Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7290
Revised: July 27, 2012
Accepted: July 29, 2012
Published online: December 28, 2012
AIM: To evaluate the results of hepatic resection with ex-situ hypothermic perfusion and without veno-venous bypass.
METHODS: In 3 patients with liver tumor, the degree of the inferior vena cava and/or main hepatic vein involvement was verified when the liver was dissociated in the operation. It was impossible to resect the tumors by the routine hepatectomy, so the patients underwent ex-situ liver surgery, vein cava replacement and hepatic autotransplantation without veno-venous bypass. All surgical procedures were carried out or supervised by a senior surgeon. A retrospective analysis was performed for the prospectively collected data from patients with liver tumor undergoing ex-situ liver surgery, vein cava replacement and hepatic autotransplantation without veno-venous bypass. We also compared our data with the 9 cases of Pichlmayr’s group.
RESULTS: Three patients with liver tumor were analysed. The first case was a 60-year-old female with a huge haemangioma located in S1, S4, S5, S6, S7 and S8 of liver; the second was a 64-year-old man with cholangiocarcinoma in S1, S2, S3 and S4 and the third one was a 55-year-old man with a huge cholangiocarcinoma in S1, S5, S7 and S8. The operation time for the three patients were 6.6, 6.4 and 7.3 h, respectively. The anhepatic phases were 3.8, 2.8 and 4.0 h. The volume of blood loss during operation were 1200, 3100, 2000 mL in the three patients, respectively. The survival periods without recurrence were 22 and 17 mo in the first two cases. As for the third case complicated with postoperative hepatic vein outflow obstruction, emergency hepatic vein outflow extending operation and assistant living donor liver transplantation were performed the next day, and finally died of liver and renal failure on the third day. Operation time (6.7 ± 0.47 h vs 13.7 ± 2.6 h) and anhepatic phase (3.5 ± 0.64 h vs 5.7 ± 1.7 h) were compared between Pichlmayr’s group and our series (P = 0.78).
CONCLUSION: Ex-situ liver resection and liver autotransplantation has shown a potential for treatment of complicated hepatic neoplasms that are unresectable by traditional procedures.
-
Citation: Zhang KM, Hu XW, Dong JH, Hong ZX, Wang ZH, Li GH, Qi RZ, Duan WD, Zhang SG.
Ex-situ liver surgery without veno-venous bypass. World J Gastroenterol 2012; 18(48): 7290-7295 - URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7290.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7290
In 1988, ex vivo liver resection and liver autotransplantation were pioneered by Pichlmayr et al[1], who tried to open a new platform for unresectable hepatobiliary malignancies and explore new modalities for liver surgery. In 1990, the whole liver was explanted and then perfused with cold preservation solution. The hepatic resection was performed on the back table and the remnant liver was reimplanted orthotopically. The aim of the extracorporeal hypothermic parenchymal resection was to extend oncological radicality by achieving an R0 margin with a better tumor-free cutting edge. But ex-situ liver surgery with veno-venous bypass aggravated ischemia-reperfusion injury of liver and resulted in the liver failure, which is the most common reason for the operation abortion. Subsequently, Hannoun et al[2] and Sauvanet et al[3] endeavored to improve the ex-situ technique. However, it is still difficult to avoid the ischemia-reperfusion injury and liver failure. To resolve this problem, we performed ex-situ liver surgery without veno-venous bypass in three patients with tumors involving inferior vena cava (IVC) based on the animal experiments. We introduce our experience in this operation as follows.
From December 1999 to December 2008, a total of 1700 patients with hepatic tumors underwent routine hepatectomy at our hepatobiliary department. The extent of IVC and/or main hepatic vein (MHV) involved by the tumors was assessed by preoperative radiographic inspection. However, the degree of the tumor-involved IVC and/or main hepatic vein could not be confirmed by the preoperative inspection in three patients. Finally, it was verified when the liver was dissociated during the operation. The tumors were unresectable by the routine hepatectomy, so we performed ex-situ liver surgery, vein cava replacement and hepatic autotransplantation without veno-venous bypass. All surgical procedures were carried out or supervised by a senior surgeon (Dong JH). Patients’ conditions are summarized in Table 1. There were two males and one female, with a mean age of 60 years (range, 55-65 years). Two patients suffered from chloangiocarcinoma and the other one from hemangioma.
| No. | Sex | Age (yr) | Disease | Segments involved | Tumor diameter(cm) | Vessels involvedor infiltrated |
| 1 | Female | 60 | Hemangioma | 1, 4, 5, 6, 7, 8 | 20 | IVC (circum 190°, length 5 cm) |
| 2 | Male | 64 | Cholangiocarcinoma | 1, 2, 3, 4 | 6 | IVC (circum 80°, length 3 cm) |
| 3 | Male | 55 | Cholangiocarcinoma | 1, 5, 7, 8 | 5 | IVC (circum 60°, length 2 cm) |
| RHV, MHV, RPV |
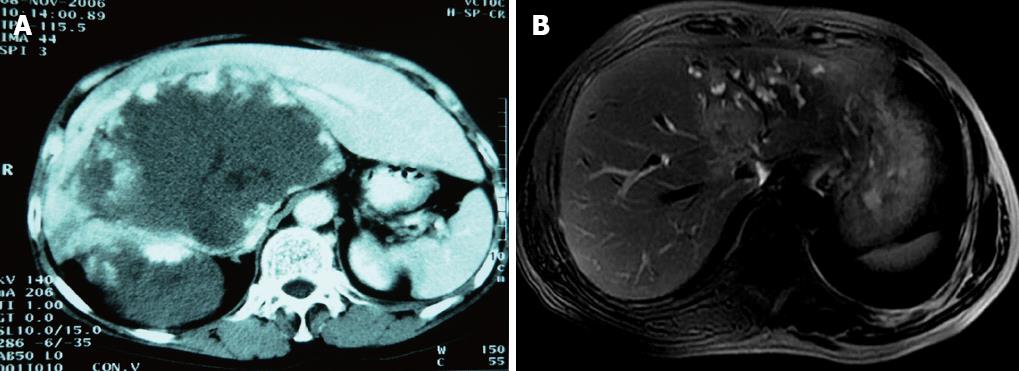
All patients were examined with computed tomography (CT) as indicated to exclude extrahepatic malignant disease of the abdomen, pelvis or chest. The location of the tumor and extent of involvement of intrahepatic vessel and IVC (Table 1) were assessed before surgery with CT (Figure 1A) and magnetic resonance imaging (Figure 1B). Cardiovascular, renal and pulmonary fitness for surgery was evaluated with an exercise electrocardiogram, chest radiography, and pulmonary function testing in all patients.
This type of surgery should be performed always with the presence of anesthesiologists who have experience with liver resection and are able to manage prolonged IVC occlusion and the changes that occur with liver reperfusion. During surgery, all patients were monitored by standard non-invasive techniques. In addition, a Swann-Ganz catheter and an arterial line should be placed before surgery in the patients who planned to undergo total vascular exclusion (TVE) with hypothermic perfusion. Body warmers were routinely employed to prevent hypothermia intraoperatively.
We routinely used a bilateral subcostal incision which provided adequate exposure for almost all types of liver resection. After mobilization of liver, a double examination of the liver by palpation and ultrasonography was performed to confirm the number and size of the lesions, to define their relationship to intrahepatic vascular structures, exclude undiagnosed liver metastases and lastly to determine the resection line.
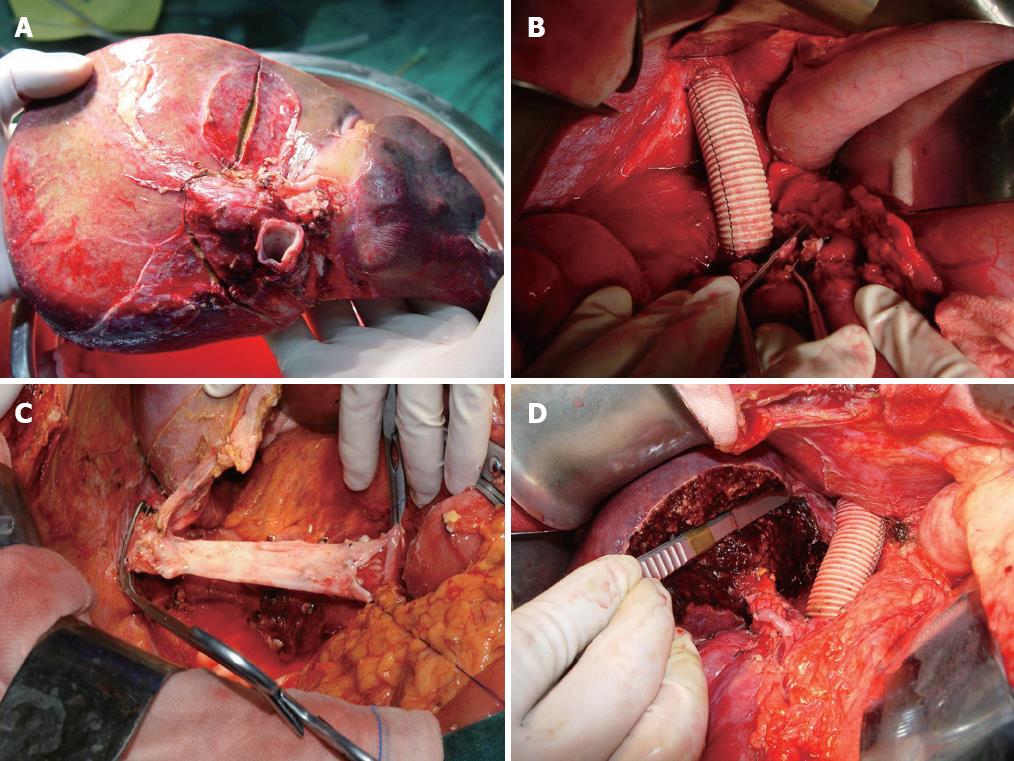
The approach of vascular control was chosen depending on the tumor location and extent of tumor involvement. The retrohepatic cava was partially occluded by a side clamp[4] if the extent of involved IVC was small (≤ 60° circumferentially and ≤ 2 cm longitudinally). However, the TVE was needed to guarantee clear surgical field[4-8] if the IVC was involved in a larger extent. The technique of TVE we used involves mobilization of the liver, exposure and control of the suprahepatic and infrahepatic vena cava as well as the portal structures (portal vein and hepatic artery)[9,10]. En bloc liver and a segment of the vena cava were removed finally (Figure 2A).
IVC was quickly replaced with a 20-mm ringed polytetrafluoroethylene (PTFE) graft if it was infiltrated by the tumor. After that, IVC was unclamped (Figure 2B).
Hypothermic hepatic perfusion and bench hepatectomy: After parenchymal transection was completed, the liver was perfused and harvested as described for whole organ procurement. The hilar structures were divided on the bench. After cannulation of the portal and arterial systems, the liver was flushed with preservation solution [histidine-tryptophan-ketoglutarate (HTK)-Bretschneider] at 4 °C and packed with ice immediately. The volume of HTK used was 1 L. Proper hepatic artery and common bile duct were flushed respectively as well. The tumor was excised with cavipulse ultrasonic surgical aspirator and dipolar electrosurgical unit under intraoperative ultrasonographic guide, and the involved blood vessels were partially exscinded, repaired, formed and reconstructed. Figure 2C shows that the involved IVC was peeled off, trimmed and replaced anastomosed PTFE graft in right trisegmentectomy in the case of hemangioma.
The hepatic resection was performed on the back table and the remnant liver was reimplanted orthotopically (Figure 2D). After meticulous hemostasis, a fibrin glue sealant was sprayed on the cut surface. After irrigation, a closed suction drain was inserted, and the wound was closed in layers.
Fisher’s exact tests were used to compare variables between Pichlmayr et al[1] and the present series. All statistical analyses were performed using Stat View 5.0J (Abacus Concepts, Berkeley, CA). P < 0.05 was defined as significant difference.
Neither major perioperative surgical nor anesthesiological complications were observed and no respiratory complications were noticed during surgery or the immediate postoperative period. Patient general features and tumor characteristics are summarized in Table 1. The intraoperative clinical outcomes and postoperative clinical course are summarized in Table 2. The first case was a 60-year-old female with a huge hemangioma located in S1, S4, S5, S6, S7 and S8 of the liver; the second was a 64-year-old man with cholangiocarcinoma in S1, S2, S3 and S4 and the third one was a 55-year-old man with a huge chloangiocarcinoma in S1, S5, S7 and S8 (Table 1). The operation time for the three patients were 6.6, 6.4 and 7.3 h, respectively. The anhepatic phases were 3.8, 2.8 and 4.0 h. The volume of blood loss during operation were 1200, 3100 and 2000 mL, respectively. The survival period without recurrence was 22 and 17 mo in the first two cases, respectively. As for the third case, which was complicated with hepatic vein outflow obstruction after operation, emergency hepatic vein outflow extending operation and assistant living donor liver transplantation (LDLT) were performed the next day, and finally he died of liver and renal failure on the third day. Operation time (6.7 ± 0.47 h vs 13.7 ± 2.6 h) and anhepatic phase (3.5 ± 0.64 h vs 5.7 ± 1.7 h) were compared between Pichlmayr et al[1] and our series (Table 3) (P = 0.78).
| No. | Hepatectomy | Segments resected | IVC reconstruction | Operation time (h) | Anhepatic phase (h) | Blood loss (mL) | Complication | Hospital stay (d) | Outcome |
| 1 | Ex vivo hepatectomy with reimplantation of segment 2 and 3 | 1, 4, 5, 6, 7, 8 | Synthetic graft and autologous IVC | 6.6 | 3.8 | 1200 | Pleural effusion | 28 | Alive without disease 22 mo after operation |
| 2 | Ex vivo hepatectomy with reimplantation of segment 4b, 5, 6, 7, 8 | 1, 2, 3, 4 | Synthetic graft | 6.4 | 2.8 | 3100 | No | 38 | Alive without disease 17 mo after operation |
| 3 | Ex vivo hepatectomy with reimplantation of segment 2, 3, 4 | 1, 5, 6, 7, 8 | Synthetic graft | 7.3 | 4.0 | 2000 | Liver and renal failure | 11 | Died |
| n | Operation time (h) | Anhepatic phase (h) | Postoperativedeath | |
| Pichlmayr’s | 9 | 13.7 ± 2.6 | 5.7 ± 1.7 | 3 |
| (11-18) | (4-9) | |||
| Present series | 3 | 6.7 ± 0.47 | 3.5 ± 0.64 | 1 |
| (6.4-7.3) | (2.8-4.0) |
Ex-vivo liver resection and liver autotransplantation represents a new approach for liver surgery. We simplified the operation by omitting the veno-venous bypass, which shortened the operative time and anhepatic period.
Most liver tumors can be removed with conventional resection techniques using partial or total vascular occlusion when needed. But it is difficult to resect the central liver or posterior segments where hepatic veins are involved. The procedure would result in massive hemorrhage or air embolism if it was performed in such patients.
The accessory left hepatic artery should be occluded carefully, or it will lead to hepatic congestion or excessive bleeding. Huguet et al[9] and Choi et al[10] reported that TVE resulted in blood congestion of IVC and portal vein definitely and caused a series of physiopathologic problems. The congested blood re-enters the circulation and aggravates the injury of ischemia-reperfusion when the influx was recovered, especially in patients with liver cirrhosis. The mortality can reach up to 75% after the hepatectomy using the TVE method.
With the development of surgical techniques, the veno-venous bypass has been applied to complicated hilum hepatectomy[1,11]. The blood in portal vein and IVC was by-passed by the bio-pump to ensure the stability of general circulation and lessen the ischemia-reperfusion injury when the TVE was performed. The total veno-venous bypass technique enhances the tolerance of liver to warm ischemia. In this condition, the safe time limit of liver tolerance to total vascular occlusion seems to be 30-120 min[7,12,13], which is short for extensive and complex tumors in a direct contact with major vascular structures.
To overcome this limitation and to reduce liver damage, the concept of hypothermic perfusion has come into consideration. This technique enables liver resection to proceed in situ, ante situm or ex vivo. The liver was filled by Belzer’s University of Wisconsin solution. The tolerance to the ischemia phase is extended to 4 h with hypothermic liver perfusion. Azoulay et al[14] completed complicated liver resections using combined veno-venous bypass and in situ hypothermic perfusion, and they testified that the duration of liver tolerance to ischemia could last 90 min at least in 85% cases. The IVC must be replaced and reconstructed if it is infiltrated by tumors extensively.
Ante situm liver resection (in vivo hypothermic perfusion and veno-venous bypass) has been applied widely to resect the tumors located in hepatic hilum, confluence of hepatic vein and IVC, but veno-veinous bypass was used during the operation which led to severe water, electrolyte and acid-base imbalance during the first postoperative week. Azoulay et al[14] reported that the mortality from ex-situ liver resection with hypothermic perfusion (28%) was higher than that from in vivo hepatectomy (8%). We reviewed the pertinent literature published from 1992 to 2007 and found that 41 cases underwent ex-situ liver surgery with hypothermic perfusion. But only one patient underwent ex-situ liver surgery with hypothermic perfusion without veno-venous bypass, and the patient is still alive[15]. However, 10 patients died after the resection with veno-venous bypass with a mortality of 24%[15-22]. Cabezuelo et al[23] suggested that, in the resection with veno-venous bypass, the hemodynamic stability of the body and regional organs (kidney, gastrointestinal tract) was improved during the anhepatic phase, but the rate of kidney failure was not decreased. The procedure without veno-venous bypass has been verified to be feasible and safe during the past few years. The cost was reduced because this technique shortens the total operation time, reduces hemorrhage during operation and decreases the rates of reperfusion injury.
Taking into account these controversial facts and the deficiency of liver graft, we performed ex-situ liver surgery, vein cava replacement and hepatic autotransplantation without veno-venous bypass in three cases. The general conditions of the three cases are described in Table 1.
The first case is a patient with a huge hemangioma located in S1, S4, S5, S6, S7 and S8 (Figure 1A) where IVC was involved and compressed severely. It is likely to lead to grave hemorrhea if IVC is dissected in the operation because of involvement of IVC (circumference 190°, longitude 5 cm). The other two cases were patients with cholangiocarcinoma located at S1, S2, S3 and S4 and S1, S5, S7 and S8, respectively. The extension of infiltrated IVC was large (circumference ≥ 60°, longitude ≥ 2 cm)[6,24].
Although the tumor diameter was about 5 cm, the IVC, right hepatic vein, MHV and right portal vein were involved in the third patient. And the aim of cholangiocarcinoma resection was to achieve an R0 margin with a better tumor-free cutting edge. So it is difficult to complete the resection in a short time if the in situ or ante situm procedure was performed. But the ex-situ liver resection with hypothermic perfusion could decrease the chance of blood disseminated, and provide more chances to achieve an R0 margin[25].
We have accumulated much experience in performing complicated hepatectomy and liver transplantation. Before we performed the ex-situ liver surgery under hypothermic perfusion and without veno-venous bypass, we had done the operation in the animal experiments with the protocol approved by the ethics committee of the hospital. In order to shorten the operation time, we repaired the IVC, and performed the ex-vivo hepatectomy when the IVC was resected at back table in the first case. The segment S2 and S3 was autotransplanted when the PTFE graft was removed. The operation time and the anhepatic phase were 6.6 and 3.8 h, respectively.
For the other two patients with cholangiocarcinoma, we performed ex- vivo hepatectomy with liver reimplantation. The segment 4b, 5, 6, 7 and 8 and segment 2, 3 and 4 were autotransplanted, respectively. Their operation time was 6.4 and 7.3 h and anhepatic phase was 2.8 and 4.0 h, respectively.
We compared our data with the Pichlmayr et al[1] of 9 cases undergoing ex-vivo hepatectomy with veno-veno bypass. Our procedure shortened the time of surgery and anhepatic phase because we avoided the veno-venous bypass, although there was no significant difference between the different techniques (P = 0.78), possibly due to the small number of cases. Furthermore, no hepatic dysfunction resulting from the delayed liver hemoperfusion occurred in our group after operation. But in the 9 cases of Pichlmayr et al[1], four cases suffered from hepatic dysfunction because of the delayed liver hemoperfusion. Although 3 cases received liver transplantation, they were all died of liver failure finally. In our study, the patients 1 and 2 are still alive without recurrence. The third case was complicated with acute liver failure because of outflow obstruction of hepatic vein after operation. Although emergency hepatic vein outflow extending operation and assistant LDLT were performed the next day, he died of liver and kidney failure at last. Therefore, it is important to reconstruct the outflow tract of hepatic vein for ex-vivo hepatectomy.
In conclusion, the ex-situ liver surgery under hypothermic perfusion and without veno-venous bypass extends the group of surgical patients, and offers a chance for the patients with unresectable liver tumor by traditional procedures. But further clinical studies are needed to verify the feasibility of this technique in clinical practice.
Primary liver malignancies together with metastatic liver tumors are the most common tumor types in human. Experimental studies are being conducted in hepatic resection with ex-situ hypothermic perfusion if huge lesions are located in hepatic hilum or hepatic vein confluence, which involved and/or infiltrated main hepatic vein and inferior vena cava (IVC).
In some patients, the degree of the IVC and/or main hepatic vein involved by tumors is so extensive that it is impossible to resect the tumors by the routine hepatectomy. The ex-situ liver surgery, vein cava replacement and hepatic autotransplantation without veno-venous bypass have been performed.
The extracorporeal hypothermic parenchymal resection was to extend the oncological radicality by achieving an R0 margin with a better tumor-free cutting edge. But ex-situ liver surgery with veno-venous bypass aggravates ischemia-reperfusion injury of liver and results in liver failure. To resolve this problem, the authors performed ex-situ liver surgery without veno-venous bypass in three cases with tumors involving IVC based on animal experiments.
Ex-vivo liver resection and liver autotransplantation has shown a potential for treatment of complicated hepatic neoplasms that are unresectable by traditional procedures.
The authors described their experience in hepatic resection with total vascular exclusion, ex-situ liver surgery, without the need of veno-venous bypass because of immediate replacement of inferior vena cava. The topic is of interest in the specific surgical field.
Peer reviewer: Chih-Chi Wang, MD, Department of Surgery, Chang Gung Memorial Hospital- Kaohsiung Medical Center, 123 Ta-Pei Road, Niao-Sung, Kaohsiung 833, Taiwan, China
S- Editor Lv S L- Editor Ma JY E- Editor Li JY
| 1. | Pichlmayr R, Grosse H, Hauss J, Gubernatis G, Lamesch P, Bretschneider HJ. Technique and preliminary results of extracorporeal liver surgery (bench procedure) and of surgery on the in situ perfused liver. Br J Surg. 1990;77:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 164] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Hannoun L, Panis Y, Balladur P, Delva E, Honiger J, Levy E, Parc R. Ex-situ in-vivo liver surgery. Lancet. 1991;337:1616-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Sauvanet A, Dousset B, Belghiti J. A simplified technique of ex situ hepatic surgical treatment. J Am Coll Surg. 1994;178:79-82. [PubMed] |
| 4. | MacKenzie S, Dixon E, Bathe O, Sutherland F. Intermittent hepatic vein--total vascular exclusion during liver resection: anatomic and clinical studies. J Gastrointest Surg. 2005;9:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Ding YT, Sun XT, Xu QX. Non-bleeding technique in resection of hepatoma: report of 49 cases. Hepatobiliary Pancreat Dis Int. 2002;1:52-56. [PubMed] |
| 6. | Berney T, Mentha G, Morel P. Total vascular exclusion of the liver for the resection of lesions in contact with the vena cava or the hepatic veins. Br J Surg. 1998;85:485-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Mizuno S, Kato H, Azumi Y, Kishiwada M, Hamada T, Usui M, Sakurai H, Tabata M, Shimpo H, Isaji S. Total vascular hepatic exclusion for tumor resection: a new approach to the intrathoracic inferior vena cava through the abdominal cavity by cutting the diaphragm vertically without cutting the pericardium. J Hepatobiliary Pancreat Sci. 2010;17:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Malde DJ, Khan A, Prasad KR, Toogood GJ, Lodge JP. Inferior vena cava resection with hepatectomy: challenging but justified. HPB (Oxford). 2011;13:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Huguet C, Nordlinger B, Galopin JJ, Bloch P, Gallot D. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689-693. [PubMed] |
| 10. | Choi J, Lee YJ, Hwang DW, Chon SH, Nagpal A, Park KM. Surgical treatment of giant hepatic hemangiomas: technical point of view. Am Surg. 2011;77:48-54. [PubMed] |
| 11. | Knubben K, Thiel C, Schenk M, Etspüler A, Schenk T, Morgalla MH, Königsrainer A. A new surgical model for hepatectomy in pigs. Eur Surg Res. 2008;40:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Maeba T, Okano K, Mori S, Karasawa Y, Goda F, Wakabayashi H, Usuki H, Maeta H. Retrohepatic vena cava replacement of hepatic malignancies without using total hepatic vascular exclusion or extracorporeal bypass. Hepatogastroenterology. 2001;48:1455-1460. [PubMed] |
| 13. | Facciuto ME, Singh MK, Rocca JP, Rochon C, Rodriguez Davalos MI, Eshghi M, Schwalb DM, Choudhury M, Sheiner PA. Benefits of liver transplantation surgical techniques in the management of extensive retroperitoneal tumors. World J Surg. 2008;32:2403-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Azoulay D, Eshkenazy R, Andreani P, Castaing D, Adam R, Ichai P, Naili S, Vinet E, Saliba F, Lemoine A. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg. 2005;241:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Brekke IB, Mathisen Ø, Line PD, Hauss HJ. Hepatic autotransplantation with ex situ neoplasm extirpation and vena cava replacement. Hepatogastroenterology. 2003;50:2169-2172. [PubMed] |
| 16. | Gruttadauria S, Marsh JW, Bartlett DL, Gridelli B, Marcos A. Ex situ resection techniques and liver autotransplantation: last resource for otherwise unresectable malignancy. Dig Dis Sci. 2005;50:1829-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Kumada K, Yamaoka Y, Morimoto T, Tanaka K, Moriyasu F, Yamaguchi T, Mori K, Tanaka A, Ozawa K. Partial autotransplantation of the liver in hepatocellular carcinoma complicating cirrhosis. Br J Surg. 1992;79:566-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Oldhafer KJ, Lang H, Schlitt HJ, Hauss J, Raab R, Klempnauer J, Pichlmayr R. Long-term experience after ex situ liver surgery. Surgery. 2000;127:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Azoulay D, Andreani P, Maggi U, Salloum C, Perdigao F, Sebagh M, Lemoine A, Adam R, Castaing D. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Yagyu T, Shimizu R, Nishida M, Nakashima K, Uchiyama T, Suzuki T. Reconstruction of the hepatic vein to the prosthetic inferior vena cava in right extended hemihepatectomy with ex situ procedure. Surgery. 1994;115:740-744. [PubMed] |
| 21. | Hemming AW, Reed AI, Langham MR, Fujita S, van der Werf WJ, Howard RJ. Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg. 2002;235:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Lechaux D, Megevand JM, Raoul JL, Boudjema K. Ex vivo right trisegmentectomy with reconstruction of inferior vena cava and "flop" reimplantation. J Am Coll Surg. 2002;194:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Cabezuelo JB, Ramirez P, Acosta F, Torres D, Sansano T, Pons JA, Bru M, Montoya M, Rios A, Sánchez Bueno F. Does the standard vs piggyback surgical technique affect the development of early acute renal failure after orthotopic liver transplantation? Transplant Proc. 2003;35:1913-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Lodge JP, Ammori BJ, Prasad KR, Bellamy MC. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Raab R, Schlitt HJ, Oldhafer KJ, Bornscheuer A, Lang H, Pichlmayr R. Ex-vivo resection techniques in tissue-preserving surgery for liver malignancies. Langenbecks Arch Surg. 2000;385:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |