Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7362
Revised: October 4, 2012
Accepted: November 11, 2012
Published online: December 28, 2012
Processing time: 138 Days and 20.9 Hours
AIM: To quantitatively assess the relationship between energy intake and the incidence of digestive cancers in a meta-analysis of cohort studies.
METHODS: We searched MEDLINE, EMBASE, Science Citation Index Expanded, and the bibliographies of retrieved articles. Studies were included if they reported relative risks (RRs) and corresponding 95% CIs of digestive cancers with respect to total energy intake. When RRs were not available in the published article, they were computed from the exposure distributions. Data were extracted independently by two investigators and discrepancies were resolved by discussion with a third investigator. We performed fixed-effects meta-analyses and meta-regressions to compute the summary RR for highest versus lowest category of energy intake and for per unit energy intake and digestive cancer incidence by giving each study-specific RR a weight that was proportional to its precision.
RESULTS: Nineteen studies consisting of 13 independent cohorts met the inclusion criteria. The studies included 995 577 participants and 5620 incident cases of digestive cancer with an average follow-up of 11.1 years. A significant inverse association was observed between energy intake and the incidence of digestive cancers. The RR of digestive cancers for the highest compared to the lowest caloric intake category was 0.90 (95% CI 0.81-0.98, P < 0.05). The RR for an increment of 239 kcal/d energy intake was 0.97 (95% CI 0.95-0.99, P < 0.05) in the fixed model. In subgroup analyses, we noted that energy intake was associated with a reduced risk of colorectal cancer (RR 0.90, 95% CI 0.81-0.99, P < 0.05) and an increased risk of gastric cancer (RR 1.19, 95% CI 1.08-1.31, P < 0.01). There appeared to be no association with esophageal (RR 0.96, 95% CI 0.86-1.07, P > 0.05) or pancreatic (RR 0.79, 95% CI 0.49-1.09, P > 0.05) cancer. Associations were also similar in studies from North America and Europe. The RR was 1.02 (95% CI 0.79-1.25, P > 0.05) when considering the six studies conducted in North America and 0.87 (95% CI 0.77-0.98, P < 0.05) for the five studies from Europe.
CONCLUSION: Our findings suggest that high energy intake may reduce the total digestive cancer incidence and has a preventive effect on colorectal cancer.
- Citation: Yu XF, Wang YQ, Zou J, Dong J. A meta-analysis of the effects of energy intake on risk of digestive cancers. World J Gastroenterol 2012; 18(48): 7362-7370
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7362
An important discovery in recent years is that lifestyle and environmental factors affect cancer initiation, promotion, and progression. Epidemiological studies strongly suggest that the majority of cancer deaths can be attributed to factors such as unhealthy diets, tobacco, alcoholism, infections, and occupational exposure. In particular, data from several observational studies support the theory that diet plays an important role in the initiation of many common cancers[1]. Calorie restriction (CR) is an experimental mode in which test animals receive a lower-calorie diet than ad libitum-fed controls. It has emerged as the most potent, broadly acting dietary intervention for preventing carcinogenesis in rodent models of cancer[2]. Recent reports of extended life span and delayed cancer development in response to CR in rhesus monkeys[3] and observations that CR during the premenopausal years decreases postmenopausal breast cancer risk in women[4] suggest that the anticancer effects of CR reported in rodent models extend to primates, including humans.
Although animal models have clearly demonstrated a protective effect of CR on cancer risk, it is less clear and there is little direct evidence that such a protective effect exists in humans. A study of normal-weight humans found that a 20% energy restriction for 10 wk did not reduce oxidative DNA damage[5]. In free-living populations, it is difficult to answer the important question of whether such an effect exists within the range of energy intake by humans. In human populations, energy intake is determined by physical activity, body size, and metabolic efficiency, and all these factors may be related to cancer risk, which makes the relationship between energy intake and cancer in humans complex.
Very few studies have assessed the relationship between CR and the risk of various cancer sites because of ethical issues. One study of the 1944-1945 Dutch famine and subsequent overall cancer incidence[6] found no evidence that the short famine affected overall cancer risk. However, higher energy intake in childhood may increase the risk of developing cancer in adulthood[7]. Data from case-control studies may be subject to recall bias with respect to energy intake and to selection bias with respect to the control group. Additional prospective cohort studies excluding those biases would be more useful for observing energy-cancer associations. We therefore systematically reviewed and performed a meta-analysis of prospective cohort studies to quantitatively assess the association between energy intake and digestive cancer risk in free-living human populations.
We searched the electronic databases MEDLINE (1966 to May, 2012), EMBASE (1985 to May, 2012), and Science Citation Index Expanded (1945 to May, 2012), using the Medical Subject Heading term energy intake combined with digestive system neoplasms. Furthermore, we reviewed reference lists of retrieved articles to search for additional studies. Only studies published as full-length articles in English were considered.
For inclusion, studies had to fulfill the following criteria: have a prospective cohort design, report relative risks (RR) or hazard ratios and their corresponding 95%CIs (or data to calculate them) of digestive cancers relating to every category of energy intake, and provide the categories or total intake of calories. Studies were excluded if a case-control design was used, the experimental participants were children or adolescents, energy intake from special food was reported in which the total intake of calories could not be calculated, or adequate classification of intake could not be determined because categories of energy intake were not reported. If multiple published reports from the same study cohort were available, we included only the one with the most detailed information for both outcome and energy intake. If there were multiple articles on different types of digestive cancers in the same cohort, we combined the outcomes to calculate the summary RR and its corresponding 95%CIs.
Data were extracted independently by two investigators (Yu XF and Dong J) according to the meta-analysis of observation studies in epidemiology guidelines[8], and discrepancies were resolved by discussion with a third investigator (Zou J). For each study, the following information was extracted: first author’s last name, year of publication, country of origin, follow-up period, number of patients and cases, digestive cancer sites, category amounts of energy intake, outcome assessment, RR or hazard ratios of cancer and the corresponding 95%CIs for every category of energy intake, and covariates adjusted for in the statistical analysis.
The measures of interest were the RR and the corresponding 95%CIs for included cohort studies. When RRs were not available in the published article, they were computed from the exposure distributions. We computed the summary RR for highest versus lowest category of energy intake and for per unit energy intake and digestive cancer incidence by giving each study-specific RR a weight that was proportional to its precision (i.e., the inverse of the variance was derived, when necessary, from the reported 95%CIs).
Statistical heterogeneity among studies was estimated using Q and I2 statistics. For the Q statistic, heterogeneity was considered present for P < 0.1. We pooled the study-specific estimates using both the fixed-effect model and the random-effect model proposed by DerSimonian and Laird; when a significant heterogeneity was found, the random-effect model results were presented. A sensitivity analysis was also conducted, in which one study at a time was removed and the rest were analyzed to estimate whether the results could have been markedly affected by a single study.
Finally, publication bias was evaluated with funnel plot visual analysis and with the Begg’s and Egger’s tests. P < 0.05 was considered statistically significant. All statistical analyses were performed with STATA (Version 9.0; Stata Corp., College Station, TX).
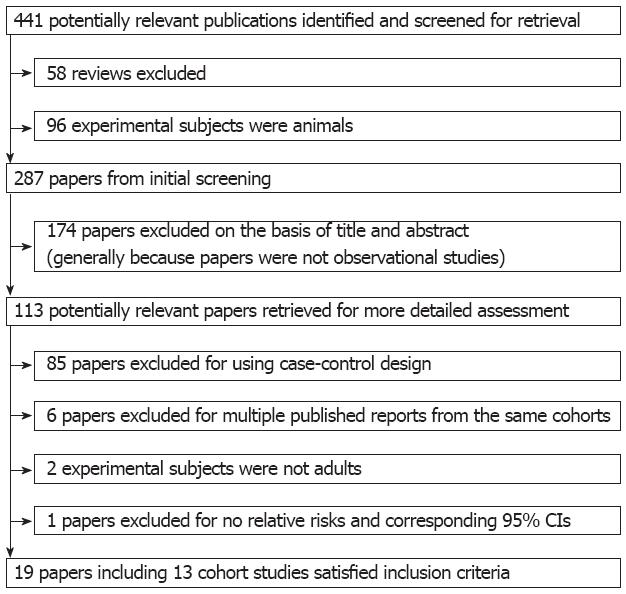
Using the predefined search strategy, we identified 19 publications and 13 prospective cohort studies (Figure 1), including 995 577 participants and 5620 incident cases of digestive cancer with an average follow-up of 11.1 years, which were eligible for inclusion in the meta-analysis[9-27]. The characteristics of the included cohorts are summarized in Table 1. Initial agreement between the two reviewers on whether a study was eligible for inclusion occurred for 108/113 manuscripts (95.6%; κ = 0.912). Of the 13 cohorts included in the meta-analysis, 6 were conducted in Europe, 6 in North America (United States), and 1 in Asia (Singapore).
| Ref. | Country | Follow-upperiod (yr) | Age (yr) | Cohort size | Cases | Exposure details (kcal/d) | Outcome | Contrast between groups (kcal) | Relative risk (95%CI) | Adjustments |
| Giovannucci et al[11] | United States | 6 | 40-75 | 47 949 | 205 | Caloric intake | Colon cancer incidence | 1229 | 1 | Age |
| 1586 | 1.92 (1.28-2.90) | |||||||||
| 1884 | 1.33 (0.85-2.08) | |||||||||
| 2308 | 1.12 (0.70-1.80) | |||||||||
| 2820 | 0.94 (0.57-1.55) | |||||||||
| Goldbohm et al[12] | Netherlands | 3.3 | 55-69 | 120 852 | 215 | Caloric intake | Colon cancer incidence | 1510 (M); 1163 (F) | 1 | |
| 1836 (M); 1435 (F) | 0.88 (0.57-1.69) | |||||||||
| 2096 (M); 1626 (F) | 1.12 (0.75-1.70) | |||||||||
| 2364 (M); 1848 (F) | 0.84 (0.54-1.31) | |||||||||
| 2791 (M); 2200 (F) | 0.74 (0.47-1.18) | |||||||||
| Chyou et al[13] | United States | 24 | 45-68 | 7903 | 695 | Caloric intake | Upper digestive Tract, colorectal Cancer incidence | < 2000 | 1 | Age, alcohol, number of cigarettes day, number of years smoked |
| 2000-2499.9 | 0.91 (0.60-1.22) | |||||||||
| ≥ 2500 | 0.94 (0.64-1.24) | |||||||||
| Gaard et al[15] | Norway | 11.4 | 20-54 | 50 535 | 143 | Energy intake kJ/d | Colon cancer incidence | ≥ 9999: highest quintile | 1.24 (0.56-1.92) | Age, height, BMI, attained age, smoking status |
| ≥ 6654 (F) | ||||||||||
| ≤ 6857: lowest quintile | ||||||||||
| ≤ 4453 (F) | ||||||||||
| Martínez et al[16] | United States | 12 | 30-55 | 89 448 | 501 | Caloric intake | Colon cancer incidence | 5th: highest quintile | 1.18 (0.89-1.57) | Age |
| 1st: lowest quintile | ||||||||||
| Harnack et al[17] | United States | 9 | 55-69 | 33 976 | 355 | Caloric intake | Esophageal gastric, Pancreatic, colon Cancer incidence | ≤ 1450 | 1 | Age, alcohol use, pack-years of smoking, yellow/orange vegetables, grains intake |
| 1451-1900 | 0.69 (0.49-0.88) | |||||||||
| > 1900 | 0.73 (0.53-0.92) | |||||||||
| Kato et al[18] | United States | 7.1 | 34-65 | 14 727 | 100 | Energy intake | Colorectal cancer incidence | Quintile 1 | 1.0 | Age, educational level, place at enrollment |
| Quintile 2 | 1.16 (0.67-2.00) | |||||||||
| Quintile 3 | 0.85 (0.47-1.53) | |||||||||
| Quintile 4 | 1.20 (0.69-2.08) | |||||||||
| Järvinen et al[21] | Finland | 24 | ≥ 15 | 9959 | 109 | Energy intake | Colorectal cancer incidence | 4th: highest quintile 1st: lowest quintile | 0.78 (0.42-1.44) | Age, sex, BMI, smoking, occupational group, geographical area |
| Stolzenberg-Solomon et al[23] | Finland | 13 | 50-69 | 27 111 | 459 | Energy intake | Gastric, pancreatic, colorecal cancer incidence | ≤ 2155 | 1 | Age, BMI, educational level, calcium intake, smoking years, alcohol consumption, physical activity at work |
| > 2155 and ≤ 2541 | 1.18 (0.78-1.58) | |||||||||
| > 2541 and ≤ 2917 | 1.19 (0.76-1.85) | |||||||||
| > 2917 and ≤ 3410 | 0.99 (0.62-1.35) | |||||||||
| > 3410 | 0.75 (0.42-1.09) | |||||||||
| Tiemersm et al[24] | Netherlands | 8.5 | 20-59 | > 36 000 | 102 | Energy intake kJ/d | Colorectal cancer incidence | mean cases: 6895 | ||
| mean controls: 6773 | ||||||||||
| Wong et al[25] | Singapore | 7 | 45-74 | 63 257 | 482 | Energy intake | Colorectal cancer incidence | mean cases: 1511 | ||
| mean controls: 1492 | ||||||||||
| Friedenreich et al[26] | 10 European countries | 6.4 | 35-70 | 413 044 | 1693 | Energy intake | Colorectal cancer incidence | < 1827 | 1 | Age, center, education, smoking, fiber |
| 1827-2351 | 0.91 (0.78-1.05) | |||||||||
| > 2351 | 0.90 (0.78-1.02) | |||||||||
| Prentice et al[27] | United States | 12 | 50-79 | 80 816 | 561 | Energy intake | Pancreatic, colorectal cancer incidence | Quartile 1 | 1 | |
| Quartile 2 | 1.19 (0.81-1.57) | |||||||||
| Quartile 3 | 1.18 (0.82-1.54) | |||||||||
| Quartile 4 | 1.47 (0.99-1.94) |
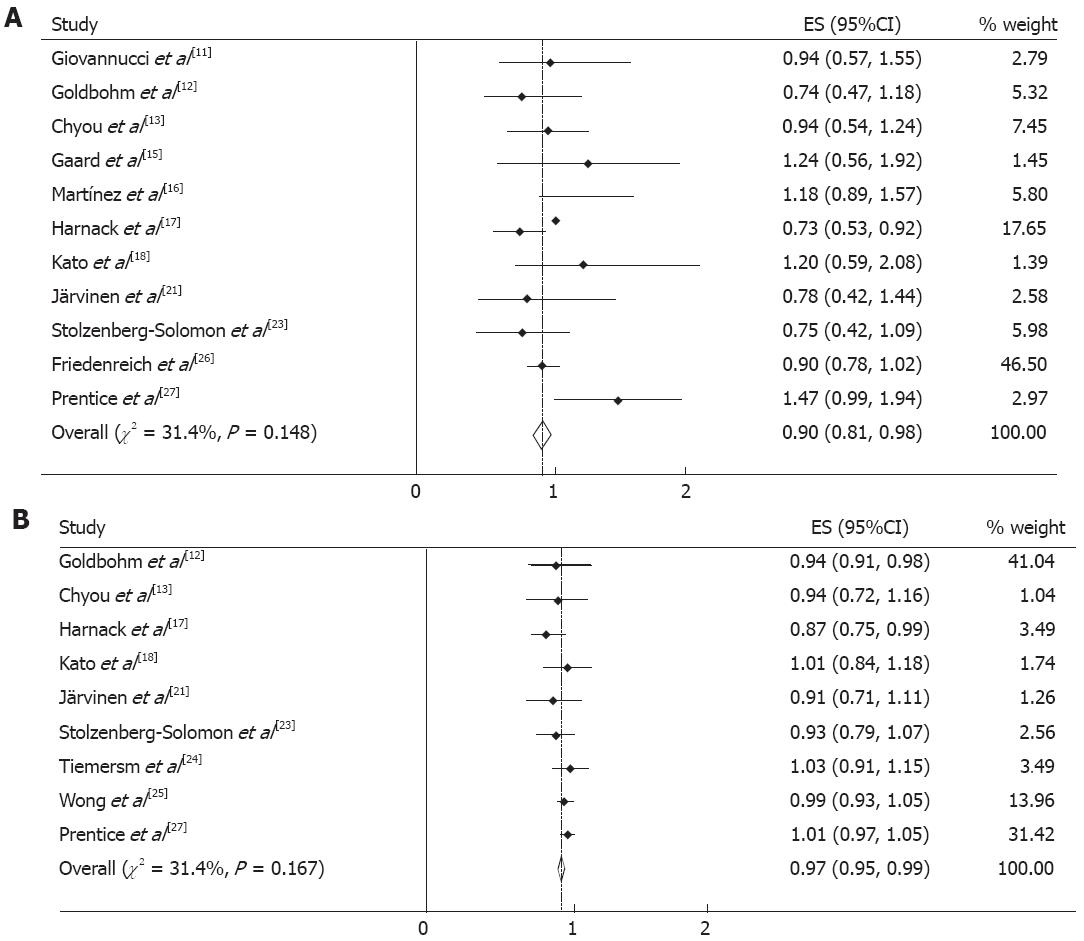
From the 13 cohorts reporting energy intake, 11 cohorts could be used for the qualitative meta-analyses for the highest versus the lowest category of exposure and digestive cancer incidence. Figure 2A shows the estimated RRs for the highest versus lowest category of energy intake from cohort studies. The summary RR of digestive cancers from all combined studies was 0.90 (95%CI 0.81-0.98). There was no significant heterogeneity across the studies (Q = 14.6, P = 0.148, I2 = 31.4%).
From the included cohorts, nine studies could be used in the per unit energy intake meta-analysis. The summary RR of digestive cancers for an increment of 239 kcal/d energy intake was 0.97 (95%CI 0.95-0.99), and no significant heterogeneity between studies was present (Q = 11.7, P = 0.167, I2 = 31.4%) (Figure 2B).
When stratified by the site of digestive cancer, we noted that energy intake was associated with a reduced risk of colorectal cancer (RR 0.90, 95%CI 0.81-0.99) and an increased risk of gastric cancer (RR 1.19, 95%CI 1.08-1.31). There appeared to be no association with esophageal (RR 0.96, 95%CI 0.86-1.07) or pancreatic (RR 0.79, 95%CI 0.49-1.09) cancer. Associations were also similar in studies from North America and Europe. The RR was 1.02 (95%CI 0.79-1.25) when considering the six studies conducted in North America and 0.87 (95%CI 0.77-0.98) for the five studies from Europe.
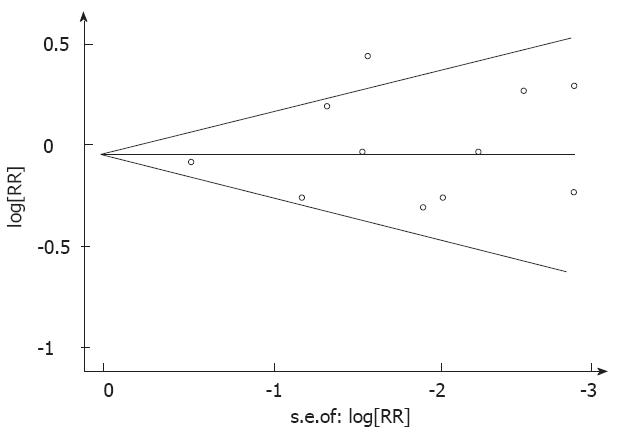
There was no indication of publication bias from either visualization of the funnel plot or Egger’s (P = 0.661) and Begg’s (P = 0.533) (Figure 3) tests. A sensitivity analysis, in which one study was removed at a time, was performed to evaluate the stability of the results. This analysis confirmed the stability of our results.
Over the past 30 years, CR has emerged as the most potent, broadly acting dietary intervention for preventing carcinogenesis in rodent models of cancer. Some observational studies further support the hypothesis that CR has beneficial effects on longevity and cancer risk in humans[28]. However, physical activity and body size are highly related to total energy intake, and it is difficult to assess the independent effect of energy intake on cancer risk. In addition, energy intake is also difficult to assess in large-scale epidemiologic studies. Animal experimental studies have suggested the importance of energy balance as a determinant for cancer risk[28,29]. Although very few studies have assessed the relationship between CR and the risk of various cancer sites in humans because of ethical issues, we quantitatively assessed the relationship between energy intake and the incidence of digestive cancers in a meta-analysis of cohort studies. Our meta-analysis yielded an inconsistent result in former studies and showed that energy intake was inversely associated with the risk of digestive cancers. The summary RR of digestive cancers was 0.97 (95%CI 0.95-0.99) for an increment of 239 kcal calorie intake per day.
Data from countries that experienced varying degrees of energy restriction during World War II may support our results. For example, a cohort of Norwegians showed reduced breast cancer risk when exposed to acute (< 1 year) energy restriction (50% reduction in caloric intake without significant changes in diet quality)[30]. In contrast, survivors of the Dutch famine of 1944, during which energy restriction (70% reduction in rations for adults; 50% reduction in rations for children) was more severe than in the Norwegian study, experienced higher breast cancer rates but no apparent change in risk of any other cancer[6]. Cohorts exposed to even longer and more severe (> 80% reduction in normal energy intake) energy restriction, such as European Jewish survivors exposed to the Holocaust[31] or Russian survivors of the Siege of Leningrad[32], show increased risk of some cancers. The confounding effects of severe physical and psychosocial stress, malnutrition, infection, and other factors associated with war conditions make these studies challenging to interpret. However, based on data from animal and human studies, it seems clear that although CR typically decreases cancer risk, the anticancer effects associated with reduced energy intake can be neutralized or overcome in the presence of extreme stressors, such as what occurred during World War II.
The mechanisms responsible for CR-mediated beneficial effects on cancer are thought to involve metabolic adaptations to CR itself, including (1) decreased production of growth factors and anabolic hormones[33,34]; (2) decreased production of reactive oxygen species and modulation of the endogenous antioxidant systems that decrease oxidative stress and free radical-induced DNA damage[35,36]; (3) decreased plasma concentrations of inflammatory cytokines and an increase in circulating corticosteroids, ghrelin, and adiponectin that results in reduced inflammation[37-40]; and (4) protection against aging-associated deterioration in immunosurveillance[41]. In addition, CR simultaneously affects multiple processes that are involved in cancer pathogenesis, including DNA repair processes, removal of damaged cells through apoptosis, autophagy, and protection from the effects of damaging agents (e.g., toxic and genotoxic compounds)[42,43]. Many of the effects of CR are probably mediated by regulation of gene expression, including upregulation of tumor suppressor genes and genes promoting DNA and cellular repair, protein turnover, stress resistance and antioxidant genes, downregulation of proinflammatory genes, and modulation of energy metabolism pathways[44,45]. Whether CR with adequate nutrition reduces the cancer incidence in humans is unknown, but data from studies of long-term CR suggest that the metabolic and physiological responses to CR in humans are similar to those in rodents and monkeys[46-49].
To further elucidate the relationship between energy intake and risk of digestive cancer at various sites, we performed subgroup analysis and noted that energy intake was associated with a reduced risk of colorectal cancer and an increased risk of gastric cancer. There appeared to be no association with esophageal or pancreatic cancer. Colorectal cancer is one of the most common cancers worldwide. Only one study assessed the association between energy restriction and colorectal cancer risk[50]. This study observed no significant relationship between energy restriction early in life and subsequent colon carcinoma risk in men and women who had lived in a western city in 1944-1945 (hunger winter) in the Netherlands. Interestingly, of studies that have examined the relationship between energy intake and colon cancer, many prospective investigations have similarly found inverse associations with greater energy intake, whereas case-control studies have observed positive associations[51-55].
A combined analysis of 13 case-control studies demonstrated a positive association with total energy intake in 11 of the 13 studies. The association was similar between men and women, between younger (< 50 years old) and older (> 50 years old) people, between colon and rectal cancer, and between right and left colon cancer sites[54]. On the other hand, cohort studies have usually reported a weak or null association[15,18]. In the study by Bostick et al[10], a decreasing risk of colon cancer with increasing total energy intake was seen following age-adjusted analysis. The RR comparing the highest quintile (> 2.238 kcal/d) with the lowest quintile (< 1.301 kcal/d) was 0.60 (95%CI 0.39-0.92). Martínez et al[16] reported a weak positive association between energy intake and colorectal cancer. The age-adjusted RR for the highest compared with the lowest quintile of energy intake was 1.18 (95%CI 0.89-1.57). Among 63 257 Asian participants followed for an average of 7 year in which 310 incident cases of colorectal cancer were identified, no significant difference was found between the median total caloric intake of patients with colorectal cancer (1494.0 kcal/d) and controls (1483.5 kcal/d)[56].
In our meta-analysis, 10 cohorts were identified from Finland, the Netherlands, Norway, and the United States. The summary RR of colorectal cancer was 0.90 (95%CI 0.81-0.99) for the highest versus lowest category of energy intake. We observed an inverse association between energy intake and the risk of colorectal cancer. The inverse association observed in some of these prospective studies may be explained by the greater energy intake associated with energy expenditure from greater physical activity, which is protective against colon cancer[57].
There were over 20 case-control studies concerning the relationship between total energy intake and the risk of gastric cancer. In all studies, total energy consumed during adulthood was assessed. Some studies reported a positive association between total energy intake and the risk of gastric cancer[58-62]. Regarding cohort studies, Ahn et al[63] reported a approximately 60% decreased risk of gastric cancer with increased total energy intake in Korea. Kasum et al[22] studied the association between energy intake and the risk of gastric cancer in postmenopausal women in the United States. Among 34 651 participants followed for an average of 14 years in which 56 incident cases of gastric cancer were identified, the summary RR of stomach cancer was 1.10 for an increment of 250 kcal/d energy intake. Our meta-analysis including two cohort studies suggested a significant positive relationship between energy intake and gastric cancer (RR = 1.19; 95%CI 1.08-1.31).
Over the past three decades, many studies have been conducted to examine the relationship between energy intake and pancreatic cancer. Harnack et al[17] found that in 33 976 postmenopausal women in the United States, the summary RR of pancreatic cancer for individuals with an energy intake of > 1900 kcal/d was 1.20 (95%CI 0.67-2.15) compared with those having an intake of < 1450 kcal/d. In 27, 111 male smokers in Finland, Stolzenberg-Solomon et al[23] studied the association between energy intake and the risk of pancreatic cancer. After following participants for an average of 10.2 years, 163 incident cases of exocrine cancer of the pancreas were identified. The RR comparing the highest quintile (> 3410 kcal/d) with the lowest quintile (< 2155 kcal/d) was 0.62 (95%CI 0.36-1.07). After a pooled analysis of three cohort studies, we found that the summary RR of pancreatic cancer was 0.79 (95%CI 0.49-1.09) for the highest versus lowest category of energy intake. Thus, there appears to be no obvious association between energy intake and pancreatic cancer risk.
Some limitations of this meta-analysis should be acknowledged. First, as in all observational studies of diet and disease, the possibility of bias and confounding factors cannot be excluded. However, cohort studies, which are less susceptible to bias because of the prospective design, also showed an inverse association between energy intake and risk of digestive cancers, suggesting that the finding is not likely attributable to recall and selection bias. Individual studies may have failed to adjust for potential known or unknown confounders. Second, energy intake in our study may be a marker for greater nutrient intake and better nutritional status, because energy is correlated with many nutrients, and their combined effect may also explain the protective association that we observed. Dietary data do not necessarily reflect absorbed or biologically active doses and may contain measurement error from nutritional assessment techniques and nutrient databases, and participants may have changed their diets since baseline. All these parameters may have attenuated risk estimates. Third, we extracted the risk estimates that reflected the greatest degree of the control potential confounders because it was difficult to obtain raw data from each study to conduct standardized adjustments. Therefore, the results based on adjustment for different confounders were likely different from those based on standardized adjustments. Finally, only published studies were included in our meta-analysis. Therefore, publication bias may have occurred, although no publication bias was indicated from both visualization of the funnel plot and Egger’s test.
This meta-analysis presents epidemiologic evidence about the relationship between energy intake and risk of digestive cancers. In summary, we observed an inverse association between energy intake and the risk of digestive cancers. High energy intake may increase the risk of gastric cancer and decrease that of colorectal cancer. However, because physical activity, body size, and metabolic efficiency are highly related to total energy intake and expenditure, it is difficult to assess a possible independent effect of energy intake on digestive cancer risk. More investigations are needed to determine the biological mechanism of the inverse relationship between energy intake and the incidence of digestive cancers.
An important discovery in recent years is that lifestyle and environmental factors affect cancer initiation, promotion and progression. Epidemiological studies strongly suggest that the majority of cancer deaths can be attributed to factors such as unhealthy diets, tobacco, alcoholism, infections, and occupational exposure. Recent reports of extended life span and delayed cancer development in response to calorie restriction (CR) in rhesus monkeys and observations that CR during the premenopausal years decreases postmenopausal breast cancer risk in women suggest that the anticancer effects of CR reported in rodent models extend to primates, including humans.
Very few studies have assessed the relationship between CR and the risk of various cancer sites because of ethical issues. One Dutch famine study and subsequent overall cancer incidence found no evidence that the short famine affected overall cancer risk. However, higher energy intake in childhood may increase the risk of developing cancer in adulthood. Data from case-control studies may be subject to recall bias with respect to energy intake and to selection bias with respect to the control group. Prospective cohort studies excluding those biases would be more useful for observing energy-cancer associations.
This meta-analysis presents epidemiologic evidence about the relationship between energy intake and risk of digestive cancers. They observed an inverse association between energy intake and the risk of digestive cancers. High energy intake may increase the risk of gastric cancer and decrease that of colorectal cancer. However, because physical activity, body size, and metabolic efficiency are highly related to total energy intake and expenditure, it is difficult to assess a possible independent effect of energy intake on digestive cancer risk. More investigations are needed to determine the biological mechanism of the inverse relationship between energy intake and the incidence of digestive cancers.
The study results suggest that an inverse association between energy intake and the risk of digestive cancers. High energy intake may increase the risk of gastric cancer and decrease that of colorectal cancer. They could prevent digestive cancers by controlling energy intake.
CR is an experimental mode in which test animals receive a lower-calorie diet than ad libitum-fed controls. It has emerged as the most potent, broadly acting dietary intervention for preventing carcinogenesis in rodent models of cancer.
This manuscript presents a meta-analysis of selected studies about the effects of energy intake on risk of digestive cancer. It is well designed with the use of only prospective studies. Appropriate statistical methods are used for each reported meta-analysis for the assessment of effects on outcome.
Peer reviewers: Stefan Riss, MD, Division of General Surgery, Department of General Surgery, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; Dr. Francesco Manguso, MD, PhD, UOC di Gastroenterologia, AORN A. Cardarelli, Via A. Cardarelli 9, 80122 Napoli, Italy
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2007;56:254-281; quiz 313-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 411] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 2. | Hursting SD, Lashinger LM, Colbert LH, Rogers CJ, Wheatley KW, Nunez NP, Mahabir S, Barrett JC, Forman MR, Perkins SN. Energy balance and carcinogenesis: underlying pathways and targets for intervention. Curr Cancer Drug Targets. 2007;7:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1855] [Cited by in RCA: 1642] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 4. | Howell A, Chapman M, Harvie M. Energy restriction for breast cancer prevention. Recent Results Cancer Res. 2009;181:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Loft S, Velthuis-te Wierik EJ, van den Berg H, Poulsen HE. Energy restriction and oxidative DNA damage in humans. Cancer Epidemiol Biomarkers Prev. 1995;4:515-519. [PubMed] |
| 6. | Elias SG, Peeters PH, Grobbee DE, van Noord PA. The 1944-1945 Dutch famine and subsequent overall cancer incidence. Cancer Epidemiol Biomarkers Prev. 2005;14:1981-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Uauy R, Solomons N. Diet, nutrition, and the life-course approach to cancer prevention. J Nutr. 2005;135:2934S-2945S. [PubMed] |
| 8. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16776] [Article Influence: 671.0] [Reference Citation Analysis (0)] |
| 9. | Nomura A, Grove JS, Stemmermann GN, Severson RK. A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res. 1990;50:627-631. [PubMed] |
| 10. | Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, McKenzie DR, Gapstur SM, Folsom AR. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control. 1994;5:38-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 305] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390-2397. [PubMed] |
| 12. | Goldbohm RA, van den Brandt PA, van 't Veer P, Brants HA, Dorant E, Sturmans F, Hermus RJ. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res. 1994;54:718-723. [PubMed] |
| 13. | Chyou PH, Nomura AM, Stemmermann GN. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. Int J Cancer. 1995;60:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Chyou PH, Nomura AM, Stemmermann GN. A prospective study of colon and rectal cancer among Hawaii Japanese men. Ann Epidemiol. 1996;6:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Gaard M, Tretli S, Løken EB. Dietary factors and risk of colon cancer: a prospective study of 50,535 young Norwegian men and women. Eur J Cancer Prev. 1996;5:445-454. [PubMed] |
| 16. | Martínez ME, Giovannucci EL, Colditz GA, Stampfer MJ, Hunter DJ, Speizer FE, Wing A, Willett WC. Calcium, vitamin D, and the occurrence of colorectal cancer among women. J Natl Cancer Inst. 1996;88:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 156] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Harnack LJ, Anderson KE, Zheng W, Folsom AR, Sellers TA, Kushi LH. Smoking, alcohol, coffee, and tea intake and incidence of cancer of the exocrine pancreas: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 1997;6:1081-1086. [PubMed] |
| 18. | Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women's Health Study. Nutr Cancer. 1997;28:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, Virtamo J. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control. 1999;10:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland). Cancer Causes Control. 2001;12:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Järvinen R, Knekt P, Hakulinen T, Aromaa A. Prospective study on milk products, calcium and cancers of the colon and rectum. Eur J Clin Nutr. 2001;55:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Kasum CM, Jacobs DR, Nicodemus K, Folsom AR. Dietary risk factors for upper aerodigestive tract cancers. Int J Cancer. 2002;99:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Tiemersma EW, Kampman E, Bueno de Mesquita HB, Bunschoten A, van Schothorst EM, Kok FJ, Kromhout D. Meat consumption, cigarette smoking, and genetic susceptibility in the etiology of colorectal cancer: results from a Dutch prospective study. Cancer Causes Control. 2002;13:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Wong HL, Seow A, Arakawa K, Lee HP, Yu MC, Ingles SA. Vitamin D receptor start codon polymorphism and colorectal cancer risk: effect modification by dietary calcium and fat in Singapore Chinese. Carcinogenesis. 2003;24:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Friedenreich C, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, Clavel-Chapelon F, Linseisen J, Boeing H, Bergman M. Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15:2398-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Prentice RL, Shaw PA, Bingham SA, Beresford SA, Caan B, Neuhouser ML, Patterson RE, Stefanick ML, Satterfield S, Thomson CA. Biomarker-calibrated energy and protein consumption and increased cancer risk among postmenopausal women. Am J Epidemiol. 2009;169:977-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 381] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Thompson HJ, Jiang W, Zhu Z. Mechanisms by which energy restriction inhibits carcinogenesis. Adv Exp Med Biol. 1999;470:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Tretli S, Gaard M. Lifestyle changes during adolescence and risk of breast cancer: an ecologic study of the effect of World War II in Norway. Cancer Causes Control. 1996;7:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Keinan-Boker L, Vin-Raviv N, Liphshitz I, Linn S, Barchana M. Cancer incidence in Israeli Jewish survivors of World War II. J Natl Cancer Inst. 2009;101:1489-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Koupil I, Plavinskaja S, Parfenova N, Shestov DB, Danziger PD, Vågerö D. Cancer mortality in women and men who survived the siege of Leningrad (1941-1944). Int J Cancer. 2009;124:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521-B538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 151] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540-E547. [PubMed] |
| 35. | Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2366] [Cited by in RCA: 2147] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 36. | Youngman LD, Park JY, Ames BN. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci USA. 1992;89:9112-9116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Matsuzaki J, Kuwamura M, Yamaji R, Inui H, Nakano Y. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. J Nutr. 2001;131:2139-2144. [PubMed] |
| 38. | Han ES, Evans TR, Shu JH, Lee S, Nelson JF. Food restriction enhances endogenous and corticotropin-induced plasma elevations of free but not total corticosterone throughout life in rats. J Gerontol A Biol Sci Med Sci. 2001;56:B391-B397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Yang H, Youm YH, Nakata C, Dixit VD. Chronic caloric restriction induces forestomach hypertrophy with enhanced ghrelin levels during aging. Peptides. 2007;28:1931-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98:370-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci USA. 2006;103:19448-19453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Wachsman JT. The beneficial effects of dietary restriction: reduced oxidative damage and enhanced apoptosis. Mutat Res. 1996;350:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining "clean" cells. Autophagy. 2005;1:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 617] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 44. | Park SK, Prolla TA. Gene expression profiling studies of aging in cardiac and skeletal muscles. Cardiovasc Res. 2005;66:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci USA. 2004;101:5524-5529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 351] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 47. | Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 48. | Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232-3235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr). 2010;32:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Dirx MJ, van den Brandt PA, Goldbohm RA, Lumey LH. Energy restriction early in life and colon carcinoma risk: results of The Netherlands Cohort Study after 7.3 years of follow-up. Cancer. 2003;97:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Satia-Abouta J, Galanko JA, Potter JD, Ammerman A, Martin CF, Sandler RS. Associations of total energy and macronutrients with colon cancer risk in African Americans and Whites: results from the North Carolina colon cancer study. Am J Epidemiol. 2003;158:951-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Slattery ML, Caan BJ, Benson J, Murtaugh M. Energy balance and rectal cancer: an evaluation of energy intake, energy expenditure, and body mass index. Nutr Cancer. 2003;46:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Mao Y, Pan S, Wen SW, Johnson KC. Physical inactivity, energy intake, obesity and the risk of rectal cancer in Canada. Int J Cancer. 2003;105:831-837. [PubMed] |
| 54. | Howe GR, Aronson KJ, Benito E, Castelleto R, Cornée J, Duffy S, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R. The relationship between dietary fat intake and risk of colorectal cancer: evidence from the combined analysis of 13 case-control studies. Cancer Causes Control. 1997;8:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Boutron-Ruault MC, Senesse P, Méance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer. 2001;39:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Koh WP, Yuan JM, van den Berg D, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer. 2004;90:1760-1764. [PubMed] |
| 57. | Martínez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst. 1997;89:948-955. [PubMed] |
| 58. | Lissowska J, Gail MH, Pee D, Groves FD, Sobin LH, Nasierowska-Guttmejer A, Sygnowska E, Zatonski W, Blot WJ, Chow WH. Diet and stomach cancer risk in Warsaw, Poland. Nutr Cancer. 2004;48:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055-1062. [PubMed] |
| 60. | Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer. 1997;80:1021-1028. [PubMed] |
| 61. | De Stefani E, Correa P, Boffetta P, Deneo-Pellegrini H, Ronco AL, Mendilaharsu M. Dietary patterns and risk of gastric cancer: a case-control study in Uruguay. Gastric Cancer. 2004;7:211-220. [PubMed] |
| 62. | Kaaks R, Tuyns AJ, Haelterman M, Riboli E. Nutrient intake patterns and gastric cancer risk: a case-control study in Belgium. Int J Cancer. 1998;78:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Ahn YO. Diet and stomach cancer in Korea. Int J Cancer. 1997;Suppl 10:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |