Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7327
Revised: November 7, 2012
Accepted: November 14, 2012
Published online: December 28, 2012
Processing time: 137 Days and 16.1 Hours
AIM: To evaluate the relationship between donor safety and remnant liver volume in right lobe living donor liver transplantation (LDLT).
METHODS: From July 2001 to January 2009, our liver transplant centers carried out 197 LDLTs. The clinical data from 151 cases of adult right lobe living donors (not including the middle hepatic vein) were analyzed. The conditions of the three groups of donors were well matched in terms of the studied parameters. The donors’ preoperative data, intraoperative and postoperative data were calculated for the three groups: Group 1 remnant liver volume (RLV) < 35%, group 2 RLV 36%-40%, and group 3 RLV > 40%. Comparisons included the different remnant liver volumes on postoperative liver function recovery and the impact of systemic conditions. Correlations between remnant liver volume and post-operative complications were also analyzed.
RESULTS: The donors’ anthroposomatology data, operation time, and preoperative donor blood test indicators were calculated for the three groups. No significant differences were observed between the donors’ gender, age, height, weight, and operation time. According to the Chengdu standard liver volume formula, the total liver volume of group 1 was 1072.88 ± 131.06 mL, group 2 was 1043.84 ± 97.11 mL, and group 3 was 1065.33 ± 136.02 mL. The three groups showed no statistically significant differences. When the volume of the remnant liver was less than 35% of the total liver volume, the volume of the remnant had a significant effect on the recovery of liver function and intensive care unit time. In addition, the occurrence of complications was closely related to the remnant liver volume. When the volume of the remnant liver was more than 35% of the total liver volume, the remnant volume change had no significant effect on donor recovery.
CONCLUSION: To ensure donor safety, the remnant liver volume should be greater than the standard liver volume (35%) in right lobe living donor liver transplantation.
- Citation: Shi ZR, Yan LN, Du CY. Donor safety and remnant liver volume in living donor liver transplantation. World J Gastroenterol 2012; 18(48): 7327-7332
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7327
To solve the shortage of liver grafts in adult liver transplantation, an increasing number of transplant centers have used right graft living donor liver transplantation. This surgical method can provide a greater proportion of liver grafts to meet the metabolic demands of recipients. However, right lobe graft donors take more risks and have more complications than left graft donors. This has created considerable controversy with respect to donor safety. At the present time, there have been 17 donor deaths reported, and the morbidity was reported to be in the range of 20% to 30%[1-3].
It has been reported in the literature that donor complications are closely related to remnant liver volume (RLV)[4,5]. Initial experiences from previous studies have suggested leaving a remnant of not less than 30%[6]. Other articles have reported that remnant liver volumes less than 35% do not appear to be a contraindication for right liver procurement in living donors[7].
Considering the controversy regarding safety and remnant liver volume in right-lobe living donor liver transplantation (LDLT), we analyzed our own data. We retrospectively examined the remnant liver volume in our right graft donors and compared those donors with different remnant liver volumes. Thus, the aim of the present study was to assess the relationship between donor recovery, complications and the volume of remnant liver[7,8].
From July 2001 to January 2009, our transplant centers carried out 197 LDLTs. Inclusion criteria were: (1) a healthy adult donor, age > 18 and < 60 years; (2) a right liver graft without the middle hepatic vein (MHV); (3) adult-to-adult LDLT; (4) single donors; and (5) without a history of long-term drinking. Exclusion criteria: (1) age < 18 or > 60 years; (2) a left hepatic graft or left lateral lobe graft; (3) double donor grafts; (4) adult-to-child transplants; and (5) donors that were hepatitis B virus or hepatitis C virus carriers.
After the above selection criteria eliminated some patients, eligible subjects were identified. In total, we identified 151 cases of right liver adult-to-adult living donors (not including the MHV). Ninety cases were male and 61 were female. The total liver volume was calculated using the Chengdu standard liver volume formula[9,10]. The volume of actual grafts (excluding the MHV of the right liver) was measured intraoperatively. Remnant liver volume = the total liver volume - the volume of the actual graft. According to the ratio of the remnant liver volume to the total liver volume, the cases within the study group were further subdivided into three groups: Group 1: RLV < 35% (n = 14), group 2: RLV 36%-40% (n = 20), and group 3: RLV > 40% (n = 117).
Written informed consent was obtained from all patients to include their data in this study, which was approved by the HuaXi Ethics Committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Indicator variables included: age, gender, body height (error < 1 cm), and body weight (error < 0.5 kg). Preoperative donor blood test indicators were also obtained. These indicators included: hemoglobin (HGB), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL). Donor selection was based on a designated radiologist row computerised tomography examination and determination of liver volume. All donors used the same surgery/medical team.
An LDLT was completed through a right subcostal incision with an upward midline extension. Intraoperative cholangiography via cystic duct cannulation was required to evaluate the anatomy of the bile duct. A right hilar dissection was then performed to isolate the right hepatic artery, right portal vein, and right hepatic duct[11]. The right lobe of the liver was then rotated towards the left side for division of the ligaments on the right side of the liver, the minute venous branches between the anterior surface of the inferior vena cava and the posterior surface of the paracaval portion of the caudate lobe. To prevent impeding the circulation, the right hepatic vein and the right inferior hepatic veins that were larger than 5 mm were preserved until the time of harvesting.
The transection plane was determined by intraoperative ultrasonography and the temporary occlusion of the right portal vein and right hepatic artery. After identification of the confluence of the left and right hepatic ducts, the right hepatic duct was divided near the confluence of the hepatic ducts using scissors. The divided end was closed transversely using a continuous 5-0 prolene suture. The transection was carried down to the junction of the right hepatic vein and the inferior vena cava. The right hepatic artery was then divided. To accomplish this, the right hepatic vein was clamped at the junction with the inferior vena cava and divided. The stumps of the right portal vein and right hepatic vein were closed with continuous nonabsorbable sutures. The falciform ligament was then sutured to the anterior abdominal wall. A drain was inserted into the right subphrenic cavity prior to wound closure[12].
The weight of the grafted liver was measured using a pan scale, the error was found to be less than 10 g. The volume was measured using the drainage method in a 3 L beaker full of saline; the error was found to be less than 10 mL.
Postoperatively, donors stayed in the intensive care unit (ICU) for monitoring and oxygen, and received parenteral nutrition which was rich in branched-chain amino acids. Donors bagn parenteral nutrition following intestinal function recovery. When necessary, donors received blood transfusions or plasma and human serum albumin. Postoperative monitoring of HGB, ALT, AST, TBIL, and the international standardization ratio (INR) was performed. ICU time, hospital stay, timely diagnosis, and treatment of surgical complications were recorded. Donor follow up was 6 to 48 mo, and all follow up information was recorded.
The mean ± SD of the data are presented. The SPSS program (version 15.0, SPSS Inc., United States) was used for the statistical analysis. After testing for normal distribution using Kurtosis and Skewness tests, descriptive variables including pre-operative, intra-operative, post-operative and prognostic parameters, were calculated. Fisher’s exact test was used to detect the differences among the groups for categorical variables, including gender. Independent-sample t tests were calculated to detect differences among the groups for continuous random variables including HGB, ALT, AST, TBIL, volumetric data, postoperative INR, ICU time, hospital stay, and reasonable and customary (R and C). A correlation analysis using the χ2 test was conducted to determine the incidence of complications. The difference was considered significant if P < 0.05.
The donors’ anthroposomatology data, operation time, and preoperative blood test indicators were calculated for the three groups: Group 1 RLV < 35%, group 2 RLV 36%-40%, and group 3 RLV > 40%. These data are shown in Table 1. No significant differences were observed between the donors’ gender, age, height, weight, and operation time. Preoperative data on ALT, AST, TBIL and HGB were also collected among the three groups of donors. These results suggested that the conditions of the three groups of donors were well matched in terms of the studied parameters.
| Characteristics | Patients | P value | ||||
| Group 1 (RLV < 35%) | Group 2 (40% > RLV > 35%) | Group 3 (RLV > 40%) | P1 | P2 | P3 | |
| n = 14 | n = 20 | n = 117 | ||||
| Demographic and intraoperative data | ||||||
| Gender (M/F), 90/61 | 10/4 | 12/8 | 62/46 | 0.717 | 1.000 | 0.395 |
| Age (yr) | 37.1 ± 8.7 | 41.0 ± 12.7 | 38.0 ± 9.8 | 0.337 | 0.156 | 0.431 |
| Weight (kg) | 64.3 ± 11.4 | 61.7 ± 8.4 | 63.6 ± 11.8 | 0.463 | 0.500 | 0.844 |
| Height (cm) | 164.9 ± 12.3 | 163.3 ± 9.1 | 164.5 ± 13.1 | 0.664 | 0.679 | 0.925 |
| Operation time (min) | 389.9 ± 87.2 | 373.4 ± 60.5 | 363.7 ± 71.9 | 0.581 | 0.472 | 0.765 |
| EBL (mL) | 602.8 ± 73.1 | 582.9 ± 81.6 | 531.3 ± 50.7 | 0.740 | 0.583 | 0.341 |
| Preoperative laboratory data | ||||||
| ALT (IU/L) | 28.2 ± 16.6 | 29.8 ± 15.7 | 27.8 ± 20.9 | 0.784 | 0.683 | 0.937 |
| AST (IU/L) | 28.9 ± 17.4 | 25.6 ± 9.9 | 23.3 ± 11.1 | 0.496 | 0.389 | 0.105 |
| TBIL (mg/dL) | 12.7 ± 3.8 | 13.9 ± 6.1 | 15.2 ± 7.0 | 0.529 | 0.413 | 0.189 |
| Hemoglobin (g/L) | 147.1 ± 13.2 | 142.4 ± 17.2 | 141.5 ± 16.9 | 0.392 | 0.822 | 0.229 |
| Volumetric data of Chengdu standard liver volume formula (mL) | ||||||
| Whole liver volume of formula | 1072.88 ± 131.06 | 1043.84 ± 97.11 | 1065.33 ± 136.02 | 0.463 | 0.500 | |
| Graft volume | 745.00 ± 100.22 | 653.80 ± 56.55 | 534.83 ± 89.26 | 0.006 | 0.000 | |
| Remnant liver volume of formula | 327.88 ± 61.83 | 390.04 ± 48.46 | 530.50 ± 125.83 | 0.002 | 0.000 | |
| Remnant volume/whole volume of Chengdu formula(%) | 30.56 ± 4.17 | 37.31 ± 2.06 | 49.63 ± 7.33 | 0.000 | 0.000 | |
Compared to Heinemann, Urata, Vauthey[13-16], and the Lee formulae[17], the Chengdu standard liver volume formula was demonstrated to be more reliable in LDLT. In LDLT, this formula can more accurately forecast total liver volume[10]. Standard liver volumes in the 151 cases were calculated using the Chengdu formula: SLV (mL) = 11.5 × body weight (kg) ± 334. The volumes of the actual grafts (excluding the MHV of the right liver) were measured intraoperatively. Remnant liver volume = the total liver volume - the volume of the actual graft. We determined the ratio of the remnant liver by remnant liver volume/standard liver volume. The liver volume-related parameters are shown in Table 1.
According to the Chengdu standard liver volume formula, the total liver volume in group 1 was 1072.88 ± 131.06 mL, group 2 was 1043.84 ± 97.11 mL, and group 3 was 1065.33 ± 136.02 mL. The three groups showed no statistically significant differences. However, the graft volume in group 1 was 745.00 ± 100.22 mL, group 2 was 653.80 ± 56.55 mL, and group 3 was 534.83 ± 89.26 mL, revealing a statistically significant difference between the groups. Remnant liver volume also showed significant differences.
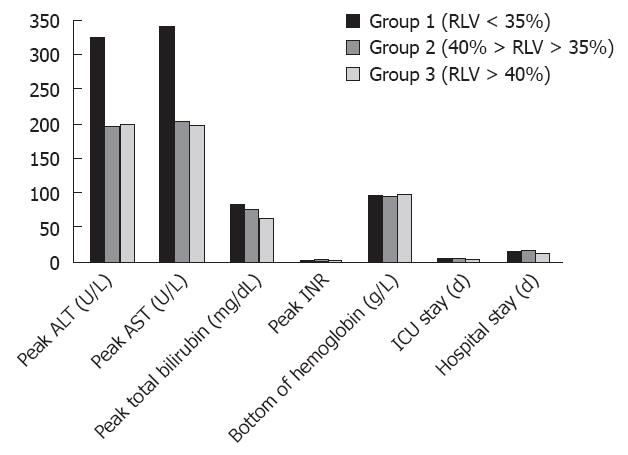
Postoperative monitoring of donor ALT peak, AST peak, TBIL peak, INR peak, and HGB value was conducted during their ICU stay and hospital stay. Postoperative characteristics are illustrated in Figure 1. The ALT peak in the smallest remnant liver volume in group 1 was 325.64 ± 202.33 U/L, this value was significantly higher than that in the other two groups (196.85 ± 130.62 U/L and 200.70 ± 150.94 U/L, respectively). The AST peak of 339.79 ± 172.91 U/L was also significantly higher than that in group 2 and group 3 (P value = 0.010 and 0.003, respectively). The ALT peak and AST peak in groups 2 and 3 showed no significant difference (P = 0.915 and 0.893, respectively). However, differences in the TBIL peak, INR peak, and HGB value among the three groups of donors were observed, but no statistical differences were found.
The ICU time for group 1 was 6.93 ± 2.13 d, significantly longer than that for group 2 and group 3 (5.10 ± 1.62 d vs 5.33 ± 1.63 d, respectively). There was no statistical difference in ICU stay between groups 2 and 3. The three groups of patients exhibited no significant difference in hospitalization time.
The Clavien classification system has been increasingly used in the analysis of post-surgical complications[18,19]. Researchers have also begun to use this classification in the LDLT donor complication category[20,21]. The donor complications were calculated according to the Clavien classification system of grading.
Fifty donors that exhibited a total of 151 complications. According to the Clavien grading system, 28 cases had grade 1 complications, 9 cases had grade 2 complications, 8 cases had grade 3a complications, and 5 cases had grade 3b complications. No serious grade 4 or 5 complications were observed. There were no donor deaths (Table 2).
| Grades, n (%) | Complications | n |
| Grade 1, 28 (18.5) | Transient bile leak treated conservatively | 7 |
| Superficial wound infection treated without antibiotics | 2 | |
| Postoperative voice change | 3 | |
| Mild pleural effusion treated conservatively | 2 | |
| Mild subphrenic effusion treated conservatively | 5 | |
| Hyperbilirubinemia > 1.3 mg/dL 7 d after operation | 9 | |
| Grade 2, 9 (6.0) | Intra-abdominal bleeding requiring blood transfusion | 1 |
| Bile leak not requiring ERCP or surgical intervention | 3 | |
| Dyspepsia | 1 | |
| Chyle leak | 1 | |
| Wound infection requiring antibiotics | 1 | |
| Pneumonia requiring antibiotics | 2 | |
| Grade 3a, 8 (5.3) | Bile leak needing ERCP | 3 |
| Pleural effusion requiring thoracic cavity puncture | 2 | |
| Pleural effusion requiring thoracic drainage | 1 | |
| Subphrenic infection requiring abdominal cavity puncture | 1 | |
| Chylothorax requiring thoracic cavity puncture | 1 | |
| Grade 3b, 5 (3.3) | Portal vein thrombosis requiring re-laparotomy | 1 |
| Biliary stricture requiring ERCP with stent placement | 2 | |
| Abdominal hematoma requiring intervention | 1 | |
| Intra-abdominal bleeding requiring re-laparotomy | 1 | |
| Grade 4a | 0 | |
| Grade 4b | 0 | |
| Grade 5 | 0 |
A correlation analysis of the χ2 test was used to compare different grades of complications among the three groups of donors (Table 3). R and C correlation analysis revealed that the complication grade had a significant relationship with remnant liver volume.
| Complications | Grades | P value | ||||
| No complications (n = 101) | Grade 1 (n = 28) | Grade 2 (n = 9) | Grade 3a (n = 8) | Grade 3b (n = 5) | ||
| Group 1 (RLV < 35%), n = 14 | 3 | 5 | 3 | 2 | 1 | 0.000 |
| Group 2 (40% > RLV > 35%), n = 20 | 10 | 4 | 3 | 1 | ||
| Group 3 (RLV > 40%), n = 117 | 88 | 19 | 3 | 5 | 2 | |
The evaluation of suitable donors is related to both donor and recipient safety. The volume of the graft liver should ensure the absolute safety of the donor, but also meet the needs of the recipient. For example, if the remnant liver volume is too small for the body, it can lead to acute liver failure in the donor. If the graft is too small, it can result in small-for-size graft syndrome[22,23]. In general, the younger the donor, the better is the liver’s regenerative capacity[24], thus donors aged between 18 and 60 years are required.
In this study, donors age ranged from 18 to 60 years. During the preoperative examination, there were no obvious abnormal liver functions, obvious blood vessels, biliary anatomical abnormalities, or intraoperative liver biopsies with serious fatty degeneration. According to the remnant liver volume, the study group was divided into three groups. Data for each group of donors was recorded, and included preoperative parameters, operative time, and intraoperative blood loss. No statistical differences between the groups were found. The three groups also had homogeneity of the body, excluding other factors of donor recovery.
Postoperative data revealed that the ALT and AST peaks in group 1 donors were significantly higher than those in the other two groups. There were no significant differences (P = 0.915 and 0.893, respectively) for the ALT peak or AST peak between groups 2 and 3. The ICU time in group 1 donors was significantly longer than that in group 2 and 3 donors. No statistical differences in ICU time between groups 2 and 3 were observed.
Postoperative indicators showed that when the remnant liver volume was greater than 35% of standard liver volume, the volume of the remnant liver had no significant effect on the recovery of liver function or the ICU time, however, when the remnant liver volume was less than 35%, this led to a much slower recovery of liver function.
In LDLT, there are three possible types of short-term complications. One type of complication includes bleeding, biliary leakage, embolization, liver failure, metabolic abnormalities caused by cholinesterase, and hypophosphatemia. The second are open surgery-related complications and include intra-abdominal infections, incisional hernias, adhesions, and intestinal obstruction. There are also complications associated with anesthesia.
An international statistical analysis showed that the incidence of complications in donors was 10%-30%, while the case fatality rate was 0.1%-0.3%[4,25-27]. In this study, 33% of the 151 complications occurred in 50 patients. According to the Clavien grading system, the patients in this study experienced 28 grade 1 complications, 9 grade 2 complications, 8 grade 3a complications, and 5 grade 3b complications. There were no serious grade 4 or grade complications and no donor deaths. R and C correlation analysis showed that complication grades had a significant relationship with remnant liver volume.
In summary, when the volume of a remnant liver was less than 35% of the standard liver volume, the volume of the remnant had a significant effect on the recovery of liver function and ICU time. In addition, the occurrence of complications was closely related to remnant liver volume. Recipients were only available if good results were expected. Therefore, the interests of the donor should be accounted for to minimize their risks during surgery.
We thank the specialists affiliated with the Chinese Liver Transplantation Registry for their assistance in data collection and analysis.
An increasing number of transplant centers have used right graft living donor liver transplantations. This surgical method could provide a greater number of liver grafts to meet the metabolic demands of recipients. However, right lobe graft donors take more risks and have more complications than left graft donors. This has created considerable controversy with respect to donor safety. At the present time, 26 donor deaths have been reported, and morbidity was reported to be in the range of 20% to 30%.
The literature has reported that donor complications are closely related to remnant liver volume (RLV). The authors retrospectively examined the RLV in right graft donors and compared those donors with other different RLVs.
This has created considerable controversy with respect to RLV and donor safety. The aim of the present study was to assess the relationship between donor recovery, complications and the volume of remnant liver.
The SPSS program (version 15.0, SPSS Inc., United States) was used for the statistical analysis. After testing for normal distributions using Kurtosis and Skewness tests, descriptive variables including pre-operative, intra-operative, post-operative and prognostic parameters were calculated. Fisher’s exact test was used to detect the differences among the groups for categorical variables, including gender. Independent-sample t tests were used to detect differences among the groups for continuous random variables including hemoglobin, alanine aminotransferase, aspartate aminotransferase, total bilirubin, volumetric data, postoperative International Normalized ratio, intensive care unit time, hospital stay, and reasonable and customary. A correlation analysis using the chi-squared test was conducted to determine the incidence of complications.
This study is an analysis of 151 cases of adult right lobe living related donor to determine the effect of volume of the remant liver on post operative complications. The conclusions reached was that volumes less than 35% of total liver volumes were associated with increased complications and length of recovery. This is a significant study adding further to the knowledge in this area of liver transplanation.
Peer reviewer: Christopher Christophi, Professor and Head of The University of Melbourne Department of Surgery, Austin Hospital. Melbourne, 145 Studley Road, Victoria 3084, Australia
S- Editor Gou SX L- Editor Webster JR E- Editor Li JY
| 1. | Trotter JF, Adam R, Lo CM, Kenison J. Documented deaths of hepatic lobe donors for living donor liver transplantation. Liver Transpl. 2006;12:1485-1488. [PubMed] |
| 2. | Middleton PF, Duffield M, Lynch SV, Padbury RT, House T, Stanton P, Verran D, Maddern G. Living donor liver transplantation--adult donor outcomes: a systematic review. Liver Transpl. 2006;12:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Chan SC, Fan ST, Lo CM, Liu CL, Wei WI, Chik BH, Wong J. A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes. Ann Surg. 2008;248:411-419. [PubMed] |
| 4. | Patel S, Orloff M, Tsoulfas G, Kashyap R, Jain A, Bozorgzadeh A, Abt P. Living-donor liver transplantation in the United States: identifying donors at risk for perioperative complications. Am J Transplant. 2007;7:2344-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Taketomi A, Kayashima H, Soejima Y, Yoshizumi T, Uchiyama H, Ikegami T, Yamashita Y, Harada N, Shimada M, Maehara Y. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation. 2009;87:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Fan ST, Lo CM, Liu CL, Yong BH, Chan JK, Ng IO. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 336] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Cho JY, Suh KS, Kwon CH, Yi NJ, Lee HH, Park JW, Lee KW, Joh JW, Lee SK, Lee KU. Outcome of donors with a remnant liver volume of less than 35% after right hepatectomy. Liver Transpl. 2006;12:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Taner CB, Dayangac M, Akin B, Balci D, Uraz S, Duran C, Killi R, Ayanoglu O, Yuzer Y, Tokat Y. Donor safety and remnant liver volume in living donor liver transplantation. Liver Transpl. 2008;14:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Li FG, Yan LN, Li B, Zeng Y, Wen TF, Xu MQ, Wang W. Estimation formula of standard liver volume for Chinese adults. Sichuan Daxue Xuebao Yixueban. 2009;40:302-306. [PubMed] |
| 10. | Shi ZR, Yan LN, Li B, Wen TF. Evaluation of standard liver volume formulae for Chinese adults. World J Gastroenterol. 2009;15:4062-4066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Wu H, Yan LN, Li B, Zeng Y, Wen TF, Zhao JC, Wang WT, Yang JY, Xu MQ, Chen ZY. Hepatic venous outflow reconstruction in right lobe graft without middle hepatic vein. Hepatol Res. 2007;37:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wen TF, Chen ZY, Yan LN, Li B, Zeng Y, Zhao JC, Wang WT, Yang JY, Ma YK, Xu MQ. Measures for increasing the safety of donors in living donor liver transplantation using right lobe grafts. Hepatobiliary Pancreat Dis Int. 2007;6:590-595. [PubMed] |
| 13. | Heinemann A, Wischhusen F, Püschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 704] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 15. | Urata K, Hashikura Y, Ikegami T, Terada M, Kawasaki S. Standard liver volume in adults. Transplant Proc. 2000;32:2093-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, Lerut J, Materne R, Wang X, Encarnacion A. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 465] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 17. | Lee SG, Park KM, Hwang S, Lee YJ, Kim KH, Ahn CS, Choi DL, Joo SH, Jeon JY, Chu CW. Adult-to-adult living donor liver transplantation at the Asan Medical Center, Korea. Asian J Surg. 2002;25:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24849] [Article Influence: 1183.3] [Reference Citation Analysis (0)] |
| 19. | Liu B, Yan LN, Li J, Li B, Zeng Y, Wang WT, Xu MQ, Yang JY, Zhao J. Using the Clavien grading system to classify the complications of right hepatectomy in living donors. Transplant Proc. 2009;41:1703-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Patel S, Cassuto J, Orloff M, Tsoulfas G, Zand M, Kashyap R, Jain A, Bozorgzadeh A, Abt P. Minimizing morbidity of organ donation: analysis of factors for perioperative complications after living-donor nephrectomy in the United States. Transplantation. 2008;85:561-565. [PubMed] |
| 21. | Marsh JW, Gray E, Ness R, Starzl TE. Complications of right lobe living donor liver transplantation. J Hepatol. 2009;51:715-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Tanaka K, Ogura Y. "Small-for-size graft" and "small-for-size syndrome" in living donor liver transplantation. Yonsei Med J. 2004;45:1089-1094. [PubMed] |
| 23. | Yoshizumi T, Taketomi A, Kayashima H, Harada N, Uchiyama H, Yamashita Y, Ikegami T, Soejima Y, Nishizaki T, Shimada M. Successful treatment for a patient with hemophagocytic syndrome after a small-for-size graft liver transplantation. Hepatogastroenterology. 2008;55:359-362. [PubMed] |
| 24. | Olthoff KM. Hepatic regeneration in living donor liver transplantation. Liver Transpl. 2003;9:S35-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Coelho JC, de Freitas AC, Matias JE, de Godoy JL, Zeni Neto C, Parolin MB, Okawa L. Donor complications including the report of one death in right-lobe living-donor liver transplantation. Dig Surg. 2007;24:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Lee SY, Ko GY, Gwon DI, Song HY, Lee SG, Yoon HK, Sung KB. Living donor liver transplantation: complications in donors and interventional management. Radiology. 2004;230:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Renz JF, Roberts JP. Long-term complications of living donor liver transplantation. Liver Transpl. 2000;6:S73-S76. [PubMed] |