Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7348
Revised: October 21, 2012
Accepted: November 14, 2012
Published online: December 28, 2012
Processing time: 155 Days and 18 Hours
AIM: To investigate the role of c-Jun N-terminal kinase (JNK) in thermotherapy-induced apoptosis in human gastric cancer SGC-7901 cells.
METHODS: Human gastric cancer SGC-7901 cells were cultured in vitro. Following thermotherapy at 43 °C for 0, 0.5, 1, 2 or 3 h, the cells were cultured for a further 24 h with or without the JNK specific inhibitor, SP600125 for 2 h. Apoptosis was evaluated by immunohistochemistry [terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)] and flow cytometry (Annexin vs propidium iodide). Cell proliferation was determined by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The production of p-JNK, Bcl-2, Bax and caspase-3 proteins was evaluated by Western blotting. The expression of JNK at mRNA level was determined by reverse transcription polymerase chain reaction.
RESULTS: The proliferation of gastric carcinoma SGC-7901 cells was significantly inhibited following thermotherapy, and was 32.7%, 30.6%, 43.8% and 52.9% at 0.5, 1, 2 and 3 h post-thermotherapy, respectively. Flow cytometry analysis revealed an increased population of SGC-790l cells in G0/G1 phase, but a reduced population in S phase following thermotherapy for 1 or 2 h, compared to untreated cells (P < 0.05). The increased number of SGC-790l cells in G0/G1 phase was consistent with induced apoptosis (flow cytometry) following thermotherapy for 0.5, 1, 2 or 3 h, compared to the untreated group (46.5% ± 0.23%, 39.9% ± 0.53%, 56.6% ± 0.35% and 50.4% ± 0.29% vs 7.3% ± 0.10%, P < 0.01), respectively. This was supported by the TUNEL assay (48.2% ± 0.4%, 40.1% ± 0.2%, 61.2% ± 0.29% and 52.0% ± 0.42% vs 12.2% ± 0.22%, P < 0.01) respectively. More importantly, the expression of p-JNK protein and JNK mRNA levels were significantly higher at 0.5 h than at 0 h post-treatment (P < 0.01), and peaked at 2 h. A similar pattern was detected for Bax and caspase-3 proteins. Bcl-2 increased at 0.5 h, peaked at 1 h, and then decreased. Furthermore, the JNK specific inhibitor, SP600125, suppressed p-JNK, Bax and caspase-3 at the protein level in SGC790l cells following thermotherapy, compared to mock-inhibitor treatment, which was in line with the decreased rate of apoptosis. The expression of Bcl-2 was consistent with thermotherapy alone.
CONCLUSION: Thermotherapy induced apoptosis in gastric cancer cells by promoting p-JNK at the mRNA and protein levels, and up-regulated the expression of Bax and caspase-3 proteins. Bcl-2 may play a protective role during thermotherapy. Activation of JNK via the Bax-caspase-3 pathway may be important in thermotherapy-induced apoptosis in gastric cancer cells.
- Citation: Xiao F, Liu B, Zhu QX. c-Jun N-terminal kinase is required for thermotherapy-induced apoptosis in human gastric cancer cells. World J Gastroenterol 2012; 18(48): 7348-7356
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7348
Gastric cancer is the second leading cause of cancer death worldwide and may remain one of the leading causes of all deaths in the near future[1-3]. At present, the majority (about 80%) of patients diagnosed with gastric cancer are in an advanced stage with limited surgical options[4]. Chemotherapy is an alternative treatment for advanced gastric cancer, however, patient outcome following chemotherapy is still very poor, with a median overall survival time of less than 1 year[5]. Chemotherapy with cytotoxic drugs usually leads to severe toxicity which lowers the quality of life of patients. Thermotherapy is a new cancer treatment which is used following surgical, radiotherapy, chemotherapy and biological treatments. Thermotherapy induces malignant cell apoptosis and has a synergistic effect with chemotherapy resulting in improved outcomes and reduced side effects of chemotherapy, particularly in the later stages of malignancy or in tumors resistant to other treatments[6,7]. A few studies have reported that induction of cellular apoptosis is important in thermotherapy, but the underlying mechanism remains to be explored.
c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinase family. Activation of JNK by phosphorylation has been implicated in a variety of processes, including embryonic development, cellular transformation and initiation of apoptosis following various stresses[8-11]. It has been reported that overexpression of JNK may cause cellular apoptosis in transfected cells[12-14]. However, the function of JNK is complicated during cell stress, because JNK also has anti-apoptotic action[15]. Thus, it is believed that JNK has either anti- or pro-apoptotic activity depending on cell type, apoptotic stimuli and other signalling pathways[16]. JNK can be activated by thermotherapy, and ultimately leads to cellular apoptosis[17]. It is unclear whether JNK involves or regulates apoptosis of gastric cancer cells in response to thermotherapy. In the current study, the human gastric cancer cell line, SGC-7901, was chosen as a model for thermotherapy-induced apoptosis in vitro. The role of JNK was determined to further investigate the underlying mechanism of thermotherapy-induced apoptosis in human gastric cancer. This may provide useful information for both clinical therapy and basic scientific research in gastric cancer.
Annexin V-fluoresceine isothiocyanate (V-FITC), propidium iodine (PI), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St. Louis, MO, United States). Mouse monoclonal antibodies against human p-SAPK/JNK (Thr183/Tyr185), Caspase-3, Bcl-2, Bax and the JNK-specific inhibitor, SP600125, were also purchased from Sigma (St. Louis, MO, United States). The M-MLV reverse transcription polymerase chain reaction (RT-PCR) kit was purchased from Promega (Beijing, China). Taq DNA polymerase was purchased from Takara (Dalian, China). Fetal bovine serum was purchased from Sijichun Bioengineering Materials, Inc. (Hangzhou, Zhejiang, China). Dulbecco's Modified Eagle Media (DMEM) culture media and 0.25% trypsin were purchased from Invitrogen-GIBCO (Carlsbad, CA, United States). dNTP and TRIzol were purchased from Invitrogen (Invitrogen, United States). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining kits was purchased from KGI (Nanjing, China). The gastric cancer cell line, SGC-7901[18], was obtained from the Jiangxi Province Digestive Institute (Jiangxi, Nanchang, China).
Gastric cancer SGC-7901 cells were cultured in DMEM media (high glucose) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained at 37 °C and 5% CO2 in an incubator, and the culture medium was changed every 2-3 d. After thermotherapy (43 °C) treatment for 0, 0.5, 1, 2 or 3 h, SGC-7901 cells were cultured for a further 24 h. In addition, the JNK-specific inhibitor, SP600125, was added to SGC-7901 cells 2 h before thermotherapy treatment to determine whether the JNK pathway was involved in thermotherapy-induced apoptosis.
The cells (2 × 105 cell/mL) were seeded onto 96-well plates with 200 μL in six wells for each thermotherapy treatment for 0, 0.5, 1, 2 and 3 h. Twenty μL of MTT solution (5 mg/mL) was added to each well and incubated at 37 °C for 4 h, and the reaction was terminated with a detergent solution to lyse the cells and solubilize the colored formazan crystals. The supernatant was centrifuged at 3000 g for 10 min to obtain a formazan pellet. The supernatant was removed, and the pellet was dissolved completely with 150 μL dimethyl sulfoxide and observed at a wavelength of 570 nm using an enzyme-linked immunosorbent assay plate reader.
The relative inhibition rate was calculated as a percentage, as follows: (1-Aexperiment/Acontrol) × 100%. Three independent experiments were performed.
The effect of thermotherapy on the cell cycle and apoptosis in SGC-7901 cells was analyzed by flow cytometry. Cells floating in medium combined with the adherent layer were collected by trypsinization and fixation with 2 mL citrate buffer for 1 h. For detection of the cell cycle, the cells were stained with propidium iodide (1500 μL) after RNase A (1500 μL) treatment. The labeled cells were immediately analyzed by flow cytometry to evaluate the cell cycle and apoptosis. TUNEL assay, in which the residue of digoxigenin-labeled dUTP was catalytically incorporated into the DNA by terminal deoxynucleotidyl transferase II, was performed according to the manufacturer’s instructions. The positive particles of diaminobenzidine staining were viewed under an optical microscope. The number of apoptotic cells was counted under a microscope (400×) and expressed as the apoptotic index (the number of apoptotic bodies/1000 cells).
Protein concentrations from isolated SGC-7901 cells were determined. The proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis gels (polyacrylamide concentration 100 g/L) and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked with 5% skimmed milk at 37 °C for 1 h and probed with the primary antibody: mouse anti-human JNK (1:2000), caspase-3, Bcl-2, Bax (1:1000), or β-actin (1:1000) monoclonal antibody overnight at 4 °C. The labeled antibody was visualized by horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (1:5000) and enhanced chemiluminescence. The blots were washed with 1 × Tris-buffered saline and Tween buffer for 10 min, 3 times between each step. The density of the targeted bands was quantified using the Quantity One 4.6.2 Imaging Analysis System.
Total RNA was extracted from the SGC-7901 cells with TRIzol following the manufacturer’s instructions. cDNA was synthesized from 2 μg total RNA using the M-MLV RT-PCR kit in a 20 μL volume, according to the manufacturer’s instructions. Two μL of cDNA, 2 μL each primer (50 pmol/L), 1 μL dNTP mix (10 mmol/L) and 1 μL Taq DNA polymerase were used for the PCR analysis. The PCR amplification cycles consisted of denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 60 s, annealing for 60 s, extension at 72 °C for 60 s, and final elongation at 72 °C for 10 min. The PCR products were separated on a 1.5% agarose gel, stained with 0.5 mg/mL ethidium bromide, and visualized by ultraviolet light. Gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase and shown as the ratio of absorbance values. The primer sequences and annealing temperature are listed in Table 1.
| Gene | Primer sequence | Size (bp) | Annealing temperature |
| β-actin | F: TCAGGTCATCACTATCGGCAAT | 432 | 57 °C |
| R: AAAGAAAGGGTGTAAAAGGCA | |||
| JNK | F: CACAGTCCTAAAACGATACC | 354 | 57 °C |
| R: CCACACAGCATTTGATAGAG |
Statistical analysis was performed using statistical software (SPSS version 13.0). Data are expressed as the mean ± SD. The means of 2 groups were compared using the t test. The means of more than 2 groups were compared using one-way analysis of variance, and P < 0.05 was regarded as statistically significant.
To evaluate the effects of thermotherapy on cell viability, cultured gastric cancer SGC-7901 cells underwent thermotherapy for different time periods. Viability inhibitory rates of gastric carcinoma SGC-7901 cells were about 30%, 30%, 43% and 53% after 0.5, 1, 2 and 3 h of thermotherapy, respectively. These data suggest that proliferation of gastric carcinoma SGC-7901 cells was significantly inhibited in a time-dependent manner.
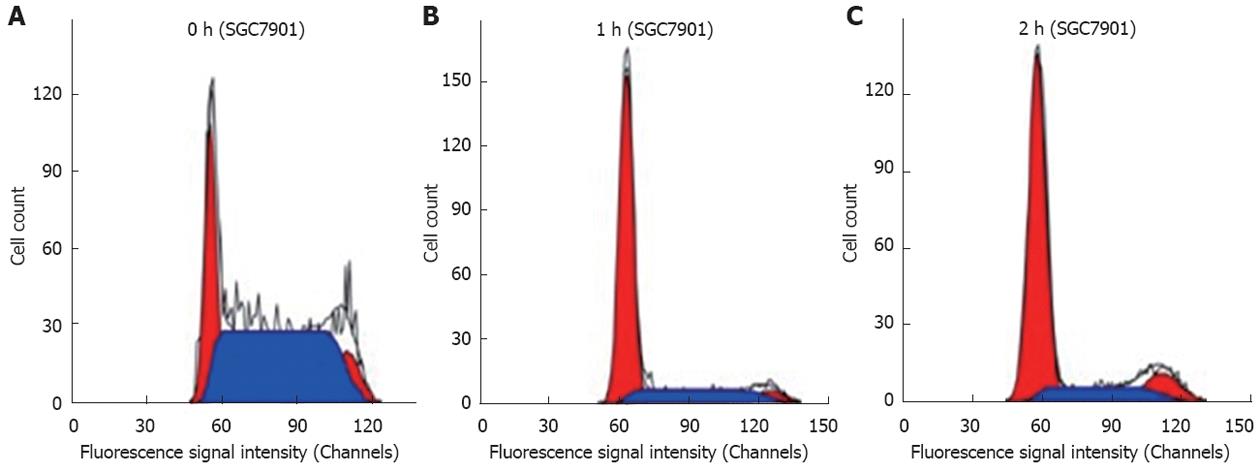
The effects of thermotherapy on the cell cycle of SGC-790l cells were determined using flow cytometry. Our results showed that thermotherapy increased the number of SGC-790l cells in G0/G1 phase, but reduced the number in S phase (P < 0.05) at 1 h and 2 h, compared with that at 0 h. These results suggest that thermotherapy arrested gastric cancer cells in G0/G1 phase, inhibiting mitosis, and subsequent proliferation (Figure 1 and Table 2).
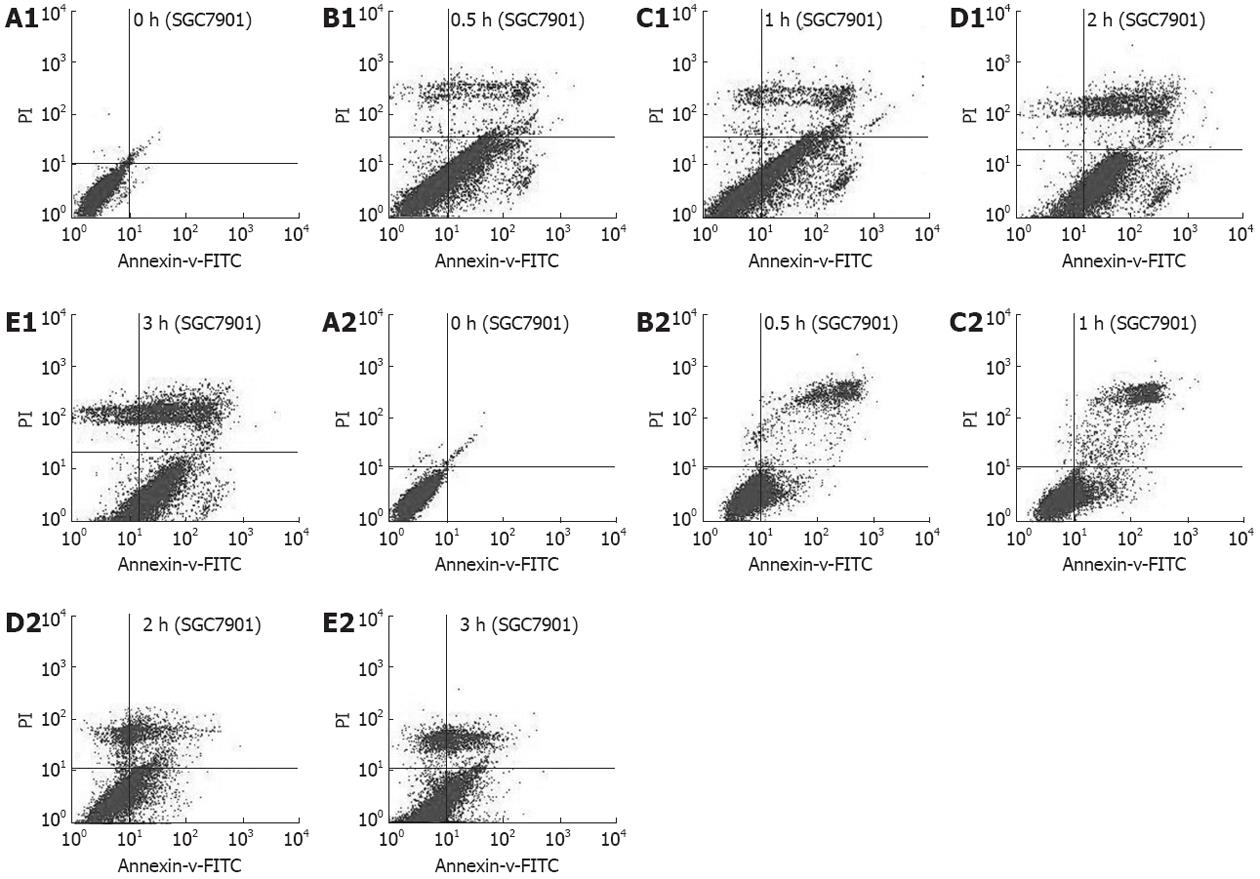
As arrest of cell cycle progression in tumor cells is usually associated with concomitant activation of cell apoptosis pathways, we investigated the effect of thermotherapy on induction of apoptosis in SGC-7901 cells by flow cytometry and TUNEL assay. Flow cytometry showed that the proportion of apoptotic cells was significantly increased after thermotherapy for 0.5, 1, 2 and 3 h, compared to untreated SGC-7901 cells, which was further confirmed by TUNEL assay (Table 3). To further address the role of JNK in thermotherapy-induced apoptosis, pretreatment of cells with the JNK-specific inhibitor, SP600125, significantly inhibited cellular apoptosis induced by thermotherapy at 0.5, 1, 2 and 3 h (P < 0.01), compared to that at the same time points in the control groups. The apoptotic rates at 0 h (P > 0.05) in the experimental group and the control group were similar (Figures 2 and 3, Table 3). These results suggested that the JNK-specific inhibitor, SP600125, significantly inhibited thermotherapy-induced apoptosis in gastric cancer cells.
| Apoptotic rate(%) | ||
| Group (h) | Control group | SP600125 group |
| Flow cytometry | ||
| 0 | 7.3 ± 0.10 | 3.2 ± 0.08 |
| 0.5 | 46.5 ± 0.23b | 21.8 ± 0.15bd |
| 1 | 39.9 ± 0.53b | 17.9 ± 0.26bd |
| 2 | 56.6 ± 0.35b | 22.4 ± 0.36bd |
| 3 | 50.4 ± 0.29b | 24.5 ± 0.72bd |
| TUNEL assay | ||
| 0 | 12.2 ± 0.22 | 11.3 ± 0.13 |
| 0.5 | 48.2 ± 0.40b | 25.8 ± 0.19ad |
| 1 | 40.1 ± 0.20b | 19.2 ± 0.09ad |
| 2 | 61.2 ± 0.29b | 26.3 ± 0.23ad |
| 3 | 52.0 ± 0.42b | 23.4 ± 0.36ad |
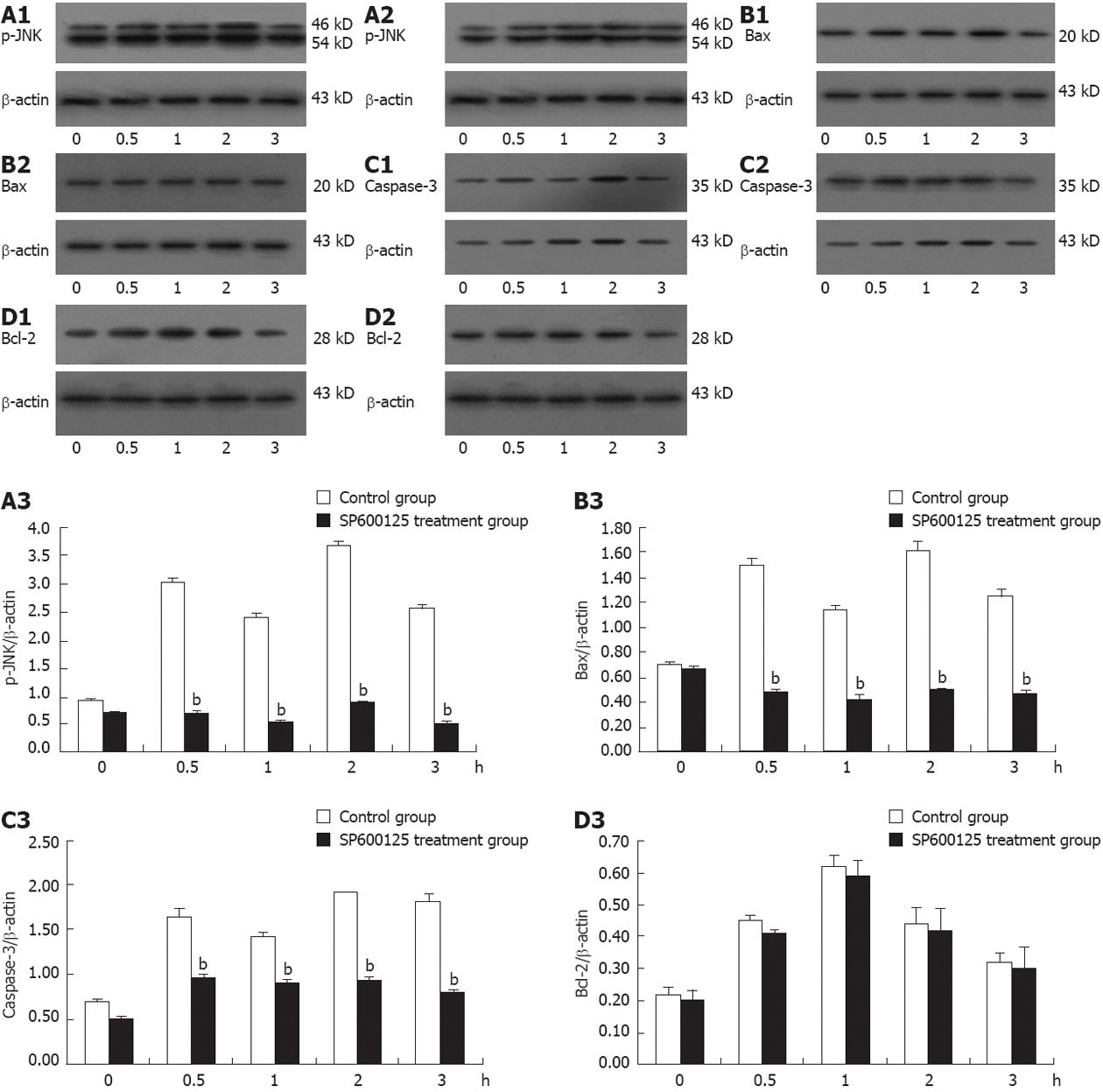
To determine whether thermotherapy activates p-JNK expression in gastric cancer cells, the expression level of p-JNK protein was determined in SGC-7901 cells following thermotherapy for 0.5, 1, 2 and 3 h using Western blotting analysis. The protein production of p-JNK was significantly higher at 0.5 h than that at 0 h post-treatment (Figure 4) (P < 0.01), peaked at 2 h, and then decreased. Interestingly, p-JNK protein was slightly lower at 1 h than that at 0.5 h post-treatment. Thus, these results clearly indicate that JNK in gastric cancer cells was activated by thermotherapy.
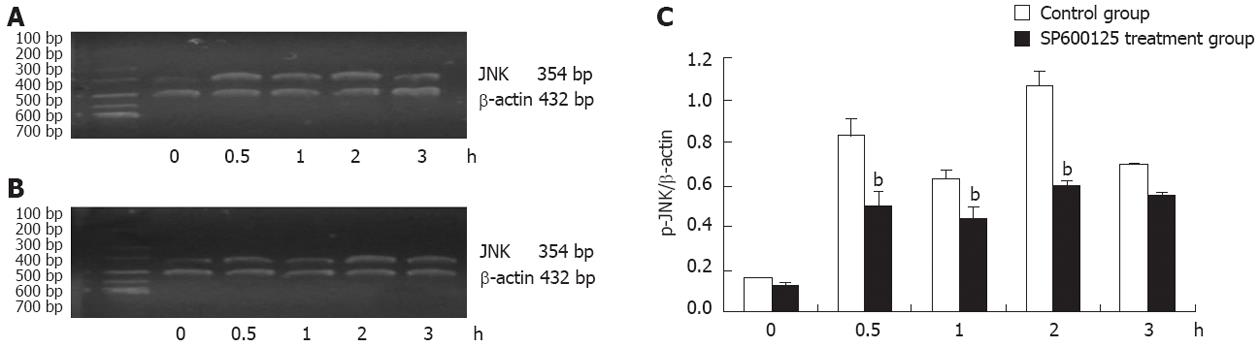
Since activation of JNK is known to induce JNK mRNA expression in several cell systems, we examined whether the expression of JNK mRNA was also induced by thermotherapy in gastric cancer cells. The expression of JNK mRNA in SGC-7901 cells was determined by RT-PCR analysis, which showed that the induction patterns of the expression of p-JNK protein and JNK mRNA were very similar in thermotherapy-induced apoptosis. Namely, the expression of JNK mRNA was significantly higher at 0.5 h than that at 0 h post-treatment (P < 0.01), reached a maximum at 2 h, followed by a decrease, and was slightly lower at 1 h than at 0.5 h post-treatment. Taken together, the datas in Figure 5 demonstrate that JNK plays a role in the effect of thermotherapy on gastric cancer cells.
Apoptosis-related proteins, such as Bax, Bcl-2 and caspase-3 are known to cause cell apoptosis. Thus, the activation of JNK may affect the expression of Bax, Bcl-2 and caspase-3 proteins in thermotherapy-induced apoptosis. To determine whether the expression of Bax, Bcl-2 and caspase-3 proteins was regulated by activation of JNK protein, we measured the expression of Bax, Bcl-2 and caspase-3 proteins in SGC-7901 cells treated with thermotherapy at various time points using Western blot analysis. As shown in Figure 4, the expression of Bax and caspase-3 proteins was significantly higher at 0.5 h than at 0 h post-treatment (P < 0.01), reached a maximum at 2 h, followed by a decrease, and was slightly lower at 1 h than at 0.5 h post-treatment. Bcl-2 was increased at 0.5 h, peaked at 1 h, and decreased thereafter.
To further understand how the activation of JNK regulates the expression of Bax, Bcl-2 and Caspase-3 proteins in thermotherapy-induced apoptosis in SGC-7901 cells we used the JNK-specific inhibitor, SP600125, which exhibits significant selectivity for JNKs leading to inhibition of phosphorylation of both c-Jun and JNKs[19]. Therefore, we treated SGC-7901 cells with thermotherapy in the absence or presence of SP600125 and analyzed the expression of p-JNK, Bax, Bcl-2 and caspase-3 proteins (Figure 4). We found that the presence of SP600125 significantly reduced the p-JNK level at various time points (P < 0.01), while the p-JNK level did not change markedly at 0 h (P > 0.05), compared to cells in the absence of SP600125. Concurrently, the expression of Bax and caspase-3 protein was also inhibited in the presence of SP600125, as well as that of p-JNK. However, there was no significant difference in the expression of Bcl-2 in SGC-7901 cells at any of the time points (P > 0.05) between the SP600125 treatment and mock treatment (Figure 4). These results collectively indicate that thermotherapy induced-apoptosis may be mediated by activation of JNK and the up-regulated expression of Bax and caspase-3 proteins, causing cellular apoptosis in gastric carcinoma cells. Bcl-2 protein did not seem to be involved and played a protective role in this process. These datas suggest that JNK plays an important role in thermotherapy-induced apoptosis in gastric carcinoma cells.
Thermotherapy is a new cancer therapy which has reemerged in the last 20 years, and plays an important role in comprehensive tumor treatment. Combined thermo- and chemo-therapy (green therapy) has become one of the important auxiliary therapies, as it improves the efficacy of cancer treatment [20,21]. Heatinduced cell apoptosis was reported in the 1990s. More recently, JNK was activated in apoptotic U937 cells by radiotherapy[22], and similar results were obtained using thermotherapy in human colon cancer[16]. Thus, the expression of phospho-JNK at the protein level and JNK at the mRNA level were determined in SGC-7901 cells treated with thermotherapy. Thermotherapy increased the expression of p-JNK at different time points, and the protein production of p-JNK was significantly higher at 0.5 h than that at 0 h post-treatment (P < 0.01), peaked at 2 h, and then decreased. The reason for this decline may be that some SGC-7901 cells were killed directly, not by induced apoptosis at 3 h, and a few SGC-7901 cells restarted proliferation after a long period of thermotherapy. It is interesting that p-JNK protein was slightly lower at 1 h than that at 0.5 h post-treatment, this indicates that SGC-7901 cells may have the ability to respond to thermotherapy. Similarly, the RT-PCR data showed that the induction patterns of the expression of p-JNK protein and JNK mRNA level were very similar in thermotherapy-induced apoptosis (Figures 4 and 5). Namely, the expression of JNK mRNA was significantly higher at 0.5 h than that at 0 h post-treatment (P < 0.01), reached a maximum at 2 h, followed by a decrease, and was slightly lower at 1 h than at 0.5 h post-treatment. Taken together, these results demonstrate that JNK plays a role in the effects of thermotherapy in gastric cancer cells. However, the mechanism of JNK activation in thermotherapy-induced apoptosis is still largely unknown in gastric cancer cells.
JNK is thought to induce mitochondria-dependent apoptosis mainly through direct or indirect activation of Bax and down-regulation of Bcl-2[23-27], a pro-apoptotic Bcl-2 family member, which plays an essential role in inducing apoptosis[28]. It is well known that most cell apoptosisinducing factors eventually cause cell apoptosis through the caspase-mediated signal transduction pathway[29,30], and caspase3 is a main effector in cell apoptosis. Therefore, to investigate the roles of Bax, Bcl-2 and caspase3 proteins during thermotherapy-induced apoptosis in human gastric cancer cells, we determined the expression of Bax, Bcl-2 and caspase3 proteins in thermotherapy-stimulated SGC-7901 cells. The data showed that thermotherapy increased the expression of Bax protein at different time points, which peaked at 2 h, and then decreased. These findings were similar to those obtained for the expression of p-JNK and caspase-3 proteins after thermotherapy in SGC-7901 cells. Bcl-2 was increased at 0.5 h and peaked at 1 h. These results suggest that thermotherapy-induced apoptosis was associated with activation of JNK and increased expression of Bax and caspase-3 proteins. The JNK-specific inhibitor, SP600125, substantially inhibited thermotherapy-induced activation of JNK expression and the expression of Bax and caspase-3 proteins. No significant change in the expression of Bcl-2 was observed. Apoptosis was significantly decreased compared to that in thermotherapy-treated cells alone at different time points (Figure 4). These findings proved that thermotherapy-induced apoptosis is associated with JNK activation through up-regulation of the expression of Bax and caspase-3 proteins. These results suggest that the JNK cascade is required for apoptosis induction following thermotherapy in human gastric cancer SGC-7901 cells, which will be investigated in the future.
In summary, our study demonstrates that thermotherapy promoted phospho-JNK production, induced cell apoptosis and inhibited SGC-7901 cell viability. JNK was phosphorylated and activated, and the expression of Bax and caspase-3 was upregulated which subsequently caused cellular apoptosis in gastric cancer cells during thermotherapy. Bcl-2 protein, an anti-apoptotic protein, was not involved and played a protective role in this process. Therefore, JNK plays a critical role in thermotherapy-induced apoptosis in human gastric cancer.
Gastric carcinoma is one of the most common human cancers worldwide and is likely to remain a leading cause of death in the near future. New approaches in the treatment of gastric cancer are required. Recent studies have shown that c-Jun N-terminal kinase (JNK) is involved in thermotherapy-induced apoptosis in colon cancer cells, and is involved in vitamin E succinate-induced apoptosis in gastric carcinoma cells. However, it is unknown whether JNK is involved in thermotherapy-induced gastric carcinoma cell apoptosis, and which pathway is affected. These problems need to be explored in further studies. Thermotherapy is a novel approach in the treatment of gastric cancer.
JNK, a member of the mitogen-activated protein kinase (MAPK) family, is activated through phosphorylation of JNK and has been implicated in a variety of processes in response to various stresses. It is believed that JNK has anti- or pro-apoptotic activity depending on cell type, apoptotic stimuli and other signalling pathways. However, the role of p-JNK during thermotherapy-induced apoptosis has not yet been elucidated. In this study, the authors demonstrate that activation of JNK via the Bax-caspase-3 pathway may represent an important mechanism in thermotherapy-induced apoptosis in gastric cancer cells.
Recent reports have highlighted that JNK is involved in thermotherapy-induced apoptosis in colon cancer cells. This is the first study to report that JNK is also involved in thermotherapy-induced apoptosis in gastric carcinoma cells. Furthermore, the studies show that activation of JNK via the Bax-caspase-3 pathway may represent an important mechanism in thermotherapy-induced apoptosis in gastric cancer cells.
The results of this study indicate the role of JNK in thermotherapy-induced apoptosis in gastric carcinoma cells, and are the basis for further investigations on the mechanism of thermotherapy-induced apoptosis which may be used in clinical applications.
JNK is a member of the MAPK family. Many studies have shown that JNK activation has anti- or pro-apoptotic activity depending on cell type, apoptotic stimuli and other signalling pathways. Unsurprisingly, activation of JNK via the Bax-caspase-3 pathway may cause cell apoptosis following thermotherapy in gastric cancer cells.
The paper describes the effects of thermotherapy on apoptosis and cell cycle progression in human gastric cancer. Further, the role of JNK was determined to investigate the underlying mechanism of thermotherapy-induced apoptosis in human gastric cancer. It would be interesting to have some pieces of information in the field of gastric cancer therapy.
Peer reviewer: Zhou LY, Reprint Author, Peking Univ, Hosp 3, Dept Gastroenterol, 49 Huayuanbei Rd, Beijing 100191, China
S- Editor Jiang L L- Editor A E- Editor Li JY
| 1. | Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, Wang Y. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 2. | Oh SC. Update of adjuvant chemotherapy for resected gastric cancer. J Gastric Cancer. 2012;12:3-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 4. | Chen XZ, Jiang K, Hu JK, Zhang B, Gou HF, Yang K, Chen ZX, Chen JP. Cost-effectiveness analysis of chemotherapy for advanced gastric cancer in China. World J Gastroenterol. 2008;14:2715-2722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8:437-447. [PubMed] |
| 6. | Wang L, Yang JY, Wu YM. A study on the effect of thermotherapy with chemotherapy on lung tumor cell growth. Huanjing Yu Zhiye Yixve. 2007;24:201-203. |
| 7. | Wang JH, Yao MZ, Zhang ZL, Zhang YH, Wang YG, Liu XY. HSF1 blockade-induced tumor thermotolerance abolishment is mediated by JNK-dependent caspase-3 activation. Biochem Biophys Res Commun. 2004;321:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Schroeter H, Spencer JP, Rice-Evans C, Williams RJ. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J. 2001;358:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 9. | Fan M, Goodwin ME, Birrer MJ, Chambers TC. The c-Jun NH(2)-terminal protein kinase/AP-1 pathway is required for efficient apoptosis induced by vinblastine. Cancer Res. 2001;61:4450-4458. [PubMed] |
| 10. | Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929-4942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 400] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Qi X, Pramanik R, Wang J, Schultz RM, Maitra RK, Han J, DeLuca HF, Chen G. The p38 and JNK pathways cooperate to trans-activate vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to vitamin D(3)-induced growth inhibition. J Biol Chem. 2002;277:25884-25892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 462] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Xing YX, Li P, Miao YX, Du W, Wang CB. Involvement of ROS/ASMase/JNK signalling pathway in inhibiting UVA-induced apoptosis of HaCaT cells by polypeptide from Chlamys farreri. Free Radic Res. 2008;42:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44:918-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Lu YY, Chen TS, Wang XP, Qu JL, Chen M. The JNK inhibitor SP600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett. 2010;584:4019-4026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Deng H, Ravikumar TS, Yang WL. Bone morphogenetic protein-4 inhibits heat-induced apoptosis by modulating MAPK pathways in human colon cancer HCT116 cells. Cancer Lett. 2007;256:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Ma PF, Chen JQ, Wang Z, Liu JL, Li BP. Function of chloride intracellular channel 1 in gastric cancer cells. World J Gastroenterol. 2012;18:3070-3080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681-13686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2168] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 20. | Patel S, Sanborn RE, Thomas CR. Definitive chemoradiotherapy for non-small cell lung cancer with N2 disease. Thorac Surg Clin. 2008;18:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Hiraoka M, Masunaga S, Nishimura Y, Nagata Y, Jo S, Akuta K, Li YP, Takahashi M, Abe M. Regional hyperthermia combined with radiotherapy in the treatment of lung cancers. Int J Radiat Oncol Biol Phys. 1992;22:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Zhang D, Song L, Li J, Wu K, Huang C. Coordination of JNK1 and JNK2 is critical for GADD45alpha induction and its mediated cell apoptosis in arsenite responses. J Biol Chem. 2006;281:34113-34123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256-21265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 830] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 25. | Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 433] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Xing D, Liu L, Gao X, Chen M. TNFalpha induces apoptosis through JNK/Bax-dependent pathway in differentiated, but not naïve PC12 cells. Cell Cycle. 2007;6:1479-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Keramaris E, Ruzhynsky VA, Callaghan SM, Wong E, Davis RJ, Flavell R, Slack RS, Park DS. Required roles of Bax and JNKs in central and peripheral nervous system death of retinoblastoma-deficient mice. J Biol Chem. 2008;283:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 3076] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 29. | Hu YR, Liu SQ, Tian YX, Yu LH, Cui XY, Zhao BC. Effects of a disintegrin, adinbitor, from gloydius blomhoffi brevicaudus on Akt signal pathway and proliferation, migration and apoptosis of SSMC7721 cells. Zhongguo Shengwuhuaxve Yu Fenzishengwu Xvebao. 2009;25:662-668. |
| 30. | Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |