levels of alanine aminotransferase, suppressing inflammatory infiltration to liver tissue, and improving the histological architecture of liver. SKLB010 inhibited the activation of NF-κB by suppressing the degradation of IκB, and prevented the secretion of pro-inflammatory mediators such as tumor necrosis factor-α, interleukin-1β, and the reactive free radical, nitric oxide, at the transcriptional and translational levels. In this chronic liver fibrosis model, treatment with 100 mg/kg per day SKLB010 attenuated the degree of hepatic fibrosis and area of collagen, and blocked the accumulation of smooth-muscle actin-expressed cells.
CONCLUSION: These results suggest that SKLB010 is a potent therapeutic agent for the treatment of CCl4-induced hepatic injury.
- Citation: Chen ZZ, Wang ZL, Deng CY, Zheng H, Wang XH, Ma L, Ye X, Ma YH, Xie CF, Chen LJ, Wei YQ. (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione protects rats from carbon tetrachloride-induced liver injury and fibrogenesis. World J Gastroenterol 2012; 18(7): 654-661
- URL: https://www.wjgnet.com/1007-9327/full/v18/i7/654.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i7.654
Hepatic damage, i.e., acute liver injury and chronic liver fibrogenesis, has become a severe health problem worldwide. Despite considerable and continuous efforts, effective treatment strategies against this disease resulting in fewer side effects are still lacking.
Carbon tetrachloride (CCl4), is an acknowledged hepatotoxin and can cause acute or chronic liver injury characterized by centrilobular necrosis, inflammatory cell infiltration, centrilobular fatty changes, and apoptosis[1]. CCl4-induced hepatic inflammatory response was found to be mediated by the action of cytokines, especially tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and the free radical, nitric oxide (NO)[2]. CCl4 also induced the peroxidation of lipids or lipid membranes, and up-regulated the serum level of alanine aminotransferase (ALT). Sustained hepatic inflammation provoked by long-term exposure to CCl4 is believed to induce hepatic fibrosis through ongoing hepatocytic necrosis and the production of fibrogenic cytokines acting on fibroblasts [e.g., activated hepatic stellate cells (HSC)][3].
On the other hand, oxidative stress is capable of stimulating nuclear factor-κB activation (NF-κB)[4]. NF-κB is an inducible transcription factor whose activity is primarily regulated by phosphorylation and degradation of IκB[5]. CCl4-induced oxidative stress can stimulate the phosphorylated effect of IκB, and degradation of IκB leads to the translocation of NF-κB to the nucleus[5]. This process further regulates the expression of inducible inflammatory cytokines (such as TNF-α, NO, and IL-1β)[6]. The up-regulation of TNF-α and IL-1β conversely activates the expression of NF-κB. Therefore, the up-regulation of these inflammatory mediators induces a vicious cycle, which eventually alters the structure of hepatocytes and impairs their biological functions[7]. Consequently, prolonged activation of NF-κB results in the perpetuation of inflammatory responses. Hence, it is regarded as the potential target for the treatment of anti-inflammatory disease including hepatic damage[8].
In our previous study, we found that several analogues of thiazolidinediones (TZDs) showed high inhibitory effects on the chemotaxis of RAW264.7 cells. We observed that (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione (SKLB010) exhibited the strongest inhibitory activity against chemotaxis in MCP-1-stimulated macrophage-like RAW 264.7 cells with an IC50 value of 0.72 μmol/mL, and evaluated its protective effects on Con A-induced acute hepatitis[9]. In the present study, we further investigate the therapeutic effect of SKLB010 on CCl4-induced acute hepatitis and chronic liver fibrosis, and explore its underlying mechanisms.
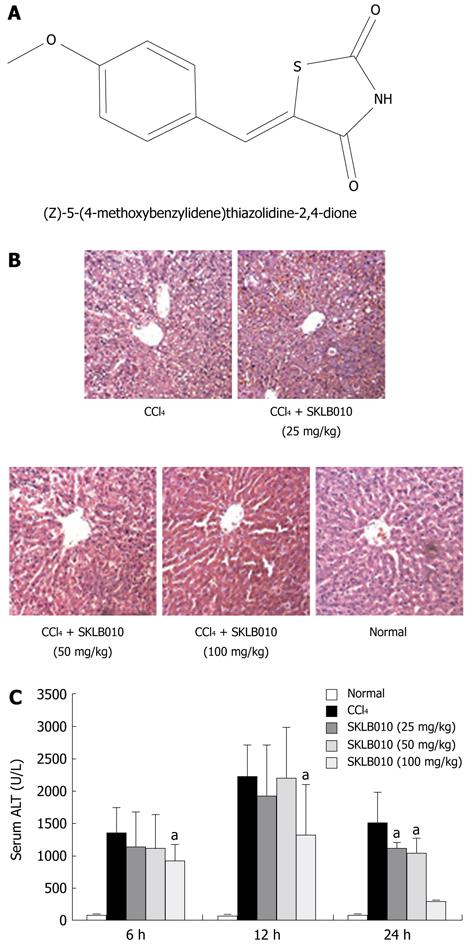
SKLB010 (Figure 1A) was synthesized with purity of more than 99.5% in our lab, and dissolved in saline containing 5% (v/v) Tween-80 (Sigma-Aldrich, St. Louis, MO, USA). The structure and purity were identified by high performance liquid chromatography, a Q-TOF Premier Mass Spectrometer (Waters, Milford, MA, United States) and nuclear magnetic resonance (Bruker Avance 400 NMR system).
Female Sprague-Dawley rats from Western China Experimental Animal Center were maintained under controlled conditions and had free access to standard chow and water. All rats received human care according to the National Institutes of Health Guidelines of China until their weight was 200 g to 220 g after which they were used in the experiments.
In the first experiment, rats were weighed and randomly divided into 5 groups (five rats in each group) to assess the protective effect of SKLB010 on acute liver injury. For induction of acute injury, rats were administered a single intraperitoneal injection of 2 mL/kg of 50% (v/v) CCl4 dissolved in olive oil (1:1)[10]. Group 1 was untreated and served as the control group; group 2 received CCl4 for induction of liver injury and served as the model group. In groups 3, 4 and 5, rats received CCl4 to induce liver injury and were also treated with SKLB010 at doses of 25, 50 and 100 mg/kg, respectively.
In groups 2 to 5, blood samples were collected at 6, 12 and 24 h after CCl4 intoxication to determine the serum activity of ALT using an Olympus AU2700 multifunctional biochemistry analyzer (Olympus, Tokyo, Japan).
At 24 h after CCl4 injection, rats were killed and the livers were removed. A small portion of the liver was used for hematoxylin-eosin (HE) staining studies following fixation with 10% formalin and subsequent embedment in paraffin.
In the second experiment, rats were randomly divided into 2 groups (15 rats in each group), and liver injury in the CCl4-administered groups was induced by a single intraperitoneal injection of 2 mL/kg of 50% (v/v) CCl4 dissolved in olive oil (1:1). The SKLB010-treated groups received oral 100 mg/kg SKLB010 before CCl4 administration. Five rats in each group were sacrificed at 2 h, 6 h, 12 h after CCl4 intoxication and the livers were cut into pieces and rapidly frozen with liquid nitrogen for extraction of total RNA, hepatic proteins and glutathione (GSH) assays.
In the hepatic fibrosis model group, rats were randomly divided into 2 groups (5 rats each group). Rats were injected intraperitoneally with a mixture of CCl4 (1 mL/kg body weight) and olive oil [1:1 (v/v)] twice a week for 4 wk[11]. In the SKLB010-treated groups, SKLB010 (100 mg/kg) was given once daily by oral gavage for 4 wk after CCl4 administration. The rats were sacrificed one week after the last injection and the livers from each group were harvested and fixed in 10% formalin for HE and immunohistochemical staining.
In the study of acute liver injury, liver sections of 3 μm thickness were stained with HE using a standard procedure and analyzed by light microscopy to determine histological changes in tissue structure assessed using an optical microscope.
During the study of chronic liver fibrogenesis, liver tissues fixed in 10% formalin were embedded in paraffin, cut into 4-μm sections, and stained with HE to evaluate liver injury under an optical microscope. Sections were stained with Masson’s Trichrome Staining to detect collagen deposition. An immunohistochemistry method was used as previously described. Briefly, liver sections were treated with 3% H2O2/PBS and incubated overnight at 4°C with an anti-CD11b antibody (Cell Signaling, United States) used as the primary antibody for detecting macrophages in liver tissue and a rabbit-anti-mouse-α smooth-muscle actin (SMA) antibody (Thermo, Immunohistochemistry Specific, United States) used as the primary antibody for analyzing the activity of HSCs. The proportion of tissue stained with picrosirius red was assessed by morphometric analysis with MetaView software (Universal Imaging, Downingtown, PA, United States). Collagen staining was quantitated in random sections (under × 400 magnification, 10 fields each from sample).
Levels of hepatic GSH (γ-glutamyl-cysteinylglycine, GSH) were determined using the enzyme immune assay kit GSH (Jiancheng Bioengineering, Nanjing, China), following the protocol provided by the manufacturer.
Detection of serum TNF-α, IL-1β was performed using enzyme-linked immunosorbent assay (ELISA) kits (RandD Systems, Minneapolis, MN, United States), according to the manufacturer’s instructions. Detection of serum NO was performed using the NO assay kit (nitrate reductase progress; Jiancheng Bioengineering, Nanjing, China), which reduces nitrate to nitrite as an index of NO, and was assayed colorimetrically at 450 nm.
Cytokine transcript levels of TNF-α, IL-1β and inducible nitric oxide synthase (iNOS) in the liver tissues of rats were measured using a reverse transcriptase reverse transcription-polymerase chain reaction (RT-PCR) technique. Total RNA was isolated using Trizol reagents (Invitrogen, Carlsbad, CA, United States). The RNA was reverse transcribed and PCR-amplified by the prime script one-step RT-PCR kit (TaKaRa, Japan). The results were expressed as a ratio of the number of copies of the goal gene to the number of copies the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase, from the same RNA samples. The sequences of primers for the cytokine genes are as follows: TNF-α, sense 5′-CGGGGGCCACCACGCTCTTC-3′ and antisense 5′-GGCAAATCGGCTGACGGTGTG-3′; IL-1β, sense 5′-TCAAGGCATAACAGGCTCATC-3′ and antisense 5′-CCACGGGCAAGACATAGGTAG-3′; iNOS, sense 5′-CCCTTCCGAAGTTTCTGGCAGCAG-3′, and antisense 5′-GGGCTCCTCCAAGGTGTTGCCC-3′.
GADPH, sense 5′-GTGCTGAGTATGTCGTGGAGTCT-3′ and anti-sense 5′-GTGGAAGAATGGGAGTTGCTGT-3′.
Nuclear extracts were prepared according to the method of Hentze[12]. The oligonucleotide probes used for this experiment were sense, 5′-AGTTGAGGGGACTTTCCCAGGC-3′; antisense, 5′-GCCTGGGAAAGTCCCCTCAACT-3′; and NF-κB mutant, sense 5′-AGTTGAGGCGACTTTCCCAGGC-3′, anti-sense, 5′-GCCTGGGAAAGTCGCCTCAACT-3′.
Forty micrograms of protein from each sample, where the concentration was measured by the Bradford assay, was separated on 10% SDS-polyacrylamide gel and electrotransferred to nitrocellulose membranes (Schlecher-Schuell, Dassel, Germany). Membranes were blocked for 1 h at room temperature with 5% nonfat dry milk in TBST buffer. The reactions were then incubated at 4°C overnight with a 1:1000 dilution of anti-IκB or anti-β-actin antibody (Abcam, United States) in blocking buffer. After the membranes were washed three times, they were further incubated with a 1:4000 dilution of suitable secondary antibody (Cell Signaling Technology, United States) for 1 h at room temperature. The signals were normalized to the protein levels of the housekeeping gene β-actin.
SPSS 16.0 was used to determine the significance of differences between normal and experimental groups. All results were expressed as mean ± SD. Differences between groups were evaluated using an independent-samples T test. The results were considered significantly different at P < 0.05.
As shown in Figure 1B, SKLB010 effectively prevented the development of CCl4-induced liver injury as demonstrated by the reduction in serum ALT release. The ALT level was elevated after administration of CCl4 and peaked at 12 h. In contrast, oral administration of SKLB010 dose- and time-dependently down-regulated the level of ALT, and SKLB010 showed the most potent reduction at the dose of 100 mg/kg.
Histological changes in liver tissue shown by HE staining also confirmed the hepatoprotective effect of SKLB010 against CCl4-induced liver injury. The histopathological changes in liver tissue in CCl4-intoxicated rats at 24 h after CCl4 injection are shown in Figure 1C. Compared to the normal group, the group exposed to CCl4 exhibited extensive inflammatory cells infiltration, centrilobular fatty changes, apoptosis and widespread hepatocellular necrosis. Following treatment with SKLB010, inflammatory infiltration and the necrotic region in liver sections were reduced in a dose-dependent manner.
GSH is an indicator of oxidative stress at the hydrophilic level in the liver[13]. Hence, the content of GSH was measured at 6 h and 12 h after CCl4 injection. As shown in Table 1, the liver content of GSH in the normal group was 5.24 mg/g protein. However, SKLB010-treated hepatic GSH was enhanced from 2.99 mg/g to 3.26 mg/g protein at 6 h, and from 4.07 mg/g to 4.7 mg/g protein at 12 h compared with the CCl4-induced group, respectively. We conclude that administration of SKLB010 blocked the decrement in GSH induced by CCl4.
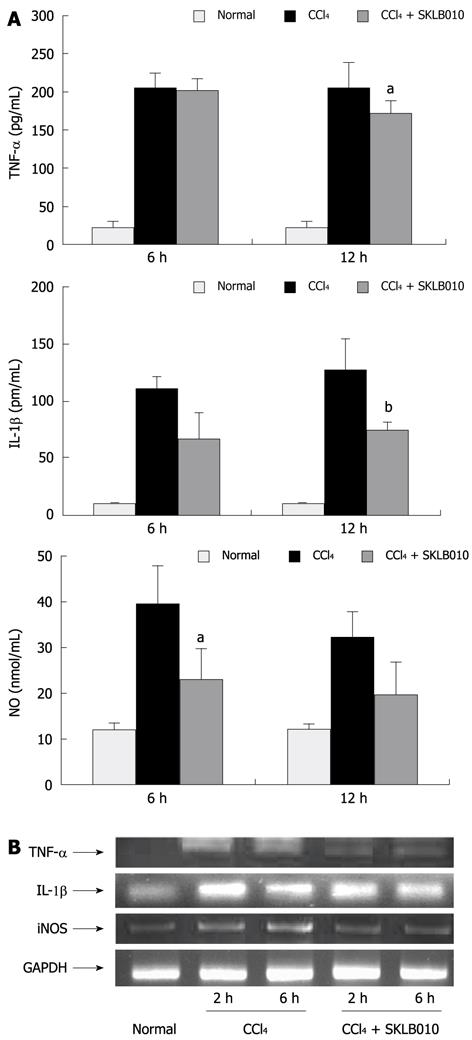
To investigate the ability of SKLB010 to modulate the production of inflammatory cytokines, the serum levels of TNF-α, IL-1β, and NO production and their corresponding liver mRNA expression were determined in rats with CCl4-induced hepatitis.
As shown in Figure 2A, treatment with SKLB010 reduced the serum levels of TNF-α and IL-1β at 12 h and NO production at 6 h compared with the CCl4-injected groups. In order to investigate whether the reduction in serum levels of inflammatory mediators was due to the changes in mRNA expression, we examined liver mRNA expression of TNF-α, IL-1β, and iNOS both at 2 h and 6 h after CCl4 injection. It can be seen from Figure 2B, that the administration of SKLB010 reduced the mRNA expression of TNF-α, IL-1β, and iNOS in contrast to the CCl4-induced groups.
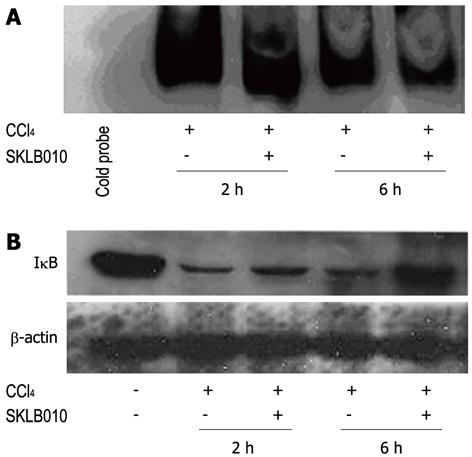
Electrophoresis mobility shift assays (EMSA) were performed in order to examine whether SKLB010 suppressed the activation of NF-κB after CCl4 injection. Correspondingly, a NF-κB-DNA binding assay was also carried out using nuclear extracts from acutely damaged liver stimulated by CCl4 in the presence or absence of SKLB010. Indeed, CCl4-mediated activation of NF-κB resulted in an increase in DNA-binding activity within 2 h, followed by inactivation of NF-κB at 6 h after stimulation which was demonstrated by a lower DNA-binding activity, suggesting that administration of SKLB010 reduced the DNA binding activity of NF-κB (Figure 3A).
As reported, degradation of IκB was required to activate NF-κB[5]. We investigated the effect of SKLB010 on the cytoplasmic level of IκB by Western blot analysis both at 2 h and 6 h after CCl4 injection. As shown in Figure 3B, treatment with SKLB010 inhibited the degradation of IκB induced by the administration of CCl4.
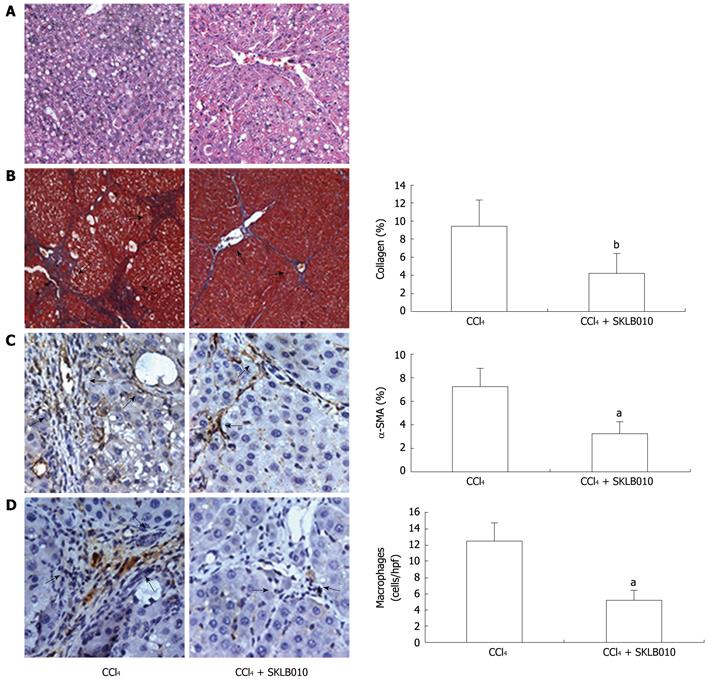
The hepatoprotective effects of SKLB010 on liver injury and fibrosis were initially evaluated by histological analyses. The results from HE staining showed that oral administration of SKLB010 daily for 4 wk significantly reduced the level of steatosis and necrosis, and suppressed hepatic fibrogenesis (Figure 4). Masson staining revealed that rats treated with SKLB010 showed histological improvement with a marked reduction in fibrosis and decreased expression of collagen I and IV. As a unique marker for activated HSC, α-SMA-positive cells around the fibrotic septa were significantly increased by CCl4. In contrast, the number of α-SMA-positive cells was reduced in the livers of SKLB010-treated rats compared with those from cirrhotic rats. These findings indicate that SKLB010 efficiently reduced the presence of activated HSC in liver fibrogenesis. Furthermore, immunohistochemical analysis of CD11b revealed fewer macrophages in the fibrotic septa between nodules after treatment with SKLB010.
In the present study, oral administration of SKLB010 improved CCl4-induced liver injury via the inhibition of NF-κB followed by suppression of the degradation of IκB. Treatment with SKLB010 also showed therapeutic effects on liver fibrogenesis. In the acute liver injury study, oral treatment with SKLB010 decreased ALT enzyme levels. Histopathological findings revealed reduced levels of inflammatory cell infiltration, centrilobular fatty changes, apoptosis, and necrosis in SKLB010-treated rats. Results on the hepatoprotective effects of SKLB010 on CCl4-induced chronic liver fibrogenesis showed that SKLB010 possessed potent ability to alleviate the development of fibrogenesis by reducing the level of steatosis and necrosis, expression of collagen I and IV, activated HSC, and macrophage infiltration of liver tissue.
SKLB010 is a novel analogue of TZDs. Recent evidence has shown that TZDs participate in the regulation of inflammation, especially in modulating the production of inflammatory mediators (such as TNF-α, IL-1β, inducible iNOS), by decreasing the activation of NF-κB while increasing the expression of IκB[1,14].
We suggest that the most likely underlying mechanism by which SKLB010 regulates the expression of proinflammatory cytokines production is through the inactivation of NF-κB.
GSH and the antioxidative enzyme system play important roles in protecting liver cells against oxidative stress[15]. CCl4-induced liver injury was associated with decreased hepatic GSH. In our experiments, treatment with SKLB010 increased the levels of GSH which were greater than those observed for the CCl4 control. Following exposure to CCl4, oxidative stress was attenuated by treatment with SKLB010. Thus, we supposed that SKLB010 possessed the properties to enhance GSH and decrease reactive metabolite formation in CCl4-induced liver injury.
These inflammatory mediators (e.g., TNF-α, IL-1β, and NO), play key roles in the induction and perpetuation of inflammation in macrophages. Our recent study using an ELISA and RT-PCR assay revealed that these mediators were inhibited by SKLB010 treatment both at the serum and mRNA level. Furthermore, the decrease in TNF-α expression occurred before the decrease in IL-1β. This is because the macrophage membrane contains the TNF-α receptor, hence the decrease in TNF-α may stimulate the expression of IL-1β. The fact that TNF-α levels initially increased rapidly and later decreased compared with the SKLB010-treated group was in accordance with the lower inactivation of NF-κB at 6 h which was able to control TNF-α production.
The expressions of these inflammatory mediators have been shown to be dependant on NF-κB activation[16,17]. Therefore, the possibility that the extract inhibited the activity of NF-κB needs to be examined further.
NF-κB, a transcription factor that regulates the expression of proinflammatory cytokines and proteins, is activated in response to several extracellular stimuli, and oxidative stress. NF-κB is sensitive to the oxidation of a particular cysteine at position 62 in p50, which is essential for DNA binding[18]. Evidence suggests that unregulated NF-κB-related gene expression in Kupffer cells contributes to CCl4-induced liver injury[19]. To support this hypothesis, the development of a pharmacological strategy to suppress the activation of NF-κB is required.
The entry of NF-κB from the cytosol to the nucleus is regulated by IκB, and its induction and binding to NF-κB which prevents its translocation into the nucleus was estimated[20].
Since phosphorylated IκB is degraded by a multisubunit protease complex, i.e., proteasome[21], we hypothesized that SKLB010 prevented the nuclear translocation of NF-κB by inhibiting degradation of IκB through inhibition of some of the proteases in the proteasome.
The EMSA assay revealed that the subsequent NF-κB-DNA binding was inhibited in the nucleus by pretreatment with SKLB010 compared to the CCl4-control group. CCl4-mediated activation of NF-κB resulted in a peak of DNA-binding activity at 2 h, followed by inactivation of NF-κB at 6 h which was confirmed by lower DNA-binding activity. These results were in accordance with previous reports on the NF-κB pathway. NF-κB translocates to the nucleus and activates a series of gene transcriptions including the IκB gene which can transform NF-κB from the activated state to the inactivated state. In addition, in the study on IκB, a lower level of degradation of IκB demonstrated that SKLB010 prevented CCl4-induced degradation of IκB. These results proved that SKLB010 inhibited the CCl4-induced activation of NF-κB by suppressing the degradation of IκB.
In summary, based on the above results, SKLB010 had significant potent inhibitory effects on CCl4-induced acute liver injury by reducing the activity of serum ALT, and improving the histology of liver tissue. Furthermore, the development of CCl4-induced chronic fibrogenesis was alleviated by treatment with SKLB010, which was demonstrated by histological staining. Importantly, the underlying mechanisms of action were proved, in that SKLB010 possessed properties to enhance GSH and anti-oxidative activity, and attenuated inflammatory mediators such as TNF-α, IL-1β, and NO through inactivation of NF-κB. These observations may have potential therapeutic value in the treatment of hepatitis.
Hepatic damage, including acute liver injury and chronic liver fibrogenesis, constitute a major problem to human health worldwide. Despite extensive efforts, effective treatment strategies resulting in fewer side effects are still lacking.
Our previous study demonstrated that (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione (SKLB010), a derivative of thiazolidinediones (TZDs) exhibits protective effects on Con A-induced acute hepatitis and adjuvant-induced arthritis without side effects.
SKLB010 inhibited the carbon tetrachloride (CCl4)-induced activation of nuclear factor-κB (NF-κB) by suppressing degradation of IκB. A dose- and time-dependent relationship was found. The protection effects of SKLB010 on liver injury and fibrogenesis were initially evaluated using histological analyses.
These observations may contribute to the therapeutic value of novel small molecule drugs in the treatment of hepatitis.
NF-κB is a transcription factor that regulates the expression of proinflammatory cytokines and proteins.
In this manuscript, the author examined the role of SKLBO10, a derivative of TZDs, in protecting the liver against CCl4 induced acute and chronic liver injury in rats. They found that orally administration of SKLBO10 at relatively high dose (100 mg per kg body mass per 24 h) protect the liver from acute injury (reduce serum alanine aminotransferase activity and inflammatory infiltration as well as improve histological architecture of the liver). Moreover, they have shown that SKLB010 inhibit: (1) degradation of IκB; (2) secretion of proinflammatory mediators; and (3) NO synthesis.
Peer reviewer: Shashi Bala, Dr., Department of Medicine, Umass Medical school, 364 plantation street, LRB270l, Worcester, MA 01605, United States
S- Editor Sun H L- Editor Webster JR E- Editor Zheng XM
| 1. | Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2807] [Cited by in RCA: 2816] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 2. | Simpson KJ, Lukacs NW, Colletti L, Strieter RM, Kunkel SL. Cytokines and the liver. J Hepatol. 1997;27:1120-1132. [PubMed] |
| 3. | Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950-1960. [PubMed] |
| 5. | Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4738] [Cited by in RCA: 4816] [Article Influence: 166.1] [Reference Citation Analysis (0)] |
| 6. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1147] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 7. | Neuman MG. Cytokines--central factors in alcoholic liver disease. Alcohol Res Health. 2003;27:307-316. [PubMed] |
| 8. | Oakley F, Mann J, Nailard S, Smart DE, Mungalsingh N, Constandinou C, Ali S, Wilson SJ, Millward-Sadler H, Iredale JP. Nuclear factor-kappaB1 (p50) limits the inflammatory and fibrogenic responses to chronic injury. Am J Pathol. 2005;166:695-708. [PubMed] |
| 9. | Luo Y, Ma L, Zheng H, Chen L, Li R, He C, Yang S, Ye X, Chen Z, Li Z. Discovery of (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione, a readily available and orally active glitazone for the treatment of concanavalin A-induced acute liver injury of BALB/c mice. J Med Chem. 2010;53:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Bansal MB, Kovalovich K, Gupta R, Li W, Agarwal A, Radbill B, Alvarez CE, Safadi R, Fiel MI, Friedman SL. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J Hepatol. 2005;42:548-556. [PubMed] |
| 11. | Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Hentze H, Gantner F, Kolb SA, Wendel A. Depletion of hepatic glutathione prevents death receptor-dependent apoptotic and necrotic liver injury in mice. Am J Pathol. 2000;156:2045-2056. [PubMed] |
| 13. | Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489-492. [PubMed] |
| 14. | Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82-86. [PubMed] |
| 15. | Zheng S, Yumei F, Chen A. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med. 2007;43:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [PubMed] |
| 17. | McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435-459. [PubMed] |
| 18. | Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821-3830. [PubMed] |
| 19. | Geier A, Kim SK, Gerloff T, Dietrich CG, Lammert F, Karpen SJ, Stieger B, Meier PJ, Matern S, Gartung C. Hepatobiliary organic anion transporters are differentially regulated in acute toxic liver injury induced by carbon tetrachloride. J Hepatol. 2002;37:198-205. [PubMed] |
| 20. | Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |