Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7285
Revised: August 8, 2011
Accepted: August 15, 2011
Published online: December 28, 2012
AIM: To investigate the growth-inhibiting and apoptosis-inducing effects of the gene MOB2 on human hepatic carcinoma cell line SMMC-7721.
METHODS: The full-length cDNA of the MOB2 gene was amplified from human umbilical vein endothelial cells. The correct full-length MOB2 cDNA was subcloned into the eukaryotic expression vector pEGFP-C1. After lipofection of the MOB2 gene into cancer cells, the levels of MOB2 protein in the cancer cells were detected by immunoblotting. To transfect the recombined plasmid vector pEGFP-CI-MOB2 into SMMC-7721 cells, the cells were cultured in Dulbecco’s Modified Eagle’s Medium with 10% fetal calf serum and glutamine, and then mixed with liposomes, Lipofectamine 2000 and the plasmid vector pEGFP-CI-MOB2.
RESULTS: We observed the growth and proliferation of SMMC-7721 cells containing pEGFP-CI-MOB2 and analyzed their apoptosis and growth cycle phases by flow cytometry. We successfully transfected the recombined plasmid vector pEGFP-CI-MOB2 into SMMC-7721 cells and screened for a single clone cell containing MOB2. After transfection, MOB2 enhanced growth suppression, induced apoptosis, increased the ratio of G0/G1, significantly inhibited the advance of cell cycle phase, and arrested cells in G0/G1 phase.
CONCLUSION: MOB2 overexpression induces apoptosis and inhibits the growth of human hepatic cancer cells, which may be useful in gene therapy for hepatic carcinoma.
- Citation: Leng JJ, Tan HM, Chen K, Shen WG, Tan JW. Growth-inhibitory effects of MOB2 on human hepatic carcinoma cell line SMMC-7721. World J Gastroenterol 2012; 18(48): 7285-7289
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7285.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7285
Mps-one-binder (Mob) family proteins belong to a conserved gene family found in yeast[1-3], Drosophila[4,5] and mammals[6]. Mob1 protein was originally identified as a regulator of mitotic exit and cytokinesis in yeast[1]. In Drosophila, Mob1 is a key component of the Hippo signaling pathway[5,7] and thus plays a role in tumor suppression. Mob2 binds to Cbk1, a conserved protein kinase that is similar to human myotonic dystrophy kinase. Mob2 promotes polarized cell growth and induces asymmetric cell fate in fission yeast[3,8-10]. In Drosophila, the Mob2 protein binds directly to the Tricornered protein, an ortholog of nuclear Dbf2-related (Ndr) kinase[11]. Previous work has also shown that Dmob2 is involved in photoreceptor morphogenesis by regulating the subcellular localization of Crumbs, a cell polarity gene, and phosphorylated moesin[12]. Knockdown of Dmob2 expression causes abnormal actin rearrangement and results in abnormal rhabdomere formation, which suggests that Dmob2 may regulate actin dynamics during rhabdomere formation[12].
Ndr kinase belongs to a subfamily of serine/threonine protein kinases that control cell division and morphogenesis in various cell types[13]. Ndr2 is induced in the mouse amygdala during fear memory consolidation. Other studies have demonstrated that this kinase plays an important role in neurite formation[14]. Tricornered, the Ndr kinase ortholog in Drosophila, interacts with actin filaments and regulates dendritic tiling and branching[11]. SAX-1, the ortholog of Ndr kinase in nematodes, activates the RhoA-GTPase signaling pathway during neurite formation[14]. These results strongly indicate the importance of Ndr kinases in neurite formation. Mob proteins have been shown to bind Ndr kinases, making them important in kinase activation[6]. In this study, we investigated what role Mob proteins play in the human hepatic carcinoma cell line SMMC-7721.
The eukaryotic expression vector pEGFP-C1 and competent cells of Escherichia coli DH5α were routinely preserved by the central laboratory of our hospital. We used the following materials in our studies: RNeasy Protect mini kit (Qiagen, Germany); SMARTTM PCR cDNA synthesis kit (Clontech, United States); DNA gel extraction kit (Dalian TaKaRa, China); plasmid mini preparation kit (Shanghai Huasun Biotechnology, China); KOD-Plus DNA polymerase (TOYOBO, United States); T4 DNA ligase and restriction enzymes (HindIII and KpnI) (New England Biolabs, United States); Lipofectamine 2000 (Invitrogen, United States); rabbit anti-MOB2 monoclonal antibody (Santa Cruz Biotechnology, United States); and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Zhongshan, China). Nucleic acid sequencing was performed by Shanghai Yingjun Bioengineering (China). MOB2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were synthesized by Shanghai Yingjun Bioengineering.
Total RNA was isolated from human umbilical vein endothelial cells. Full-length MOB2 cDNA was amplified by reverse-transcriptase polymerase chain reaction. The primer pairs for MOB2 fragment amplification were designed based on the MOB2 mRNA sequence provided by GenBank using the Premier Primer 5.0 software package. The primer sequences used were as follows:
5’-CCCAAGCTTCCATGGACTGGCTCATGGGGAAGTC-3’ (forward primer) and 5’-CGGGGTACCTCATCTCCTTCACGTGGTTCTG-3’ (reverse primer). The reaction conditions were as follows: 94 °C for 1 min, 94 °C for 30 s, 60 °C for 30 s, and 68 °C for 75 s. After 35 amplification cycles, the product was extended at 68 °C for 2 min. After removal of the pEGFP cDNA with HindIII and KpnI, the amplified fragment was inserted into pEGFP-C1 to make the expression vector pEGFP-C1-MOB2.
SMMC-7721 cells were maintained in Dulbecco’s Modified Eagle’s Medium containing 10% calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified chamber at 37 °C with 5% CO2. Transient transfections were performed. Gene transfer was conducted using the liposome-mediated method (Lipofectamine 2000; Invitrogen, United States) according to the manufacturer’s instructions. Blank control, empty vector (pEGFP-C1) and pEGFP-C1-MOB2 transfection groups were constructed in this experiment.
To harvest cells under non-denaturing conditions, we removed the medium and rinsed the cells with ice-cold PBS. Ice-cold lysis buffer (500 µL) was added to each well. The cells were scraped from the surface and transferred to an Eppendorf tube on ice. Each sample was then sonicated four times for 5 s while being kept cold. The mixture was centrifuged for 10 min at 4 °C, and the supernatant was saved as lysate for later use. The proteins (150 µg) in the lysate were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (10% polyacrylamide gel containing 0.1% SDS) and then transferred to a polyvinylidene difluoride membrane by electrophoretic blotting. The membranes were probed with specific antibodies and then treated with anti-rabbit IgG-HRP (1:2000). Protein bands were visualized by enhanced chemiluminescence using the procedure recommended by the manufacturer (Thermo Scientific, United States). The expression of GAPDH was measured as a control.
Cell cycle parameters were determined by propidium iodide labeling of nuclear DNA. Cells (106) were trypsinized and resuspended in 70% ethanol before being stored for later use. The cells were treated with RNase and Triton X-100 as well as with propidium iodide to label the nuclear DNA. The labeled cells were analyzed by flow cytometry at 630 nm for propidium iodide. The cell cycle parameters were determined using the DNA cell cycle analysis in the software provided by the manufacturer (Invitrogen).
SMMC-7721 cells were trypsinized and seeded into 24-well plates at a density of 105/mL. After transfection, cells were digested and counted using trypan blue staining under an inverted light microscope for five consecutive days.
Approximately 100 cells were added to each well of a six-well culture plate. After incubation at 37 °C for 15 d, cells were washed twice with PBS and stained with Giemsa solution. The number of colonies containing ≥ 50 cells was counted under a microscope [plate clone formation efficiency = (number of colonies/number of cells inoculated) × 100%]. Each experiment was performed in triplicate.
Student’s t test and analysis of variance were performed for statistical analysis with SPSS version 10.0.
After the recombinant plasmid pEGFP-C1-MOB2 was digested by both HindIII and KpnI, two bands appeared in the gels, with the larger band at about 3945 bp and the smaller one at 734 bp, showing that the eukaryotic expression vector had been successfully constructed. The results of nucleic acid sequencing indicated that the full-length Chinese MOB2 cDNA had been cloned successfully. BLAST analysis demonstrated that the cloned sequence was 100% homologous to the previously reported MOB2 sequence.
Western blotting indicated that uninfected SMMC-7721 cells and empty-vector-infected SMMC-7721 cells showed only faint traces of MOB2 gene product. In contrast, a bright MOB2 band was obtained from pEGFP-C1-MOB2-infected SMMC-7721 cells. Western blotting indicated that 72 h after transfection with pEGFP-C1-MOB2, MOB2 protein expression increased by 3.8 times compared with that of the control group (P < 0.01). These results suggested that the transduction of the MOB2 gene was successful and that expression of the protein was significantly higher after transfection (Figure 1).
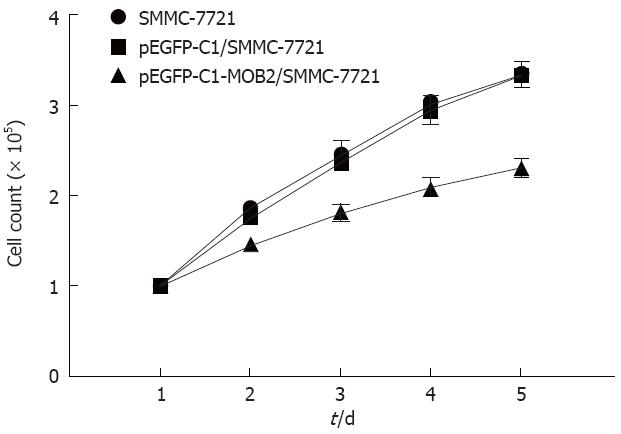
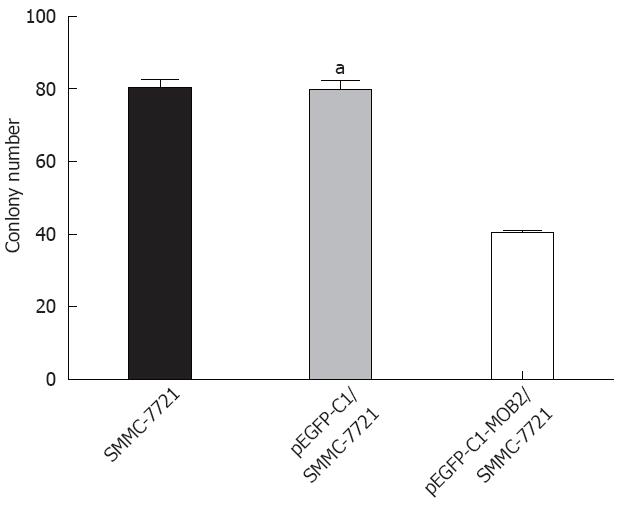
To analyze the function of MOB2, we studied the rate of cell proliferation of MOB2-expressing SMMC-7721 cells. The growth curves, as determined by a trypan blue staining assay, showed that MOB2 significantly inhibited proliferation of SMMC-7721 cell lines when compared with parental SMMC-7721 cells and control clone cells (Figure 2). A colony formation assay showed that MOB2-overexpressing SMMC-7721 cells formed significantly fewer colonies than control clone cells (P < 0.001) (Figure 3), suggesting an inhibitory effect of MOB2 on human hepatic carcinoma SMMC-7721 cells.
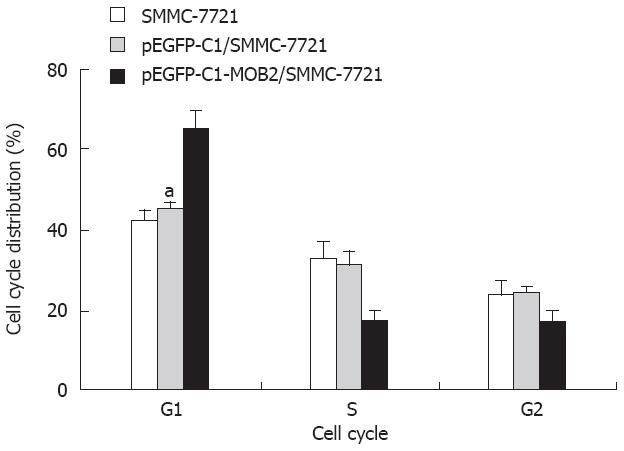
To determine the effect of MOB2 on the cell cycle, we measured cell cycle distribution in MOB2-expressing SMMC-7721 cells. In these cell lines, the S-phase population was markedly decreased while the G1 population was significantly increased compared with the control-vector cells and the SMMC-7721 cells (P < 0.01). Neither cell line had significant changes in the G2 population (Figure 4, Table 1).
| Group | Cell cycle | ||
| G1 | S | G2 | |
| SMMC-7721 | 42.41 ± 2.32 | 32.52 ± 4.53 | 23.54 ± 3.42 |
| pEGFP-Cl/SMMC-7721 | 45.45 ± 1.32 | 31.3 ± 3.11 | 24.24 ± 1.32 |
| pEGFP-Cl-MOB2/SMMC-7721 | 65.33 ± 4.34 | 17.33 ± 2.65 | 16.82 ± 3.14 |
Ndr kinase belongs to a subfamily of serine/threonine protein kinases that control cell division and morphogenesis in various cell types[13]. Ndr2 is induced in the mouse amygdala during fear memory consolidation. Further studies have demonstrated that this kinase plays an important role in neurite formation[14]. Tricornered, the Ndr kinase ortholog in Drosophila, interacts with actin filaments and regulates dendritic tiling and branching[11]. SAX-1, the ortholog of Ndr kinase in nematodes, activates the RhoA-GTPase signaling pathway during neurite formation[14]. These results strongly indicate the importance of Ndr kinases in neurite formation. Mob proteins have been shown to bind Ndr kinases, making them important in kinase activation[6]. These results suggest that MOB2 might play a suppressive role in tumor pathogenesis.
To examine whether MOB2 plays a suppressive role in cancer cells, we applied a gain-of-function approach by introducing MOB2 into cells to investigate its biological function. SMMC-7721 cells were transfected with a MOB2-pEGFP-expressing eukaryotic vector and selected using G418. We successfully established cell lines that stably expressed MOB2 protein at dramatically elevated levels compared with control cells. Subsequent functional studies demonstrated that overexpression of MOB2 led to G1/S transition blockage and significantly reduced in vitro cell growth.
A tumor suppressor gene is a gene that slows down cell growth and thus reduces the probability that a cell in a multicellular organism will turn into a tumor cell. A mutation or deletion of such a gene increases the probability of the formation of a tumor and fails to keep cancer from growing. The results of the present study indicate that the MOB2 gene plays a role in carcinogenesis and may be a candidate tumor suppressor gene. Furthermore, our results clearly suggest that overexpression of MOB2 can inhibit proliferation and result in apoptosis of ovarian cancer cells. Therefore, reactivation of the MOB2 signaling pathway might provide an effective gene therapy strategy for human hepatic carcinoma.
Some reports suggest that knockdown of MOB2 expression causes abnormal actin rearrangement and results in abnormal rhabdomere formation in Drosophila. However, few reports exist regarding the effects in MOB2 on cancer cells. In this study, the authors investigated what role the MOB2 proteins play in the human hepatic carcinoma cell line, SMMC-7721.
In this study, by cloning the MOB2 gene and transferring it into hepatocellular carcinoma cell line SMMC-7721, the authors investigated the growth-inhibiting and apoptosis-inducing effects of MOB2 gene on carcinoma cells and concluded that the over-expression of MOB2 gene could induce apoptosis and inhibit the growth of hepatic carcinoma cell line SMMC-7721.
These results suggest that MOB2 functions as an inhibitor of hepatic carcinoma and might serve as a new novel biological target to cure hepatic carcinoma.
Mps-one-binder (Mob) family proteins belong to a conserved gene family found in yeast, Drosophila, and mammals. In Drosophila, MOB2 acts as a tumor suppressor through its function as a key component of the Hippo signaling pathway.
In this study, the authors applied a gain-of-function approach to examine the biological processes regulated by MOB2 in SMMC-7721 cells. They demonstrated the functional importance of MOB2 in the suppression of SMMC-7721 cell growth. This is an important result, and it can bring out a new field in clinical tumor treatment.
S- Editor Lv S L- Editor Kerr C E- Editor Zhang DN
| 1. | Luca FC, Winey M. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol Biol Cell. 1998;9:29-46. [PubMed] |
| 2. | Salimova E, Sohrmann M, Fournier N, Simanis V. The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signalling the onset of septum formation. J Cell Sci. 2000;113:1695-1704. [PubMed] |
| 3. | Colman-Lerner A, Chin TE, Brent R. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell. 2001;107:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 271] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | He Y, Emoto K, Fang X, Ren N, Tian X, Jan YN, Adler PN. Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol Biol Cell. 2005;16:4139-4152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor. Mats Cell. 2005;5:675-685. |
| 6. | Devroe E, Erdjument-Bromage H, Tempst P, Silver PA. Human Mob proteins regulate the NDR1 and NDR2 serine-threonine kinases. J Biol Chem. 2004;279:24444-24451. [PubMed] |
| 7. | Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772-1781. [PubMed] |
| 8. | Racki WJ, Bécam AM, Nasr F, Herbert CJ. Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae. EMBO J. 2000;19:4524-4532. [PubMed] |
| 9. | Weiss EL, Kurischko C, Zhang C, Shokat K, Drubin DG, Luca FC. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J Cell Biol. 2002;158:885-900. [PubMed] |
| 10. | Hou MC, Wiley DJ, Verde F, McCollum D. Mob2p interacts with the protein kinase Orb6p to promote coordination of cell polarity with cell cycle progression. J Cell Sci. 2003;116:125-135. [PubMed] |
| 11. | Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245-256. [PubMed] |
| 12. | Liu LY, Lin CH, Fan SS. Function of Drosophila mob2 in photoreceptor morphogenesis. Cell Tissue Res. 2009;338:377-389. [PubMed] |
| 13. | Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253-264. [PubMed] |
| 14. | Stork O, Zhdanov A, Kudersky A, Yoshikawa T, Obata K, Pape HC. Neuronal functions of the novel serine/threonine kinase Ndr2. J Biol Chem. 2004;279:45773-45781. [PubMed] |