Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.6900
Revised: June 14, 2012
Accepted: June 28, 2012
Published online: December 21, 2012
A number of congenital and acquired disorders require esophageal tissue replacement. Various surgical techniques, such as gastric and colonic interposition, are standards of treatment, but frequently complicated by stenosis and other problems. Regenerative medicine approaches facilitate the use of biological constructs to replace or regenerate normal tissue function. We review the literature of esophageal tissue engineering, discuss its implications, compare the methodologies that have been employed and suggest possible directions for the future. Medline, Embase, the Cochrane Library, National Research Register and ClinicalTrials.gov databases were searched with the following search terms: stem cell and esophagus, esophageal replacement, esophageal tissue engineering, esophageal substitution. Reference lists of papers identified were also examined and experts in this field contacted for further information. All full-text articles in English of all potentially relevant abstracts were reviewed. Tissue engineering has involved acellular scaffolds that were either transplanted with the aim of being repopulated by host cells or seeded prior to transplantation. When acellular scaffolds were used to replace patch and short tubular defects they allowed epithelial and partial muscular migration whereas when employed for long tubular defects the results were poor leading to an increased rate of stenosis and mortality. Stenting has been shown as an effective means to reduce stenotic changes and promote cell migration, whilst omental wrapping to induce vascularization of the construct has an uncertain benefit. Decellularized matrices have been recently suggested as the optimal choice for scaffolds, but smart polymers that will incorporate signalling to promote cell-scaffold interaction may provide a more reproducible and available solution. Results in animal models that have used seeded scaffolds strongly sug- gest that seeding of both muscle and epithelial cells on scaffolds prior to implantation is a prerequisite for complete esophageal replacement. Novel approaches need to be designed to allow for peristalsis and vascularization in the engineered esophagus. Although esophageal tissue engineering potentially offers a real alternative to conventional treatments for severe esophageal disease, important barriers remain that need to be addressed.
- Citation: Totonelli G, Maghsoudlou P, Fishman JM, Orlando G, Ansari T, Sibbons P, Birchall MA, Pierro A, Eaton S, De Coppi P. Esophageal tissue engineering: A new approach for esophageal replacement. World J Gastroenterol 2012; 18(47): 6900-6907
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/6900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.6900
Recent years have witnessed great interest in regenerative medicine, the replacement, repair and regeneration of tissues and organs[1,2]. Particular interest has focused on the potential for this new field to offer new solutions for failing tissues and organs, and alternatives to transplantation, implants and reconstructive surgery, all of which have limitations.
Several conditions, both congenital and acquired, may require esophageal tissue replacement. In the pediatric population the primary indication for esophageal replacement is long-gap esophageal atresia (EA) with insufficient length for primary anastomosis. Patients with long-gap EA, which fail a primary repair, receive a denervated gastric pull-up or interposition graft using either jejunum or colon, with many associated early and late post-operative complications, such as stricture formation and the potentially carcinogenic effect of acid reflux[3,4]. In children, gastric transposition and intestinal interposition can also be used in esophageal strictures not responsive to dilatation following failed EA repair or caustic ingestion, or for rare neoplastic conditions such as inflammatory pseudotumor, leiomyosarcoma and teratoma[5]. By contrast, the commonest indication for esophageal replacement in adults is cancer, a condition whose incidence is escalating[6], whilst colon interposition is sometimes indicated for diffuse Barrett’s esophagus, a premalignant condition. Unfortunately, all of these methods of esophageal replacement severely impair the quality of life of recipient adults and children[7,8] and present problems related to donor site morbidity. Even recent developments in endoluminal resection, which removes the diseased inner layers of the esophagus through an endoscope, whilst reducing morbidity, still results in a high rate of stenosis and consequent dysphagia[9]. Despite its 60-year history, conventional organ transplantation is not a solution for the failure of every organ, due to technical and ethical issues, and is specifically unable to address the unmet needs of esophageal replacement. Thus, regenerative medicine techniques, which extend the boundaries of reconstruction and do not, in most applications, require immunosuppression, present attractive alternatives[10].
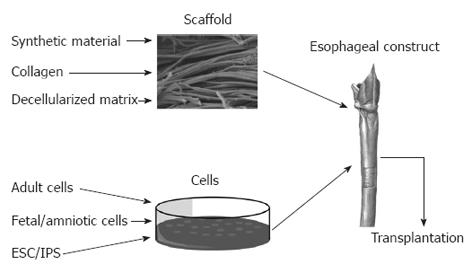
Regenerative medicine has been used to describe the use of natural human substances, such as genes, proteins, cells, and biomaterials to regenerate diseased or damaged human tissue[11,12] in order to restore normal function[2]. Tissue engineering with the end-point of organogenesis has been successful through a combination of appropriate cells with a scaffold[13-17] as well as the use of only one of these two components, for example in the repair of urethra[18] and skin[19] (Figure 1).
We review the literature relating to esophageal tissue engineering and suggest areas where research may lead to the most rapid clinical gains.
We searched Medline, Embase, the Cochrane Library, National Research Register and ClinicalTrials.gov databases, using the search terms stem cell and esophagus, esophageal replacement, esophageal tissue engineering, esophageal substitution. The reference lists of papers identified in this way were searched and further papers identified. All full-text articles in English of potentially relevant abstracts were reviewed. Finally, acknowledged experts in this field were contacted for information on gaps in our review and information on unpublished studies.
Seventy-four papers were identified and are reviewed in this manuscript. Two broad categories of intervention were identified: the use of scaffolds alone, and a combination of cells and scaffolds.
The majority of identified studies transplanted acellular scaffolds with the aim that host epithelial and smooth muscle cells will migrate to repopulate the new conduit. Acellular scaffolds studied to date conform to one of three categories: synthetic, collagen alone and decellularized matrix.
Synthetic scaffolds: Acellular synthetic scaffolds such as polyethylene plastic[20-22] and silicon[23,24] have been used for esophageal replacement, but the nature of the materials did not allow cellular migration and led to poor results in animal models. When polyvinylidene fluoride (PVDF) and polyglastin-910 (Vicryl®) were compared for the regeneration of patch defects in rabbits, PVDF was shown to lead to improved results with an absence of strictures and neo-epithelialization[25]. However, in a different study, the combination of Vicryl® and collagen brought about positive results both for patch and tubular defects in dogs, with a low mortality of 8.3%[26]. The successful use of synthetic polymers in other organs such as the trachea[27], suggests that this approach may appear attractive and further development of appropriate materials is needed.
Collagen scaffolds: In a series of experiments performed by a research group in Japan, porcine dermal collagen scaffolds were used to produce porous tubular structures (Table 1)[28-33]. The general methodology involved the use of these scaffolds to replace 5-10 cm tubular defects in the cervical or intra-thoracic portion of the esophagus in dogs. A silicon tube was used as a stent to support the scaffold until repopulation occurred. Aiming to avoid complications such as stenosis[28,29], the research group compared whether this was related to the time for which the scaffold was supported by the stent. In an experiment where three groups of dogs had a 5-cm cervical surgically created defect, the stent was removed at either 2, 3 or 4 wk. With increasing stent duration, it was observed that greater epithelial and muscle cell densities were achieved in the collagen scaffold, and this correlated with decreased stenosis and mortality[31]. However, when the collagen scaffold replaced 10-cm portions of the esophagus there was poor cellular migration in the muscular layer, suggesting that there are limitations to the size of defect that may be replaced by this methodology[30]. Moreover, when the same methods were used to replace intra-thoracic portions of the esophagus in dogs, muscular regeneration was completely absent, something the authors attributed to the lack of a vascular supply in the thorax[32]. In an attempt to address this, the scaffold was wrapped in omentum[33], as has been successfully applied to tracheal tissue engineering[34,35]. However, muscular regeneration remained absent, whilst an increase in mid-portion stenosis and mortality was observed[33].
| Animal model (n) | Results | Ref. | |||
| Scaffold type | Size | Scaffold regeneration | Clinical course | ||
| Canine (19) | Collagen with silicon stent (not removed) | 5 cm circumferential gap, cervical esophagus | Partial epithelial regeneration | 26% mortality | [28] |
| Canine (26) | Collagen with silicon stent (removed between 2 and 8 wk) | 5 cm circumferential gap, cervical esophagus | Epithelial regeneration, no stenosis | 0% mortality when stent dislodged after 4 wk (n = 4) | [29] |
| Canine (7) | Collagen with silicon stent (removed at 6 wk) | 10 cm circumferential gap, cervical esophagus | Epithelial and partial muscular regeneration, no stenosis | 29% mortality | [30] |
| Canine (43) | Collagen with silicon stent (removed either at 2, 3 or 4 wk) | 5 cm circumferential gap, cervical esophagus | Epithelial and muscular regeneration, no stenosis | 0% mortality when stent was removed at 4 wk (n = 16) | [31] |
| Canine (9) | Collagen with silicon stent (removed at 4 wk) | 5 cm circumferential gap, thoracic esophagus | Epithelial but no muscular regeneration, mid-portion stenosis | 11% mortality | [32] |
| Canine (14) | Collagen with silicon stent (removed at 4-8 wk) +/- OMPx | 5 cm circumferential gap, thoracic esophagus | Epithelial regeneration, mid-portion stenosis | 11% mortality in control group, 80% in OMPx group | [33] |
| Canine (15) | Extracellular matrix scaffold from either small intestine (n = 12) or urinary bladder submucosa (n = 3) | 5 cm semi-circumferential or 5 cm circumferential, cervical esophagus | Mucosal and muscular regeneration. Stenosis in case of complete circumferential defects | 0% mortality | [44] |
| Pigs (10) | Elastin based acellular biomaterial patch (from porcine aorta) | 2-cm circular defect, abdominal esophagus | Mucosal and muscular regeneration | 0% mortality. No complications reported in treatment groups | [43] |
| Canine (12) | Urinary bladder matrix scaffold | Complete transection with replacement of endomucosa with matrix | Mucosal and muscular regeneration | 0% mortality. No complications reported in treatment groups | [48] |
| Rats (67) | Small intestinal submucosa patch graft | Semi-circumferential defect, cervical or abdominal esophagus | Mucosal and muscular regeneration at 150 d | 94% survival at 150 d | [50] |
| Rats (85) | Small intestinal submucosa patch graft, or tube interposition | Semi-circumferential defect or segmental esophageal excision | Tube interposition unsuccessful. Mucosal and muscular regeneration at 150 d in patch-group | 100% survival for patch-group (and no complications reported), 0% survival for tube interposition group at 28 d | [52] |
| Rats (27) | Gastric acellular matrix scaffold | Patch defects, abdominal esophagus | Mucosal regeneration seen at 2 wk. No muscular regeneration seen up to 18 mo | 11% complication rate | [49] |
| Pigs (14) | Small intestinal submucosa (tubular) | 4-cm defect, cervical esophagus | Prosthesis not found either macroscopically or histologically | Only 1 pig survived the full 4 wk study. The other pigs have to be sacrificed prematurely due to severe stenosis | [51] |
| Human (5) | Porcine small intestinal mucosa | 8-cm to 13-cm en-bloc resection of mucosa and submucosa for superficial carcinoma | Restoration of normal mucosa as early as 4 mo | Strictures; perforation in one patients | [45] |
| Human (1) | Porcine small intestinal mucosa | 5 cm × 3 cm defect cervical esophagus | Intact esophagus with normal calibre | No complications encountered | [46] |
Decellularized matrix: Decellularized matrices are derived from human and animal organs and tissues that have been treated to remove cells and immunogeneic material[36]. Importantly, however, they retain the macro- and micro-architecture of the tissue of origin, and the molecular components of its natural extracellular matrix[37-40]. They have the added hypothetical advantages over synthetic scaffolds of not producing potentially toxic degradation products or inducing inflammation characteristics that may be important in the prevention of stenosis[20,41,42]. Decellularized scaffolds that have been used for esophageal organs originated from the esophagus as well as from other tissues such as the small intestinal submucosa (SIS)[28-31,43-47].
Significant heterogeneity exists among studies, both with respect to the type of scaffold, extent of surgery and species used, which partly explains the range of results reported. Thus, regeneration of the muscularis propria layer is seen to take place in some studies[43,44,48], but not others[49]. Studies that have attempted tube-interposition with SIS report the development of esophageal stenosis and increased mortality[44,50,51]. By contrast, studies applying SIS as a patch repair demonstrated encouraging results[44,50-53]. Badylak et al[45] laid sheets of SIS onto the raw internal surface of esophagus following endoscopic submucosal resection in five patients with superficial cancers. With a follow-up of 4 to 24 mo, the scaffold promoted physiological remodelling as evident by endoscopy and histological characterisation following biopsy. Strictures still formed, but only at areas outside those lined by SIS, suggesting that possible technical improvements in scaffold delivery could ameliorate this. In fact, when SIS was used to completely cover a 3 cm × 5 cm mucosal defect in the cervical esophagus, there was no stenosis and endoscopy at 4 wk demonstrated good integration of the scaffold[46].
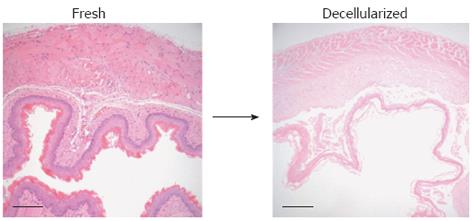
Hypothetically, decellularized esophageal tissue should retain the signals, both chemical and structural, that will direct the appropriate migration and differentiation of host cells, in a way unlikely to occur with scaffolds originating outside the esophagus, such as SIS. Ozeki et al[54] compared two methods of decellularization of adult rat esophagus based on deoxycholate and Triton X-100 respectively and assessed the resulting scaffolds using routine histology and biocompatibility. Those treated with deoxycholate showed superior mechanical properties, maintenance of the extracellular matrix and a lower DNA content than those treated with Triton X-100. Bhrany et al[55] found a combination of 0.5% sodium dodecyl sulphate and Triton X-100 to be effective in decellularization, albeit with a loss of tensile strength as measured by burst pressure studies. Our experience with the detergent-enzymatic treatment in the decellularization of the intestine[39] allowed us to use the same methodology in the esophagus (Figure 2), leading to an improved preservation in microarchitecture[56].
To reduce complications arising from acellular approaches, some authors have seeded the scaffolds prior to transplantation. As mentioned, the two main cell types that are important for esophageal tissue engineering are those that will reconstitute the epithelium and the muscle layer on the luminal and extra-luminal sides respectively. Also important in the formation of a functional esophagus are the vascular and neuronal cell components but we could locate no studies that have studied these in engineered esophagus.
A number of in vitro experiments have examined the seeding and culture of esophageal epithelial cells and different scaffolds to assess the optimal combination. When a matrix composed of decellularized human skin was compared to synthetic scaffolds in vitro for the capacity to support cultured epithelial cells, the decellularized scaffold exhibited cell differentiation and surface confluence similar to native esophagus, whereas synthetic scaffolds demonstrated a discontinuous epithelial lining[57]. Another study compared the growth of human esophageal squamous cells on human decellularized esophagus, porcine decellularized esophagus, human decellularized dermis, and collagen[58]. Interestingly the porcine matrix and collagen gave better results leading to the formation of a mature stratified epithelium. When rat esophageal epithelial cells (EEC) were seeded onto 3-dimensional (3-D) collagen scaffolds they were shown to be viable for up to 8 wk in vitro but did not fully integrate within the scaffold, remaining on the surface as individual cells or small clusters[59]. Seeding of sheep EEC on the same 3-D collagen scaffold resulted in the absence of epithelium sheet formation, which was attributed to cellular penetration into the scaffold and loss of cell-to-cell contact[60]. However, when the same cells were seeded on the 2-D collagen scaffolds a single layer of epithelium was evident following 3 wk of in vitro culture that remained viable up to 6 wk. The same group has also performed in vivo studies of vascularization of the EEC-scaffold construct by omental transplantation in lambs for 8-12 wk[61]. Positive selection of the epithelial population could increase proliferative capacity as demonstrated by Kofler et al[62], who selected ovine EEC for expression of pancytokeratin (PCK) using fluorescence activated cell sorting. The PCK-negative subpopulation had minimal cell attachment on the collagen scaffolds, whereas the PCK-positive cells had a uniform distribution.
In vivo experiments using EEC-scaffold constructs, similarly to results in acellular approaches, have shown more promise for regeneration of partial rather than circumferential defects in rats and dogs[63-65]. An innovative approach recently described seeded cells on a temperature-responsive dish that became hydrophilic at 20 °C and allowed harvesting of a single-cell sheet[63]. When the cell sheets were transplanted in dogs that had undergone endoscopic submucosal resection, complete wound healing was observed at 4 wk with no signs of stricture and an intact epithelium. Wei et al[64] obtained mucosal epithelial cells from oral biopsy, or esophageal organoid units created following digestion of rat esophagi[65]. These were seeded onto scaffolds and implanted as complete esophageal substitutes, but histology of the resultant muscle layers showed poor architecture.
To overcome the limitations of using EEC in isolation, esophageal constructs prepared using EEC-seeded collagen scaffolds were placed on the latissimus dorsi muscle of athymic mice with the intention to harvest and tubularize the muscle once the epithelial side has matured[66,67]. Miki et al[68] found an increase in the number of epithelial layers from 2 when EEC seeded alone, to 18 when co-seeded with fibroblasts. A more recent study by Hayashi et al[69] cultured both epithelial and fibroblast cells on a bed of smooth muscle cells (SMC) embedded in a collagen gel in vitro, prior to transplanting them on the latissimus dorsi of athymic rats. Nakase et al[70] also aimed to combine different cell lines and scaffolds into one tubular structure in dogs. They used oral keratinocytes and fibroblasts cultured on human amniotic membrane and SMC seeded on poly (glycolic acid). These two scaffolds were then rolled together and implanted into the omentum for 3 wk, following which they were transplanted into a 3-cm intrathoracic esophageal defect. Both muscular and epithelial layers were present at 420 d of follow-up, although no peristaltic activity was observed.
Based on the above literature, it is clear that although tissue engineering has been proposed as a solution for the current treatments of esophageal defects, currently, there is no clear strategy for recreating all the portions of the esophagus in man[71,72]. The problems that need to be solved are related to the optimal scaffold, the cell sources for the epithelial and muscular components, peristalsis and vascularization (Table 1). The stenotic changes that are the main complication encountered with esophageal constructs are likely related to poor regeneration of natural architecture.
The recent trend in organ tissue engineering has been to use decellularized scaffolds. It has been suggested that they would be an advantageous choice due to their enhancement of cellular proliferation, migration and differentiation. However, the lack of positive results when trying to replace a tubular defect, confirms that the use of biomaterials alone as a means of esophageal repair is unsuccessful. We envisage a point where “smart polymers” may replace scaffolds of biological origin and facilitate an “off-the-shelf” approach to esophageal tissue-engineering. Our group and collaborators in Sweden have used polyhedral oligomeric silsesquioxane-poly (carbonate-urea) urethane, a synthetic material used in clinical trials of vascular grafting, as an alternative to biologic scaffolds in the generation of tracheal scaffolds[73]. These have the added advantages of being tailor-made and retain biomechanical properties indefinitely, whilst there is no need for an organ donor, with all the attendant convenience, infection and ethical issues of the latter. However, early experience shows that these scaffolds do not epithelialize or vascularize easily[27]. The study of cell-scaffold interactions is likely to substantially inform the development of better biomaterials for organ and tissue regeneration. Ritchie et al[74] found that esophageal muscle cells seeded onto collagen membranes required mechanical stimulation to retain normal contractile properties in a bioreactor, showing the importance of a multidisciplinary engineering approach to this problem, but we could find no other references to the application of bioreactors to esophageal tissue-engineering. Ex vivo models, such as bioreactors and microfluidic organotypic chambers, are urgently required in order to explore the effects of varying stem cell/cell-scaffold-signaling combinations in the generation of functional esophageal tissue pre-implantation.
The general consensus indicates a significant advantage in repopulating scaffolds with cells prior to implantation. Studies that have seeded EEC have had positive results in repopulating the epithelial layer, both as an onlay patch[63,64] and as a total interposition graft[65]. Nevertheless, as with cell-free approaches, in cases in which only the lumen was seeded, there was a poor regeneration of the muscular layer, indicating a need for co-seeding with SMC. This is not a surprise, since esophageal strictures can be managed clinically easily with an intestinal patch (free graft) as partial substitution while they have very high chance of recurrence when such material is used to repair the whole circumference. Studies are required to identify the optimal cell types and sources to repopulate esophageal scaffolds. Ideally, cell sources should be autologous, easy to harvest, highly proliferative, and should have the ability to differentiate into many specialized cell types.
Equally important to muscular regeneration is the challenge of replicating peristaltic contractility and a vascular supply in an artificial esophagus. Watanabe et al[75] developed nickel-titanium, shaped-memory, alloy coils, which were placed in an annular manner on a Gore-Tex vascular graft for esophageal replacement. Interestingly, low-voltage electrical current passing through the coils generated peristaltic movements in the artificial esophagus implanted in a goat model, suggesting that re-provision of appropriate muscular stimuli, either by enhanced neural regeneration or by electrical means, may be a profitable route for investigation if functionally normal swallowing is to be achieved. What is more, we propose that the physiological contribution of neural crest cells is a pre-requisite for functional peristalsis.
Regarding the vascular component, the esophagus holds an additional challenge due to the tenuous intrinsic vascular anatomy of the esophagus in man and the association of stenosis with poor vascularization. Wrapping the engineered esophagus in the omentum prior to thoracic transplantation is one potential solution, as proposed by Nakase et al[70]. However, results in this instance as well as in our use of omental wrapping for transplantation of tissue-engineered tracheas in humans[35] were sub-optimal. More preclinical work on revascularization strategies is required. The use of intraluminal stents is another solution to avoid stenosis. Where collagen scaffolds were used in the above studies, the stenosis and mortality was inversely correlated to the length of stay of the intraluminal stent[29,31]. The use of stents allows time for epithelial and muscular migration onto the cell-free scaffolds. In our recent pediatric tissue engineered trachea transplant[35] we also used bio-absorbable stents, which were engineered using large mesh that allows epithelial ingrowth and persists for about 6 wk before complete degradation[76,77].
In the near future, tissue engineering may represent a valid therapeutic alternative to treat severe congenital or acquired esophageal disorders. We present possible lines for investigation that could indicate what such products will look like, but propose that, in the short- to medium-term, a combination of decellularized scaffolds with muscle and epithelial cells of autologous (including autologous stem cell) origin are likely to be the most expeditious route. Major questions of vascularity, cell-cell and cell-scaffold interaction, and motility remain outstanding, however, before the bioengineered neo-esophagus becomes an established, effective treatment for complex congenital and acquired malformations in adults and children.
Peer reviewer: Masaki Nagaya, MD, PhD, Department of Developmental Engineering, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki 214-8571, Japan
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Orlando G, Wood KJ, De Coppi P, Baptista PM, Binder KW, Bitar KN, Breuer C, Burnett L, Christ G, Farney A. Regenerative medicine as applied to general surgery. Ann Surg. 2012;255:867-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 387] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Ludman L, Spitz L. Quality of life after gastric transposition for oesophageal atresia. J Pediatr Surg. 2003;38:53-57; discussion 53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Ure BM, Slany E, Eypasch EP, Gharib M, Holschneider AM, Troidl H. Long-term functional results and quality of life after colon interposition for long-gap oesophageal atresia. Eur J Pediatr Surg. 1995;5:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Spitz L, Kiely E, Pierro A. Gastric transposition in children--a 21-year experience. J Pediatr Surg. 2004;39:276-281; discussion 276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 7. | Deurloo JA, Ekkelkamp S, Hartman EE, Sprangers MA, Aronson DC. Quality of life in adult survivors of correction of esophageal atresia. Arch Surg. 2005;140:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Somppi E, Tammela O, Ruuska T, Rahnasto J, Laitinen J, Turjanmaa V, Järnberg J. Outcome of patients operated on for esophageal atresia: 30 years' experience. J Pediatr Surg. 1998;33:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | van Vilsteren FG, Pouw RE, Herrero LA, Peters FP, Bisschops R, Houben M, Peters FT, Schenk BE, Weusten BL, Visser M. Learning to perform endoscopic resection of esophageal neoplasia is associated with significant complications even within a structured training program. Endoscopy. 2012;44:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Zani A, Pierro A, Elvassore N, De Coppi P. Tissue engineering: an option for esophageal replacement? Semin Pediatr Surg. 2009;18:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Kemp P. History of regenerative medicine: looking backwards to move forwards. Regen Med. 2006;1:653-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Atala A. Engineering organs. Curr Opin Biotechnol. 2009;20:575-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Macchiarini P, Walles T, Biancosino C, Mertsching H. First human transplantation of a bioengineered airway tissue. J Thorac Cardiovasc Surg. 2004;128:638-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1175] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 15. | Laurance J. British boy receives trachea transplant built with his own stem cells. BMJ. 2010;340:c1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 992] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 17. | Orlando G, Wood KJ, Stratta RJ, Yoo JJ, Atala A, Soker S. Regenerative medicine and organ transplantation: past, present, and future. Transplantation. 2011;91:1310-1317. [PubMed] |
| 18. | Atala A, Guzman L, Retik AB. A novel inert collagen matrix for hypospadias repair. J Urol. 1999;162:1148-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Chalmers RL, Smock E, Geh JL. Experience of Integra(®) in cancer reconstructive surgery. J Plast Reconstr Aesthet Surg. 2010;63:2081-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Freud E, Efrati I, Kidron D, Finally R, Mares AJ. Comparative experimental study of esophageal wall regeneration after prosthetic replacement. J Biomed Mater Res. 1999;45:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Berman EF. The experimental replacement of portions of the esophagus by a plastic tube. Ann Surg. 1952;135:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Lister J, Altman RP, Allison WA. Prosthetic Substitution of Thoracic Esophagus in Puppies: Use of Marlex Mesh with Collagen or Anterior Rectus Sheath. Ann Surg. 1965;162:812-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Fryfogle JD, Cyrowski GA, Rothwell D, Rheault G, Clark T. Replacement of the middle third of the esophagus with a silicone rubber prosthesis. An experiment and clinical study. Dis Chest. 1963;43:464-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Watanabe K, Mark JB. Segmental replacement of the thoracic esophagus with a Silastic prosthesis. Am J Surg. 1971;121:238-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Lynen Jansen P, Klinge U, Anurov M, Titkova S, Mertens PR, Jansen M. Surgical mesh as a scaffold for tissue regeneration in the esophagus. Eur Surg Res. 2004;36:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Shinhar D, Finaly R, Niska A, Mares AJ. The use of collagen-coated vicryl mesh for reconstruction of the canine cervical esophagus. Pediatr Surg Int. 1998;13:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozóky B, Crowley C, Einarsson O, Grinnemo KH, Gudbjartsson T. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378:1997-2004. |
| 28. | Natsume T, Ike O, Okada T, Takimoto N, Shimizu Y, Ikada Y. Porous collagen sponge for esophageal replacement. J Biomed Mater Res. 1993;27:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Takimoto Y, Okumura N, Nakamura T, Natsume T, Shimizu Y. Long-term follow-up of the experimental replacement of the esophagus with a collagen-silicone composite tube. ASAIO J. 1993;39:M736-M739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Takimoto Y, Nakamura T, Teramachi M, Kiyotani T, Shimizu Y. Replacement of long segments of the esophagus with a collagen-silicone composite tube. ASAIO J. 1995;41:M605-M608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Takimoto Y, Nakamura T, Yamamoto Y, Kiyotani T, Teramachi M, Shimizu Y. The experimental replacement of a cervical esophageal segment with an artificial prosthesis with the use of collagen matrix and a silicone stent. J Thorac Cardiovasc Surg. 1998;116:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Yamamoto Y, Nakamura T, Shimizu Y, Matsumoto K, Takimoto Y, Kiyotani T, Sekine T, Ueda H, Liu Y, Tamura N. Intrathoracic esophageal replacement in the dog with the use of an artificial esophagus composed of a collagen sponge with a double-layered silicone tube. J Thorac Cardiovasc Surg. 1999;118:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Yamamoto Y, Nakamura T, Shimizu Y, Matsumoto K, Takimoto Y, Liu Y, Ueda H, Sekine T, Tamura N. Intrathoracic esophageal replacement with a collagen sponge--silicone double layer tube: evaluation of omental-pedicle wrapping and prolonged placement of an inner stent. ASAIO J. 2000;46:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Teramachi M, Okumura N, Nakamura T, Yamamoto Y, Kiyotani T, Takimoto Y, Matsuda S, Ikada Y, Shimizu Y. Intrathoracic tracheal reconstruction with a collagen-conjugated prosthesis: evaluation of the efficacy of omental wrapping. J Thorac Cardiovasc Surg. 1997;113:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, Vondrys D. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994-1000. [PubMed] |
| 36. | Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 37. | Baiguera S, Jungebluth P, Burns A, Mavilia C, Haag J, De Coppi P, Macchiarini P. Tissue engineered human tracheas for in vivo implantation. Biomaterials. 2010;31:8931-8938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Fishman JM, Ansari T, Sibbons P, De Coppi P, Birchall MA. Decellularized rabbit cricoarytenoid dorsalis muscle for laryngeal regeneration. Ann Otol Rhinol Laryngol. 2012;121:129-138. [PubMed] |
| 39. | Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, Sebire NJ, Smith VV, Fishman JM, Ghionzoli M. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33:3401-3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 40. | Tan JY, Chua CK, Leong KF, Chian KS, Leong WS, Tan LP. Esophageal tissue engineering: an in-depth review on scaffold design. Biotechnol Bioeng. 2012;109:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Mertsching H, Schanz J, Steger V, Schandar M, Schenk M, Hansmann J, Dally I, Friedel G, Walles T. Generation and transplantation of an autologous vascularized bioartificial human tissue. Transplantation. 2009;88:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 692] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 43. | Kajitani M, Wadia Y, Hinds MT, Teach J, Swartz KR, Gregory KW. Successful repair of esophageal injury using an elastin based biomaterial patch. ASAIO J. 2001;47:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 46. | Clough A, Ball J, Smith GS, Leibman S. Porcine small intestine submucosa matrix (Surgisis) for esophageal perforation. Ann Thorac Surg. 2011;91:e15-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Freud E, Greif M, Rozner M, Finaly R, Efrati I, Kidron D, Odes M, Mares AJ. Bridging of esophageal defects with lyophilized dura mater: an experimental study. J Pediatr Surg. 1993;28:986-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Nieponice A, Gilbert TW, Badylak SF. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg. 2006;82:2050-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Urita Y, Komuro H, Chen G, Shinya M, Kaneko S, Kaneko M, Ushida T. Regeneration of the esophagus using gastric acellular matrix: an experimental study in a rat model. Pediatr Surg Int. 2007;23:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Lopes MF, Cabrita A, Ilharco J, Pessa P, Paiva-Carvalho J, Pires A, Patrício J. Esophageal replacement in rat using porcine intestinal submucosa as a patch or a tube-shaped graft. Dis Esophagus. 2006;19:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Doede T, Bondartschuk M, Joerck C, Schulze E, Goernig M. Unsuccessful alloplastic esophageal replacement with porcine small intestinal submucosa. Artif Organs. 2009;33:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Lopes MF, Cabrita A, Ilharco J, Pessa P, Patrício J. Grafts of porcine intestinal submucosa for repair of cervical and abdominal esophageal defects in the rat. J Invest Surg. 2006;19:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | Ozeki M, Narita Y, Kagami H, Ohmiya N, Itoh A, Hirooka Y, Niwa Y, Ueda M, Goto H. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res A. 2006;79:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Bhrany AD, Beckstead BL, Lang TC, Farwell DG, Giachelli CM, Ratner BD. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng. 2006;12:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Totonelli G, Maghsoudlou P, Georgiades F, Garriboli M, Koshy K, Turmaine M, Ashworth M, Sebire NJ, Pierro A, Eaton S. Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration. Pediatr Surg Int. 2012;Epub ahead of print. [PubMed] |
| 57. | Beckstead BL, Pan S, Bhrany AD, Bratt-Leal AM, Ratner BD, Giachelli CM. Esophageal epithelial cell interaction with synthetic and natural scaffolds for tissue engineering. Biomaterials. 2005;26:6217-6228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Green N, Huang Q, Khan L, Battaglia G, Corfe B, MacNeil S, Bury JP. The development and characterization of an organotypic tissue-engineered human esophageal mucosal model. Tissue Eng Part A. 2010;16:1053-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Saxena AK, Ainoedhofer H, Höllwarth ME. Esophagus tissue engineering: in vitro generation of esophageal epithelial cell sheets and viability on scaffold. J Pediatr Surg. 2009;44:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Saxena AK, Ainoedhofer H, Höllwarth ME. Culture of ovine esophageal epithelial cells and in vitro esophagus tissue engineering. Tissue Eng Part C Methods. 2010;16:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Saxena AK, Baumgart H, Komann C, Ainoedhofer H, Soltysiak P, Kofler K, Höllwarth ME. Esophagus tissue engineering: in situ generation of rudimentary tubular vascularized esophageal conduit using the ovine model. J Pediatr Surg. 2010;45:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Kofler K, Ainoedhofer H, Höllwarth ME, Saxena AK. Fluorescence-activated cell sorting of PCK-26 antigen-positive cells enables selection of ovine esophageal epithelial cells with improved viability on scaffolds for esophagus tissue engineering. Pediatr Surg Int. 2010;26:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Ohki T, Yamato M, Murakami D, Takagi R, Yang J, Namiki H, Okano T, Takasaki K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 64. | Wei RQ, Tan B, Tan MY, Luo JC, Deng L, Chen XH, Li XQ, Zuo X, Zhi W, Yang P. Grafts of porcine small intestinal submucosa with cultured autologous oral mucosal epithelial cells for esophageal repair in a canine model. Exp Biol Med (. Maywood). 2009;234:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Grikscheit T, Ochoa ER, Srinivasan A, Gaissert H, Vacanti JP. Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. J Thorac Cardiovasc Surg. 2003;126:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Sato M, Ando N, Ozawa S, Miki H, Kitajima M. An artificial esophagus consisting of cultured human esophageal epithelial cells, polyglycolic acid mesh, and collagen. ASAIO J. 1994;40:M389-M392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Sato M, Ando N, Ozawa S, Nagashima A, Kitajima M. A hybrid artificial esophagus using cultured human esophageal epithelial cells. ASAIO J. 1993;39:M554-M557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Miki H, Ando N, Ozawa S, Sato M, Hayashi K, Kitajima M. An artificial esophagus constructed of cultured human esophageal epithelial cells, fibroblasts, polyglycolic acid mesh, and collagen. ASAIO J. 1999;45:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Hayashi K, Ando N, Ozawa S, Kitagawa Y, Miki H, Sato M, Kitajima M. A neo-esophagus reconstructed by cultured human esophageal epithelial cells, smooth muscle cells, fibroblasts, and collagen. ASAIO J. 2004;50:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Nakase Y, Nakamura T, Kin S, Nakashima S, Yoshikawa T, Kuriu Y, Sakakura C, Yamagishi H, Hamuro J, Ikada Y. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg. 2008;136:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Orlando G, García-Arrarás JE, Soker T, Booth C, Sanders B, Ross CL, De Coppi P, Farney AC, Rogers J, Stratta RJ. Regeneration and bioengineering of the gastrointestinal tract: current status and future perspectives. Dig Liver Dis. 2012;44:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Orlando G, Bendala JD, Shupe T, Bergman C, Bitar KN, Booth C, Carbone M, Koch KL, Lerut JP, Neuberger JM. Cell and organ bioengineering technology as applied to gastrointestinal diseases. Gut. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Ahmed M, Ghanbari H, Cousins BG, Hamilton G, Seifalian AM. Small calibre polyhedral oligomeric silsesquioxane nanocomposite cardiovascular grafts: influence of porosity on the structure, haemocompatibility and mechanical properties. Acta Biomater. 2011;7:3857-3867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Ritchie AC, Wijaya S, Ong WF, Zhong SP, Chian KS. Dependence of alignment direction on magnitude of strain in esophageal smooth muscle cells. Biotechnol Bioeng. 2009;102:1703-1711. [PubMed] |
| 75. | Watanabe M, Sekine K, Hori Y, Shiraishi Y, Maeda T, Honma D, Miyata G, Saijo Y, Yambe T. Artificial esophagus with peristaltic movement. ASAIO J. 2005;51:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Vondrys D, Elliott MJ, McLaren CA, Noctor C, Roebuck DJ. First experience with biodegradable airway stents in children. Ann Thorac Surg. 2011;92:1870-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Lischke R, Pozniak J, Vondrys D, Elliott MJ. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg. 2011;40:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |