Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.7009
Revised: August 2, 2012
Accepted: August 4, 2012
Published online: December 21, 2012
AIM: To identify the determinants of endoscopic submucosal dissection (ESD) operation time.
METHODS: This investigation was conducted as a single-center, prospective study in which ESD was performed by the same endoscopist at the Chinese PLA General Hospital. A total of 173 patients underwent ESD operations performed by Dr. Lu from July 2007 to December 2011, and 183 lesions were enrolled. Patient gender, age, tumor location, gross type, tumor size, pathological type and adhesions were recorded prospectively. The order of treatment represented the experience of the operator. Univariate analysis and multivariate analysis were performed to evaluate the relationships between these factors and ESD procedure time.
RESULTS: Univariate analysis showed the ESD time was closely related to the gender (P = 0.0210), tumor size (P < 0.0001), location (P < 0.0001), gross type (P < 0.0001) and adhesion (P = 0.0010). The surgical proficiency level was associated with ESD time in unit area (P < 0.0001). Multivariate analysis revealed that the ESD time was positively correlated with tumor size (P < 0.0001), adhesion (P < 0.0001) and location (P < 0.0001), but negatively correlated with surgical proficiency level (P = 0.0046).
CONCLUSION: Large tumor size, adjacency to the cardia, and adhesion are predictors of a long ESD time, whereas high surgical proficiency level predicts a short ESD time.
- Citation: Lu ZS, Yang YS, Feng D, Wang SF, Yuan J, Huang J, Wang XD, Meng JY, Du H, Wang HB. Predictive factors of endoscopic submucosal dissection procedure time for gastric superficial neoplasia. World J Gastroenterol 2012; 18(47): 7009-7014
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/7009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.7009
Endoscopic submucosal dissection (ESD) has become a mature technique widely applied in the treatment of mucosal lesions. ESD has advantages compared with endoscopic mucosal resection. However, ESD is complex, and the associated high risk is another disadvantage of ESD that requires improvement[1-7]. The time required to perform ESD is one of the best indicators of operation difficulty. Studies have demonstrated ESD time is closely related to bleeding, perforation and post-operative pneumonia[8-12]. It is the goal of clinicians to overcome operation difficulty and reduce ESD time. Previous studies have reported on the determinants of ESD time, but these results reflected the operations conducted by several clinicians[13,14]. In the present study, ESD was performed by the same endoscopist. A total of 183 lesions were collected, and the determinants of ESD time were analyzed.
From July 2007 to December 2011, consecutive gastric superficial intraepithelial neoplasia (GIN) was treated by ESD that were performed by the same endoscopist at the Chinese PLA General Hospital. This study was approved by the medical ethical committee Chinese PLA General Hospital and the clinical study was registered with the Clinical Trial (NCT01378507). Partial resection, piecemeal resection and snare resection after circumferential cutting was excluded and complete en bloc resection was included in the study.
The morphology of the lesions of all patients was superficial intraepithelial neoplasia according to the Paris endoscopic classification[15]. Adenoma, noninfiltrating carcinoma or intramucosal invasive carcinoma were confirmed by histologic evaluation of forceps biopsy specimens, which corresponding to criteria of no lymph node metastases[16].
Patients were excluded from the study if they had a previous diagnosis of undifferentiated carcinoma, or Type 0-III or 0-Is adenocarcinoma. Systemic conditions were evaluated before each operation, and those with contraindications for anesthesia and endoscopy were excluded from the study. The existing strategies for the treatment of GIN and their advantages and disadvantages were explained to the patients and their family members before the operation. Written informed consent was obtained before ESD.
The patients were sedated by intravenous injection of propofol (0.1-0.2 mg/kg per minute) (Xi’an Libang Pharmaceutical Co., Ltd. Xi’an, China). A gastroscope (GIF Q260J, Olympus Optical Co., Ltd, Tokyo, Japan) was used for the treatment, which was facilitated with a soft transparent front cap (Olympus Optical Co, Ltd.) and a high-frequency surgical unit for cutting and coagulation (Erbotom VIO200; ERBE, Tübingen, Germany). A normal-saline solution of 10% glycerin and 5% fructose with epinephrine (0.025 mg/mL) was used for submucosal injections.
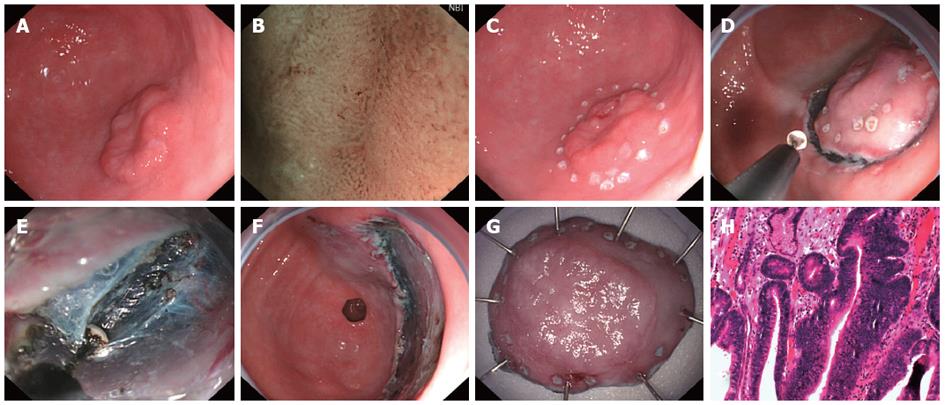
The first step was locating the lesion using gastroscopy with narrow-band imaging magnification to observe and distinguish the border between the lesion and normal mucosa. The border was marked with thermal coagulation markers at 0.5 cm intervals at a distance of 0.3 to 0.5 cm from the edge with a needle knife (KD-1L; Olympus Optical Co. Ltd., Tokyo, Japan). The glycerol fructose solution was then injected submucosally around the lesion to raise the mucosa thoroughly. Next, a pre-cut was made with needle knife to cut the mucosa through to the submucosa. Then insulation tipped (IT-2) knife (KD-610L; Olympus Optical Co, Ltd.) was inserted into the pre-cut incision to complete the incision around the lesion at 0.5 cm outside the markers (Endo cut Q 2). After the lesion was cut in a circle, submucosal injection was continued, and the mucosa was gradually dissected with an IT-2 knife (foced coag 40W) until the mucosa was completely excised. A needle knife or IT-2 knife was used for hemostasis of small blood vessels (foced coag 40W); an electric coagulation hemostat (FD-410LR; Olympus Optical Co., Ltd.) was used for hemostasis of large blood vessels (soft coag 80W). The ESD procedure is showed in Figure 1.
ESD time was defined as the time from circumferential marking around the lesion to the complete removal of GIN. The number of patients was used as the continuous numerical variable, which represented the proficiency level. The tumor size was calculated with two vertical maximum diameters.
The age, gender, number of patients, location, macroscopic appearance, tumor size, pathological type and adhesion were recorded as variables. Normally-distributed data were expressed as mean ± SD, and data not conforming to a normal distribution were expressed as medians and inter-quartile ranges. The categorical variables were presented as constituent ratios. One way analysis of variance was employed to analyze the factors influencing procedure time. Simple correlation analysis was performed to investigate the association between numerical variables and procedure time. A rank sum test was used to investigate the association between categorical variables and procedure time. A value of P < 0.05 was considered statistically significant. Factors meeting the criteria for significance in the univariate analysis were included in the multivariate analysis. Multiple linear regressions were used to analyze the association between procedure time and factors influencing procedure time. The procedure time served as the dependent variable and those from univariate analysis as independent variables. Factors with a significant difference, as determined by multivariate analysis, were included in a step-forward linear regression model to compose a predictive formula of procedural time. In addition, 0.10 and 0.05 were applied for backward selection and forward selection, respectively. Statistical analysis was performed with SPSS version 13.0.
A total of 194 GIN lesions were treated by ESD, and 183 GIN lesions were completely removed (94.3%). In addition, 178 lesions met the criteria for curative resection (91.8%). In the present study, 173 cases were recruited, and a total of 183 lesions were collected. There were 126 males and 47 females, with a mean age of 62.0 years (29-82 years). The major diameter of the resected lesions was 3.0 cm (1.5-8.0 cm). The median area of resected mucosa was 7.0 cm2 (1.5-33.25 cm2). The median procedure time was 41 min (8-221 min). Bleeding to different extents was observed during the operation but did not meet the criteria for bleeding as a complication[17]. Delayed bleeding was observed in 7 cases, which resolved after endoscopic hemostasis (n = 5) or pharmacotherapy (n = 2).
Small muscularis propria resection was identified in 2 cases, but the serosa was intact and no pneumoperitoneum was observed. Because the defect was closed with the used of endoscopic clips after the en bloc dissection, the clip closure time was not including the ESD procedure time and was not analyzed as a risk factor.
Univariate analysis showed that the procedure time was related to tumor size, but not to age or the level of surgical proficiency. The procedure time was significantly different among groups in terms of gender, location, macroscopic appearance and adhesion. No marked differences were noted among groups in terms of pathological types.
The irrelevance of some factors to ESD time may be attributed to small tumor size at early stage. For the same size lesion, the more experienced of the operator, the shorter of procedure time. Once ESD time per unit area was used as a factor, the influence of resection area was abolished. The ESD procedure time should be related to the proficiency of endoscopist because the resection size was increased as technical proficiency increased so the ESD procedure time was also increased although univariate analysis showed no correlation. If the ratio of procedure time to specimen area was transformed unit area, the ESD procedure time related to the proficiency of endoscopist and the number of procedure cases was conformed by univariate analysis because of elimination of interference of lesion area. So the number of procedure cases was independent predictors in multivariate analysis. (Tables 1 and 2).
| Predictive factors | Data | ESD time (min)median (IQR) | Analysis method | P value |
| Age | mean ± SD | 41.00 (39.00) | Pearson correlation | 0.3135 |
| 62.00 ± 11.69 | ||||
| Tumor size (cm2) | Median (IQR) | 41.00 (39.00) | Pearson correlation | < 0.0001 |
| 7.00 (8.82) | ||||
| Proficiency level | n | ESD time per unit area(min) | Pearson correlation | < 0.0001 |
| 1-183 | 5.00 ± 3.87 |
| Predictive factors | n (%) | ESD time (min)median (IQR) | Analysis method | P value |
| Location | Kruskal wallis | < 0.0001 | ||
| Upper | 44 (24.0) | 52.00 (69.00) | ||
| Middle | 52 (28.4) | 48.00 (33.00) | ||
| Lower | 87 (48.6) | 26.00 (33.50) | ||
| Macroscopic appearance | Kruskal wallis | < 0.0001 | ||
| Protrusion | 76 (41.5) | 23.00 (31.00) | ||
| Indentation | 61 (33.3) | 49.00 (31.00) | ||
| Mixed | 46 (25.2) | 50.00 (43.50) | ||
| Pathological type | Kruskal wallis | 0.2410 | ||
| Adenoma | 36 (19.7) | 25.00 (27.00) | ||
| HIN | 97 (53.0) | 29.00 (36.00) | ||
| Invasive carcinoma | 50 (27.3) | 51.00 (29.50) | ||
| Adhesion | Mann-whitney | 0.0010 | ||
| Yes | 14 (7.7) | 81.50 (149.00) | ||
| No | 179 (92.3) | 39.00 (35.00) | ||
| Gender | Mann-whitney | 0.0210 | ||
| Male | 134 (73.2) | 47.00 (41.00) | ||
| Female | 49 (26.8) | 29.00 (25.50) |
Multiple linear regression analysis revealed that ESD time was positively correlated with tumor size, adhesion and location, but was negatively associated with surgical proficiency level. After comparison with the standardized regression coefficients, the correlation intensity was in the following order: tumor size, adhesion, lesions in upper stomach and proficiency level. Although multiple linear regression analysis showed the ESD procedure time was not correlation with the lesions in the middle of the stomach, the influence of lesions in the middle stomach should be used in prediction the time because ESD procedure time was related to the lesions in the upper stomach. Statistical analysis revealed tumor size and proximity to the cardia were positively correlated with longer ESD times. In addition, a submucosal adhesion could prolong ESD time. With the increase of proficiency level, ESD time was reduced. The results of multiple linear regressions are displayed in Table 3.
| Predictive factors | Beta | Standardized coefficients beta | 95%CI for beta | P value |
| Resection area | 3.500 | 0.581 | 2.829-4.180 | < 0.0001 |
| Adhesion | 67.082 | 0.356 | 47.003-86.285 | < 0.0001 |
| Location (upper) | 39.439 | 0.345 | 25.767-50.124 | < 0.0001 |
| Proficiency level | -0.755 | -0.207 | -0.545-(-0.124) | 0.0046 |
| Location (middle) | 7.009 | 0.059 | -2.890-17.778 | 0.1408 |
In the present study, ESD was performed by a single endoscopist. The complete resection rate, curative resection rate, and incidence of complications were similar to those previously reported[18-25]. The procedures of ESD by the same operator were identical among different patients. Thus, the influence of procedural differences on ESD time was excluded. We tried our best to reduce pre-operative confounding factors, and then performed univariate and multivariate analysis. The analysis of information from operations performed by the same operator can help to identify the association between proficiency level and ESD time.
The pre-operative predictable factors served as variables for analysis, and the results can serve as a reference for predicting ESD time. Although an adhesion is only found during surgery, the scar and the cushion following submucosal injection can be identified to determine the adhesion. In the recruitment of patients, the indications were restricted and the post-operative pathology only confirmed two lesions with submucosal invasion. The remaining lesions were only found in the tunica mucosa. Lesions confined to the tunica mucosa may not affect the procedure time regardless of tumor depth. Thus, tumor depth was not applied as a viable.
Studies have shown that ESD time is related to the location and size of tumors. However, some studies are derived from empirical analysis, and some from univariate analysis[26]. Goto et al[13] investigated 222 early gastric cancers, which were resected with a Flex knife by four operators. Their results showed that location in the upper-third of the stomach, the presence of ulcerative findings, and > 20 mm in size was independent factors affecting ESD time. In the study by Ahn et al[14] complete ESDs were performed by four experts, primarily using an IT knife, for 916 early gastric cancers. The results revealed that proximal location, tumor size greater than 20 mm, submucosal fibrosis, and perforation during the procedure were independent predictors of a longer ESD time. In the present study, the multivariate analysis indicated that ESD time was positively correlated with tumor size, location and adhesion, but negatively associated with proficiency level. The correlation intensity was in the following order: resection area, adhesion, location in the upper-third of the stomach and the proficiency level.
The factors identified by the multivariate analysis can be applied to predict ESD time. However, the determinants are numerous, and some are unpredictable before an operation, including unstable anesthesia, equipment failure, and heavy bleeding. Therefore, ESD time cannot be accurately determined according to the factors identified in the present study. These factors may only be used to predict ESD time and the level of difficulty. These findings suggest that lesions with small size and without adhesion are suitable for the training of inexperienced endoscopists. The resection of lesions with larger size located in the upper to middle stomach should be performed by experienced operators or in the presence of experienced operators. Once it is determined that the predicted ESD time is relatively long, preventive measures should be prepared before operating, with the aim of preventing skin compression and deep vein thrombosis of the lower extremities. With respect to anesthesia, intubation followed by general anesthesia is preferred. Maintaining an open airway helps to assure satisfactory anesthesia status.
Although analysis of clinical information from operations performed by the same operator can exclude the influence of other confounding factors, such as different instruments and operation habits, we only analyzed the data from a single center and therefore the sample size was relatively small. Our experience revealed that the time consumed maintaining hemostasis can affect ESD time. However, this period of time is unpredictable and cannot be accurately recorded. Therefore, it was not used as a variable for analysis.
Taken together, our results revealed that large tumor size, adjacency to the cardia, and adhesion predict a long ESD time, but high a proficiency level predicts a short ESD time. Our results provide reference for the prediction of operation difficulty and ESD time.
Endoscopic submucosal dissection (ESD) is a therapeutic technique for the treatment of gastrointestinal neoplasms with high en bloc resection rate. However, owing to its technical difficulty, longer procedure time and increase risk of perforation, ESD is not as widely used in China. The procedure time of ESD is the most direct indicator of operation difficulty. An investigation of the determinants of the time required for ESD will offer guidance in predicting operation difficulty.
Many investigators have shown that ESD time is related to the location and size of tumors. In the present study, the multivariate analysis indicated that ESD time was positively correlated with tumor size, location and adhesion, but negatively associated with proficiency level.
In this study, 183 ESD procedures were performed by a single endoscopist. Analysis of clinical information from operations performed by the same operator can exclude the influence of other confounding factors, such as different instruments and operation habits.
This study revealed that large tumor size, adjacency to the cardia, and adhesion predict a long ESD time, but high a proficiency level predicts a short ESD time. The results provide reference for the prediction of operation difficulty and ESD time.
The ESD technique was first introduced in Japan. ESD is an innovative technique that improves the rate of successful en bloc resection of gastrointestinal neoplasms.
This is a study done in one center by a single endoscopist, which in this case is a strength. The manuscript is well presented and easy to read.
Peer reviewer: Javier San Martín, Chief, Gastroenterology and Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del Este 20100, Uruguay
S- Editor Lv S L- Editor A E- Editor Zhang DN
| 1. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776-782. [PubMed] |
| 3. | Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, Wada T, Kubota E, Yamada T, Mori Y. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol. 2005;3:S74-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kanao H, Kawamura T, Yoshida S, Yoshihara M, Chayama K. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996-1000. [PubMed] |
| 7. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [PubMed] |
| 8. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [PubMed] |
| 9. | Isomoto H, Ohnita K, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Nakao K, Kohno S. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer. Eur J Gastroenterol Hepatol. 2010;22:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Lee CT, Chang CY, Tai CM, Wang WL, Tseng CH, Hwang JC, Lin JT. Endoscopic submucosal dissection for early esophageal neoplasia: a single center experience in South Taiwan. J Formos Med Assoc. 2012;111:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26:2456-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Is it possible to predict the procedural time of endoscopic submucosal dissection for early gastric cancer? J Gastroenterol Hepatol. 2009;24:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH, Jung HY. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [PubMed] |
| 16. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1326] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 17. | Lee TH, Cho JY, Chang YW, Kim JO, Lee JS, Cho WY, Kim HG, Kim WJ, Park YS, Jin SY. Appropriate indications for endoscopic submucosal dissection of early gastric cancer according to tumor size and histologic type. Gastrointest Endosc. 2010;71:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy. 2009;41:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 21. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 520] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 22. | Chiu PW, Teoh AY, To KF, Wong SK, Liu SY, Lam CC, Yung MY, Chan FK, Lau JY, Ng EK. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc. 2012;26:3584-3591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Ohta T, Ishihara R, Uedo N, Takeuchi Y, Nagai K, Matsui F, Kawada N, Yamashina T, Kanzaki H, Hanafusa M. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc. 2012;75:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc. 2012;75:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Sugimoto T, Okamoto M, Mitsuno Y, Kondo S, Ogura K, Ohmae T, Mizuno H, Yoshida S, Isomura Y, Yamaji Y. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J Clin Gastroenterol. 2012;46:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |