Published online Feb 21, 2012. doi: 10.3748/wjg.v18.i7.609
Revised: October 12, 2011
Accepted: December 31, 2011
Published online: February 21, 2012
Adamantiades-Behcet’s disease (ABD) is a chronic, relapsing, systemic vasculitis of unknown etiology. It is more prevalent in populations along the ancient Silk Road from Eastern Asia to the Mediterranean Basin, and most frequently affects young adults between the second and fourth decades of life. ABD-complicated gastroenteropathy is a significant cause of morbidity and mortality, with abdominal pain as the most common symptom. The ileocecal region is affected predominantly, with ulcerations that may lead to penetration and/or perforation, whereas other parts of the gastrointestinal system including the esophagus and stomach can also be affected. Endoscopy is useful to locate the site and extent of the lesions, and tissue biopsy is often warranted to examine the histopathology that is often suggestive of underlying vasculitis of small veins/venules or, alternatively in some cases, nonspecific inflammation. Bowel wall thickening is the most common finding on computed tomography scan. Treatment is largely empirical since well-controlled studies are difficult to conduct due to the heterogeneity of the disease, and the unpredictable course with exacerbation and remission. Corticosteroids with or without other immunosuppressive drugs, such as cyclophosphamide, azathioprine, sulfasalazine, tumor necrosis factor α antagonist or thalidomide should be applied before surgery, except in emergency.
- Citation: Wu QJ, Zhang FC, Zhang X. Adamantiades-Behcet's disease-complicated gastroenteropathy. World J Gastroenterol 2012; 18(7): 609-615
- URL: https://www.wjgnet.com/1007-9327/full/v18/i7/609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i7.609
Adamantiades-Behcet’s disease (ABD) is a systemic inflammatory vasculitis of unknown etiology, characterized by relapsing episode of oral aphthous ulcers, genital ulcers, cutaneous and ocular lesions and other manifestations, including vascular, neurological and gastrointestinal involvements[1].
ABD usually occurs between the second and fourth decades of life. Recent epidemiological surveys indicate that male and female are equally affected in ABD[2-4]. ABD is particularly prevalent in ancient “Silk Route” populations from Far East to Middle East to Mediterranean, albeit it has global distribution. The marked geographic predilection of ABD can be explained by its genetics background and/or environmental triggers[1].
ABD-complicated gastroenteropathy (ABD-GE) varies in different populations, being much more frequent in the Far East than in the Middle East and Mediterranean. Several nationwide surveys and large-scale case series show that ABD-GE is frequent in Japan (16%)[5], Germany (12%)[6], Iran (7.4%)[7], Korea (7.3%)[8] and China (6.5%)[9], but rare in Turkey (1.4%)[10].
The clinical manifestation of ABD-GE may also vary greatly, from mild symptom to life-threatening complications, including perforation, infarction, and massive bleeding resulting from vasculitis and/or thrombosis. Prompt treatment with corticosteroid plus immunosuppressive agents rather than surgery can alleviate the clinical symptoms and improve prognosis of ABD-GE patients. According to the International Study Group Criteria for ABD, diagnosis of ABD requires the presence of recurrent oral ulcers and two of the followings: Genital ulcers, typical eye lesions, typical skin lesions, and positive pathergy test[11]. However, in clinical practice, it is often challenging to make prompt and correct diagnosis when gastroenteropathy is presented as the initial or predominant manifestation in ABD patients, and sometimes is misdiagnosed as inflammatory bowel disease or other disorders. This paper systematically reviews the clinical manifestation and treatment of ABD-GE for the purpose of better managing this life-threatening complication.
Among the 136 reported gastrointestinal (GI) endoscope cases in the Japanese patients with ABD who required surgery, abdominal pain was found in 92%, abdominal mass in 21%, and melena in 17%[12]. Almost all Japanese juvenile ABD-GE manifested with abdominal pain, bloody stool, high fever, and anal lesions[13]. The main manifestations of ABD-GE are described as follows (Table 1).
| Ref. | Type of manifestation | Symptom | Frequency | Complication | Therapy | Outcome |
| Zhang et al[9] Tursen et al[10] | Recurrent oral ulcer | Painful ulcer | Almost 100% | Rare | Topical measures | Excellent |
| Mori et al[15] Yi et al[16] | Esophageal ulcer/esophagitis | Substernal pain, dysphagia | Very rare | Very rare | Corticosteroid | Excellent |
| Ning-Sheng et al[29] | Gastric ulcer and/or duodenal ulcer | Epigastric pain | Variable (very rare to 45%) | Very rare | Uncertain | Excellent |
| Choi et al[35] Köklü et al[45] | Small and/or large intestinal ulcer | Abdominal pain, hematochezia | Variable (1.4% up to 16%) | Rare (perforation, massive bleeding) | Sulfalazzine, corticosteroid azathioprine, tumor necrosis factor α antagonist, thalidomide | Good |
| Bayraktar et al[53] Chubachi et al[54] | Large artery aneurysm/thrombosis in abdomen | Infarction, ischemia | Very rare | Very rare | Corticosteroid azathioprine, cyclophosphamide | Poor |
| Bismuth et al[56] | Large vein thrombosis in abdomen | Budd-Chiari syndrome | Very rare | Very rare | Corticosteroid azathioprine | Poor |
Almost all patients have recurrent oral ulceration. This is often the first symptom and occurs long before other manifestations appear. Minor aphthous ulcer (< 10 mm in diameter) is the most common type (85%), whereas major (> 10 mm in diameter) and herpetiform ulcers are less frequent. Lesions are usually painful, occur as single ulcer or in crops, with a round or oval appearance with erythematous border (pouched out), covered with grayish-white pseudo-membrane or a central yellowish fibrinous base. They usually heal without scarring. The most commonly involved sites are gingival and buccal mucosa, tongue and lips, although ulcers can also appear in the soft and hard palate, pharynx and tonsils. Histological findings suggest vasculitis with monocyte and lymphocyte infiltration at the early stage of the disease and neutrophils at later stage. Other disorders such as fibrinoid necrosis, endothelial swelling, and perivascular infiltration can also occur in ABD. Although aphthae is often multiple and occurs more frequently in ABD, they are indistinguishable from those of recurrent oral ulcers due to other causes, such as malnutrition, viral infections, inflammatory bowel disease, and Reiter’s syndrome[14].
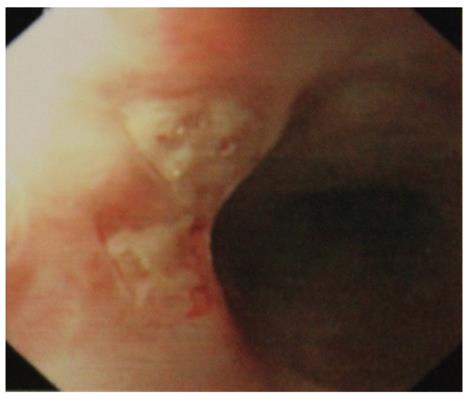
Symptomatic esophageal involvement is considered very rare[15,16], usually associated with other GI manifestations[15-19]. Only 45 retrospective case reports of esophageal involvement in ABD were found in literature. Most of the patients had symptoms severe enough to warrant upper gastrointestinal endoscopic examination[20]. The middle or lower part of the esophagus is often involved, causing substernal pain, dysphagia, and in rare case, hematemesis. Morphological findings include erosion, aphthous, linear or perforating ulcer (Figure 1), and widely distributed esophagitis[15,16]. Occasionally, there may be perforation, dissection, penetration, fistula formation, or pharyngeal stenosis causing dysphagia and dyspnea[21-25]. Esophageal varices may occur in association with superior vena cava obstruction, and portal hypertension due to portal or hepatic vein thrombosis[26]. Although some prospective studies suggest that the prevalence of asymptomatic esophageal involvement in ABD is rather high, it does not lead to severe abnormalities, and esophago-gastro-duodenoscopy is recommended only in those with clinical symptoms[20,25]. Histological findings reveal nonspecific inflammation with lymphocytic or neutrophilic infiltration rather than vasculitis. The esophageal lesions in ABD often respond to corticosteroid instead of treatment with a proton pump inhibitor[27,28]. Viral or candida esophagitis should be excluded before initiation of corticosteroid.
The stomach is the least frequently involved part of ABD, and aphthous ulcers are the most common findings. It was reported that 45% of Taiwanese patients with ABD had gastric and/or duodenal ulcers[29]. There may be pyloric stenosis due to edematous hypertrophy of pyloric ring[30,31] or Dieulafoy’s ulcer[32]. ABD patients with gastric and/or duodenal lesions usually present with epigastric pain. Duodenal ulcers may be resistant to anti-ulcer medications[31], but whether corticosteroid should be used is unclear. Such ulcers can resolve spontaneously, and corticosteroid may inhibit its healing process, so that the effect of corticosteroid is uncertain[33]. There was also report suggesting that Helicobacter pylori treatment could diminish the oral and genital ulcers of ABD patients[34].
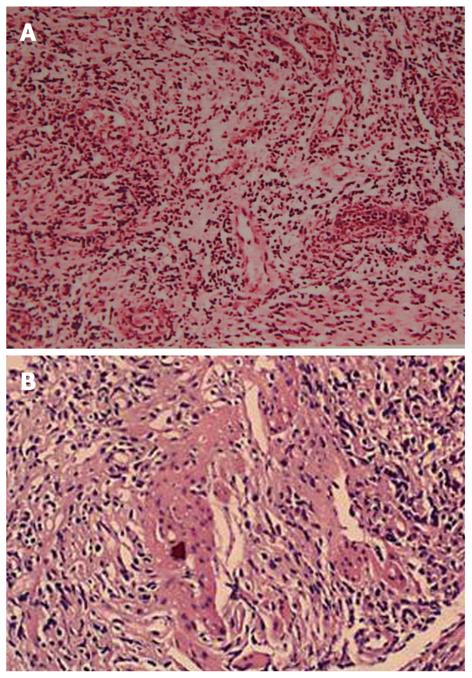
There are two forms of intestinal involvement: small vessel disease with mucosal inflammation causing ulcer (Figure 2) and large vessel disease resulting in intestinal ischemia and infarction. Mucosal ulceration is most commonly seen in the ileocecal region, and it was found in 88% patients in a study[35], usually on the antimesenteric side[36], followed by involvement of other part of colon, but rarely rectum or anus. The ulcers may be aphthous or, alternatively, deep and round with pouched-out appearance. Longitudinal ulcers are rare. The ulcers may penetrate the intestinal wall, resulting in perforation (often at multiple sites), fistula formation, or bleeding. Ulcers may resolve with medical therapy, but recur later[37]. In those ABD patients who underwent surgery while their disease was still active, the lesions tended to recur at the anastomotic site, especially along the ileal side of ileocolic anastomoses[38]. Although it is rare, there are also case reports of toxic megacolon without any precipitating factors[39] and severe proctitis with rectovaginal fistula[40].
Barium studies demonstrate single or multiple discrete ulcers with considerable thickening of the surrounding mucosal folds[18,38]. Other nonspecific findings include cecal contraction, widening of the ileocecal valve, fold thickening in the terminal ileum, and an apparent ileocecal mass with ulceration[41,42]. Barium study is a noninvasive and helpful examination to locate the GI lesions of ABD, but has limitations in defining the characteristics of lesions, and may not be applied to some cases of intestinal ABD that are inclined to penetrate the intestinal wall. In such situation, computed tomography (CT) scan and magnetic resonance imaging (MRI) will yield valuable information. CT scan shows concentric or uneven bowel wall thickening that remarkably enhances after administration of contrast agent[18,38]. This enhancement suggests stasis of blood due to vasculitis or perivasculitis affecting mainly the veins and venules of the ulcerous submucosa[38], which is supported by histopathological findings with diffuse vascular dilatation and perivascular lymphocyte infiltration. Polypoid lesions, sometimes with central ulceration, should be differentiated from malignancy by their marked contrast enhancement and involvement of both the terminal ileum and cecum. Complications such as bowel perforation and peritonitis, occur more frequently in those with a thickened bowel wall and severe perienteric infiltration than in those with polypoid lesions. MRI is helpful in revealing bowel wall thickening and increased contrast enhancement as well as extraluminal manifestations such as mesenteric infiltration around the involved bowel[43].
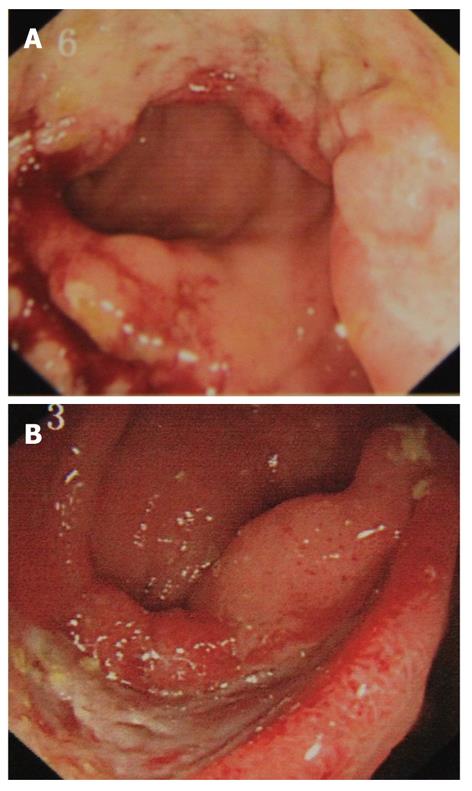
Colonoscopy is usually warranted for patients with clinical symptoms. The most common colonoscopic findings are localized single or multiple ulcers in the ileocecal region (Figure 3), and only 4% has a diffuse distribution of lesions[44,45]. The characteristics of ileocecal ulcer of ABD also vary in different regions, with multiple superficial ulcers localized prominently in the terminal ileum in Turkish patients[46], and single deep ulcer with distinct borders in the Far East[44,45]. The colonic ulcers have been classified as volcano, geographic, and aphthous types. Volcano-type ulcers are defined as well-demarcated penetrating ulcers with nodular margins, converging folds, or pseudopolyps. Geographic-type ulcers are defined as shallow ulcers with sharp edges, and aphthous-type ulcers are small, pouched-out shallow ulcers similar to oral aphthous ulceration. Volcano-type ulcers have the least favorable response to medical treatment, and are at more frequent requisition for surgery, and are more likely to recur than the other two types[47]. Therefore, volcano-type ulcers should be treated vigorously and followed up closely. In rare cases, there are vesicular lesions or single rectal ulcers with otherwise normal mucosa[48].
Wireless capsule endoscopy is also useful in the investigation of gastrointestinal symptoms in ABD. It is particularly helpful for those patients in whom conventional investigations (such as esophago-gastro-duodenoscopy, colonoscopy, and barium study) are normal or fail to account for symptoms and signs[49,50].
The diagnosis of ABD is mainly based on the typical clinical findings, no specific serum markers or pathological features are available. Diagnosis of ABD-GE can be made if there is a typical oval-shaped large ulcer in the terminal ileum, or there are ulcerations or inflammation in the small or large intestines, and clinical findings meet the diagnostic criteria of ABD. Crohn’s disease (CD), tuberculosis, vasculitis and other diseases that mimic ABD-GE should be excluded before diagnosis of ABD-GE is established[51]. Like CD, ABD-GE manifests as discrete ulcers and discontinuous bowel involvement with relative sparing of the rectum. The two diseases share extraintestinal manifestations, such as uveitis and arthritis. Unlike CD, ABD-GE is characterized by vasculitis of the small veins and venules with deep ulcerations, generally without granulomas or cobblestoning. However, both diseases may have chronic nonspecific inflammation with normal intervening mucosa. Perforation is more common in ABD-GE than in CD since the latter is characterized by intense fibrosis. Scalloping, ulceronodular patterns, and abscess formation are not observed in ABD-GE[46]. Unlike ulcerative colitis, colonic ABD consists of multiple aphthous ulcers with preservation of haustra and involvement primarily of the terminal ileum and proximal colon. Both intestinal tuberculosis and ABD-GE mainly manifest ulcerations in the ileocolonic region. Unlike ABD-GE, intestinal tuberculosis is characterized by granuloma with positive acid-fasting staining. Systemic lupus erythematosus (SLE) and ABD also share some common features, such as oral ulcerations, arthritis, and central nervous system involvement. Unlike ABD, SLE presents serum markers, such as anti-nuclear antibody, anti-ds-DNA and anti-Sm antibodies.
ABD abdominal vasculitis is more likely to affect small veins and venules than arteries or arterioles. There can be intense inflammation around the vasa vasorum, resulting in destruction of the media and fibrous thickening of the intima and adventitia[52]. Pseudoaneurysms are the most common arterial manifestation, mainly involving the aorta, the pulmonary, and the femoral arteries. Thrombosis of the superficial and deep veins is more common than thrombosis and aneurysms of the large arteries in the abdominal cavity in ABD[53], albeit there was case report showing ABD patient manifested with a large aneurysm of the superior mesenteric artery causing occlusion and ischemic enteritis[54]. Mesenteric ischemia and infarction may be due to large vessel occlusion or to mucosal disease secondary to vasculitis of the small vessels[52].
Large vessel thrombosis occurs in 11% of ABD cases, of which 26% have hepatic vein or the inferior vena cava (IVC) thrombosis. In fact, ABD is the most common cause of Budd-Chiari syndrome in Turkey, especially in young male patients[55]. It may present with hepatomegaly, ascites, and involvement of other large vessels, mostly venous. Obstruction of the IVC due to extension of the thrombus to the ostium of the hepatic veins was found in most ABD patients with Budd-Chiari[56]. It could lead to acute hepatic failure and rapid death in one-third of the patients in one series. The major predicting factor for survival was the extent of vascular thrombosis in the IVC. ABD may also contribute to development of cavernous transformation of the portal vein[57], portal vein thrombosis with splenomegaly, and superior vena cava thrombosis[58].
Hepatobiliary complications include fatty liver or congestion, acute and chronic hepatitis, cholelithiasis and cholecystitis, primary biliary cirrhosis, and hepatic abscesses[59-61]. The liver alkaline phosphatase level was elevated in 11% of ABD patients and correlated with disease activity[61]. Splenic involvement occurred in 37 of 170 autopsies in Japanese ABD patients with splenitis, splenomegaly, hemosiderosis, infarction, and auto-splenectomy, and pancreatic involvement was found in 2.9% of the ABD cases[60].
Acute ABD pancreatitis responded to treatment with corticosteroids[62]. Chronic pancreatitis was reported in an ABD patient with an alcohol history[63]. Due to the rarity of pancreatitis in ABD, other causes such as gallstone disease should be excluded.
Other rare complications include hepatic artery aneurysm causing hemobilia[64] as well as pylephlebitis and septic thrombophlebitis of the portal vein[65]. Type AA amyloidosis can also complicate with ABD, manifesting as diarrhea and malabsorption. It can also affect the kidneys with proteinuria and progress to nephrosis and renal failure. It has a 50% mortality rate after an average duration of 3.4 years[66-68].
Treatment is largely empirical since well-controlled studies are difficult to conduct due to the heterogeneity of the disease, and the unpredictable course with exacerbation and remission. Therefore, there lacks evidence-based treatment recommended for the management of ABD-GE. Agents such as corticosteroids, sulfasalazine, azathioprine, cyclophosphamide, TNFα antagonist or thalidomide should be tried first before surgery, except in emergency[69,70]. Retrospective studies suggest that corticosteroids, sulfasalazine and azathioprine are effective in achieving remission without the need for surgery in a large proportion of patients. One study reported that azathioprine could decrease re-surgery rates and suggested that it should be used as maintenance therapy in patients whose gastrointestinal complication requires emergent surgery. Mesalazine may reduce the total dose of corticosteroids required to treat intestinal disease and esophageal ulcers[71,72]. Anti-TNFα therapy such as infliximab and thalidomide were also reported useful in treating intestinal lesions in case studies[22,73], which highlighted the role of large amounts of TNFα secreted by γδ T cells in ABD patients[74].
ABD-GE can sometimes resolve spontaneously, making it difficult to precisely evaluate the effectiveness of the therapy[33,36]. On the other hand, corticosteroids may prolong the healing process, provoke colonic perforation, and worsen the pancreatitis[33,75]. In one report, perforation was noted in 41% of patients who received corticosteroids and 33% of those who did not[12].
There is also no firm evidence to guide the management of major abdominal vessel disease in ABD. For the treatment of acute deep vein thrombosis in ABD, immunosuppressive agents such as corticosteroids, azathioprine, cyclophosphamide, or cyclosporine A are recommended[69,70]. For management of arterial aneurysms, cyclophosphamide and corticosteroids are recommended[69,70]. There is no evidence that anticoagulation is beneficial and it may in fact lead to fatal pulmonary hemorrhage in those with pulmonary arteritis and aneurysm formation[76].
Patients who have a history of intestinal perforation or fistula formation have a high probability of recurrence after surgery[35]. The recommended length of resection is controversial. Some advocate wide surgical margins, while others recommend removal of only the grossly affected bowel[12,35,77]. In one report, the incidence of recurrence was 18% with resection of the right colon and 35% with resection of just the ileocecal region[12]. In another report, the length of resection did not affect the rate of recurrence or reoperation[35]. Intraoperative endoscopy, especially of the small bowel, can help determine the length of resection during surgery. Follow-up endoscopy and barium studies should be done to evaluate the anastomotic site.
ABD usually has a chronic, unpredictable course with exacerbation and remission which decrease in frequency and severity over time. Death is mainly due to major vessel disease and neurological involvement. The prognosis is worst among young males[78]. Complete remission was achieved in 38% of ABD patients with gastrointestinal involvement after 8 wk of medical treatment[35]. The rate of recurrence after surgery has been reported to be 40%-56%[12,42]. Recurrence was found in 49% of patients at 5 years, especially in those with intestinal perforation or fistula formation[35]. Recurrent lesions were at or near the anastomosis in 81% of patients. Of those who underwent surgery, 75% recurred within 2 years and were associated with a higher rate of complications such as ocular and ileal lesions than the nonsurgical group[79]. The incidence of postoperative recurrence was lower in those with normal intraoperative endoscopy than in those with observed lesions[80].
Peer reviewer: Tsianos Epameinondas, MD, PhD, Professor, 1st Division of Internal Medicine and Hepato-Gastroenterology Unit, Medical school University of Ioannina, PO Box 1186, Ioannina 45110, Greece
S- Editor Gou SX L- Editor Ma JY E- Editor Zhang DN
| 1. | Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med. 1999;341:1284-1291. [PubMed] |
| 2. | Azizlerli G, Köse AA, Sarica R, Gül A, Tutkun IT, Kulaç M, Tunç R, Urgancioğlu M, Dişçi R. Prevalence of Behçet's disease in Istanbul, Turkey. Int J Dermatol. 2003;42:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Gürler A, Boyvat A, Türsen U. Clinical manifestations of Behçet's disease: an analysis of 2147 patients. Yonsei Med J. 1997;38:423-427. [PubMed] |
| 4. | Zouboulis CC, Kötter I, Djawari D, Kirch W, Kohl PK, Ochsendorf FR, Keitel W, Stadler R, Wollina U, Proksch E. Epidemiological features of Adamantiades-Behcet's disease in Germany and in Europe. Yonsei Med J. 1997;38:411-422. [PubMed] |
| 5. | Nakae K, Masaki F, Hashimoto T, Inaba G, Mochizuki M, Sakane T. Recent epidemiological features of Behcet’s disease in Japan. Behcet’s Disease. Amsterdam: Elsevier Science Publishers B.V 1993; 145-151. |
| 6. | Altenburg A, Papoutsis N, Orawa H, Martus P, Krause L, Zouboulis CC. [Epidemiology and clinical manifestations of Adamantiades-Behçet disease in Germany -- current pathogenetic concepts and therapeutic possibilities]. J Dtsch Dermatol Ges. 2006;4:49-64; quiz 65-66. [PubMed] |
| 7. | Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Nadji A, Akhlaghi M, Faezi T, Ghodsi Z, Larimi R, Ashofteh F. Behcet's disease in Iran: analysis of 6500 cases. Int J Rheum Dis. 2010;13:367-373. [PubMed] |
| 8. | Bang D, Lee JH, Lee ES, Lee S, Choi JS, Kim YK, Cho BK, Koh JK, Won YH, Kim NI. Epidemiologic and clinical survey of Behcet's disease in Korea: the first multicenter study. J Korean Med Sci. 2001;16:615-618. [PubMed] |
| 9. | Zhang ZL, Peng J, Hou XM, Dong Y. Clinical manifestations of Behcet’s disease in Chinese patients. APLAR J Rheumatol. 2006;9:244-247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behçet's disease. Int J Dermatol. 2003;42:346-351. [PubMed] |
| 11. | Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet. 1990;335:1078-1080. [PubMed] |
| 12. | Kasahara Y, Tanaka S, Nishino M, Umemura H, Shiraha S, Kuyama T. Intestinal involvement in Behçet's disease: review of 136 surgical cases in the Japanese literature. Dis Colon Rectum. 1981;24:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 149] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Tabata M, Tomomasa T, Kaneko H, Morikawa A. Intestinal Behçet's disease: a case report and review of Japanese reports in children. J Pediatr Gastroenterol Nutr. 1999;29:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Ebert EC. Gastrointestinal manifestations of Behçet's disease. Dig Dis Sci. 2009;54:201-207. [PubMed] |
| 15. | Mori S, Yoshihira A, Kawamura H, Takeuchi A, Hashimoto T, Inaba G. Esophageal involvement in Behcet's disease. Am J Gastroenterol. 1983;78:548-553. [PubMed] |
| 16. | Yi SW, Cheon JH, Kim JH, Lee SK, Kim TI, Lee YC, Kim WH. The prevalence and clinical characteristics of esophageal involvement in patients with Behçet's disease: a single center experience in Korea. J Korean Med Sci. 2009;24:52-56. [PubMed] |
| 17. | Chung SY, Ha HK, Kim JH, Kim KW, Cho N, Cho KS, Lee YS, Chung DJ, Jung HY, Yang SK. Radiologic findings of Behçet syndrome involving the gastrointestinal tract. Radiographics. 2001;21:911-924; discussion 924-926. [PubMed] |
| 18. | Parkin JV, Wight DG. Behçet's disease and the alimentary tract. Postgrad Med J. 1975;51:260-264. [PubMed] |
| 19. | Vlymen WJ, Moskowitz PS. Roentgenographic manifestations of esophageal and intestinal involvement in Behcet's disease in children. Pediatr Radiol. 1981;10:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Houman MH, Ben Ghorbel I, Lamloum M, Khanfir M, Braham A, Haouet S, Sayem N, Lassoued H, Miled M. Esophageal involvement in Behcet's disease. Yonsei Med J. 2002;43:457-460. [PubMed] |
| 21. | Morimoto Y, Tanaka Y, Itoh T, Yamamoto S, Kurihara Y, Nishikawa K. Esophagobronchial fistula in a patient with Behçet's disease: report of a case. Surg Today. 2005;35:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Mussack T, Landauer N, Ladurner R, Schiemann U, Goetzberger M, Burchardi C, Folwaczny C, Heldwein W, Hallfeldt K. Successful treatment of cervical esophageal perforation in Behcet's disease with drainage operation and infliximab. Am J Gastroenterol. 2003;98:703-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Yashiro K, Nagasako K, Hasegawa K, Maruyama M, Suzuki S, Obata H. Esophageal lesions in intestinal Behçet's disease. Endoscopy. 1986;18:57-60. [PubMed] |
| 24. | Brookes GB. Pharyngeal stenosis in Behçet's syndrome. The first reported case. Arch Otolaryngol. 1983;109:338-340. [PubMed] |
| 25. | Bottomley WW, Dakkak M, Walton S, Bennett JR. Esophageal involvement in Behçet's disease. Is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Tavakkoli H, Asadi M, Haghighi M, Esmaeili A. Therapeutic approach to "downhill" esophageal varices bleeding due to superior vena cava syndrome in Behcet's disease: a case report. BMC Gastroenterol. 2006;6:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Anti M, Marra G, Rapaccini GL, Barone C, Manna R, Bochicchio GB, Fedeli G. Esophageal involvement in Behçet's syndrome. J Clin Gastroenterol. 1986;8:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Wedemeyer H, Kuipers JG, Streetz K, Mengel M, Schedel I, Kezmic N, Meier P, Zeidler H, Manns MP, Wagner S. A rare manifestation of Behçet's syndrome: immunological correlates and successful treatment of an esophageal ulcer. Dig Dis Sci. 2003;48:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 29. | Ning-Sheng L, Ruay-Sheng L, Kuo-Chih T. High frequency of unusual gastric/duodenal ulcers in patients with Behçet's disease in Taiwan: a possible correlation of MHC molecules with the development of gastric/duodenal ulcers. Clin Rheumatol. 2005;24:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Ozenç A, Bayraktar Y, Baykal A. Pyloric stenosis with esophageal involvement in Behçet's syndrome. Am J Gastroenterol. 1990;85:727-728. [PubMed] |
| 31. | Satake K, Yada K, Ikehara T, Umeyama K, Inoue T. Pyloric stenosis: an unusual complication of Behçet's disease. Am J Gastroenterol. 1986;81:816-818. [PubMed] |
| 32. | Arendt T, Kloehn S, Bastian A, Bewig B, Lins M, Mönig H, Fölsch UR. A case of Behçet's syndrome presenting with Dieulafoy's ulcer. Z Gastroenterol. 1997;35:935-938. [PubMed] |
| 33. | Takada Y, Saigenji K. Is intestinal Behçet's disease in fact an enterocolitis or an ulcer disease, and is steroid treatment useful or harmful? J Gastroenterol. 2003;38:1015-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Avci O, Ellidokuz E, Simşek I, Büyükgebiz B, Güneş AT. Helicobacter pylori and Behçet's disease. Dermatology. 1999;199:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Choi IJ, Kim JS, Cha SD, Jung HC, Park JG, Song IS, Kim CY. Long-term clinical course and prognostic factors in intestinal Behçet's disease. Dis Colon Rectum. 2000;43:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Takada Y, Fujita Y, Igarashi M, Katsumata T, Okabe H, Saigenji K, Takahashi T, Atari E. Intestinal Behçet's disease--pathognomonic changes in intramucosal lymphoid tissues and effect of a "rest cure" on intestinal lesions. J Gastroenterol. 1997;32:598-604. [PubMed] |
| 37. | Iida M, Kobayashi H, Matsumoto T, Okada M, Fuchigami T, Niizeki H, Yao T, Fujishima M. Intestinal Behçet disease: serial changes at radiography. Radiology. 1993;188:65-69. [PubMed] |
| 38. | Ha HK, Lee HJ, Yang SK, Ki WW, Yoon KH, Shin YM, Jung HY, Yu E, Lee SI, Kim KW. Intestinal Behçet syndrome: CT features of patients with and patients without complications. Radiology. 1998;209:449-454. [PubMed] |
| 39. | Adorian C, Khoury G, Tawil A, Sharara A. Behçet's disease complicated by toxic megacolon. Dig Dis Sci. 2003;48:2366-2368. [PubMed] |
| 40. | Teh LS, Green KA, O'Sullivan MM, Morris JS, Williams BD. Behçet's syndrome: severe proctitis with rectovaginal fistula formation. Ann Rheum Dis. 1989;48:779-780. [PubMed] |
| 41. | Rosenberger A, Adler OB, Haim S. Radiological aspects of Behçet disease. Radiology. 1982;144:261-264. [PubMed] |
| 42. | Kim JH, Choi BI, Han JK, Choo SW, Han MC. Colitis in Behçet's disease: characteristics on double-contrast barium enema examination in 20 patients. Abdom Imaging. 1994;19:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | You JK, Kim MJ, Park S, Chung JJ, Kim WH. Intestinal Behçet's disease: breath-hold MR imaging. Abdom Imaging. 2001;26:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Lee CR, Kim WH, Cho YS, Kim MH, Kim JH, Park IS, Bang D. Colonoscopic findings in intestinal Behçet's disease. Inflamm Bowel Dis. 2001;7:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Köklü S, Yüksel O, Onur I, Unverdi S, Biyikoğlu I, Akbal E, Sengül D, Unverdi H, Ekşioğlu M. Ileocolonic involvement in Behçet's disease: endoscopic and histological evaluation. Digestion. 2010;81:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Korman U, Cantasdemir M, Kurugoglu S, Mihmanli I, Soylu N, Hamuryudan V, Yazici H. Enteroclysis findings of intestinal Behcet disease: a comparative study with Crohn disease. Abdom Imaging. 2003;28:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Kim JS, Lim SH, Choi IJ, Moon H, Jung HC, Song IS, Kim CY. Prediction of the clinical course of Behçet's colitis according to macroscopic classification by colonoscopy. Endoscopy. 2000;32:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Smith GE, Kime LR, Pitcher JL. The colitis of Behcet's disease: a separate entity? Colonoscopic findings and literature review. Am J Dig Dis. 1973;18:987-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Gubler C, Bauerfeind P. Intestinal Behçet's disease diagnosed by capsule endoscopy. Endoscopy. 2005;37:689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Hamdulay SS, Cheent K, Ghosh C, Stocks J, Ghosh S, Haskard DO. Wireless capsule endoscopy in the investigation of intestinal Behçet's syndrome. Rheumatology (. Oxford). 2008;47:1231-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Kobayashi K, Ueno F, Bito S, Iwao Y, Fukushima T, Hiwatashi N, Igarashi M, Iizuka BE, Matsuda T, Matsui T. Development of consensus statements for the diagnosis and management of intestinal Behçet's disease using a modified Delphi approach. J Gastroenterol. 2007;42:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Kobayashi M, Ito M, Nakagawa A, Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Neutrophil and endothelial cell activation in the vasa vasorum in vasculo-Behçet disease. Histopathology. 2000;36:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Bayraktar Y, Soylu AR, Balkanci F, Gedikoğlu G, Cakmakçi M, Sayek I. Arterial thrombosis leading to intestinal infarction in a patient with Behçet's disease associated with protein C deficiency. Am J Gastroenterol. 1998;93:2556-2558. [PubMed] |
| 54. | Chubachi A, Saitoh K, Imai H, Miura AB, Kotanagi H, Abe T, Matsumoto T. Case report: intestinal infarction after an aneurysmal occlusion of superior mesenteric artery in a patient with Behçet's disease. Am J Med Sci. 1993;306:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Bayraktar Y, Balkanci F, Kansu E, Kayhan B, Arslan S, Eryilmaz M, Telatar H. Budd-Chiari syndrome: analysis of 30 cases. Angiology. 1993;44:541-551. [PubMed] |
| 56. | Bismuth E, Hadengue A, Hammel P, Benhamou JP. Hepatic vein thrombosis in Behçet's disease. Hepatology. 1990;11:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Bayraktar Y, Balkanci F, Kansu E, Dundar S, Uzunalimoglu B, Kayhan B, Telatar H, Gurakar A, Van Thiel DH. Cavernous transformation of the portal vein: a common manifestation of Behçet's disease. Am J Gastroenterol. 1995;90:1476-1479. [PubMed] |
| 58. | Bayraktar Y, Balkanci F, Bayraktar M, Calguneri M. Budd-Chiari syndrome: a common complication of Behçet's disease. Am J Gastroenterol. 1997;92:858-862. [PubMed] |
| 59. | Manna R, Ghirlanda G, Bochicchio GB, Papa G, Annese V, Greco AV, Taranto CA, Magaro M. Chronic active hepatitis and Behçet's syndrome. Clin Rheumatol. 1985;4:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Lakhanpal S, Tani K, Lie JT, Katoh K, Ishigatsubo Y, Ohokubo T. Pathologic features of Behçet's syndrome: a review of Japanese autopsy registry data. Hum Pathol. 1985;16:790-795. [PubMed] |
| 61. | Hisaoka M, Haratake J, Nakamura T. Small bile duct abnormalities and chronic intrahepatic cholestasis in Behçet's syndrome. Hepatogastroenterology. 1994;41:267-270. [PubMed] |
| 62. | Le Thi Huong D, Wechsler B, Dell'Isola B, Lautier-Frau M, Palazzo L, Bletry O, Piette JC, Godeau P. Acute pancreatitis in Behçet's disease. Dig Dis Sci. 1992;37:1452-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Alkim H, Gürkaynak G, Sezgin O, Oğuz D, Saritaş U, Sahin B. Chronic pancreatitis and aortic pseudoaneurysm in Behçet's disease. Am J Gastroenterol. 2001;96:591-593. [PubMed] |
| 64. | Hatzidakis A, Petrakis J, Krokidis M, Tsetis D, Gourtsoyiannis N. Hepatic artery aneurysm presenting with hemobilia in a patient with Behçet's disease: treatment with percutaneous transcatheteral embolization. Diagn Interv Radiol. 2006;12:53-55. [PubMed] |
| 65. | Gelber AC, Schachna L, Mitchell L, Schwartzman G, Hartnell G, Geschwind JF. Behçet's disease complicated by pylephlebitis and hepatic abscesses. Clin Exp Rheumatol. 2001;19:S59-S61. [PubMed] |
| 66. | Hamza M, Wechsler B, Godeau P, Hamza H, Ayed K. Intestinal amyloidosis: an unusual complication of Behçet's disease. Am J Gastroenterol. 1988;83:793-794. [PubMed] |
| 67. | Chiba M, Inoue Y, Arakawa H, Masamune O, Ohkubo M. Behçet's disease associated with amyloidosis. Gastroenterol Jpn. 1987;22:487-495. [PubMed] |
| 68. | Melikoğlu M, Altiparmak MR, Fresko I, Tunç R, Yurdakul S, Hamuryudan V, Yazici H. A reappraisal of amyloidosis in Behçet's syndrome. Rheumatology (. Oxford). 2001;40:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, Houman MH, Kötter I, Olivieri I, Salvarani C. EULAR recommendations for the management of Behçet disease. Ann Rheum Dis. 2008;67:1656-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 467] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 70. | Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, Houman MH, Kötter I, Olivieri I, Salvarani C. Management of Behçet disease: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for the management of Behçet disease. Ann Rheum Dis. 2009;68:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 71. | Fujiwara S, Shimizu I, Ishikawa M, Uehara K, Yamamoto H, Okazaki M, Horie T, Iuchi A, Ito S. Intestinal Behcet's disease with esophageal ulcers and colonic longitudinal ulcers. World J Gastroenterol. 2006;12:2622-2624. [PubMed] |
| 72. | Sonta T, Araki Y, Koubokawa M, Tamura Y, Ochiai T, Harada N, Chijiiwa Y, Nawata H. The beneficial effect of mesalazine on esophageal ulcers in intestinal Behcet's disease. J Clin Gastroenterol. 2000;30:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Hassard PV, Binder SW, Nelson V, Vasiliauskas EA. Anti-tumor necrosis factor monoclonal antibody therapy for gastrointestinal Behçet's disease: a case report. Gastroenterology. 2001;120:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Travis SP, Czajkowski M, McGovern DP, Watson RG, Bell AL. Treatment of intestinal Behçet's syndrome with chimeric tumour necrosis factor alpha antibody. Gut. 2001;49:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Toda K, Shiratori Y, Yasuda M, Enya M, Uematsu T, Shimazaki M, Fukutomi Y, Kato T, Moriwaki H. Therapeutic effect of intraarterial prednisolone injection in severe intestinal Behçet's disease. J Gastroenterol. 2002;37:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Hamuryudan V, Er T, Seyahi E, Akman C, Tüzün H, Fresko I, Yurdakul S, Numan F, Yazici H. Pulmonary artery aneurysms in Behçet syndrome. Am J Med. 2004;117:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Ketch LL, Buerk CA, Liechty D. Surgical implications of Behçet's disease. Arch Surg. 1980;115:759-760. [PubMed] |
| 78. | Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, Yurdakul S, Yazici H. The long-term mortality and morbidity of Behçet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore). 2003;82:60-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 79. | Naganuma M, Iwao Y, Inoue N, Hisamatsu T, Imaeda H, Ishii H, Kanai T, Watanabe M, Hibi T. Analysis of clinical course and long-term prognosis of surgical and nonsurgical patients with intestinal Behçet's disease. Am J Gastroenterol. 2000;95:2848-2851. [PubMed] |
| 80. | Iida M, Kobayashi H, Matsumoto T, Okada M, Fuchigami T, Yao T, Fujishima M. Postoperative recurrence in patients with intestinal Behçet's disease. Dis Colon Rectum. 1994;37:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |