INTRODUCTION
Various types of cancer arise in the setting of chronic inflammation, suggesting a strong link between inflammation and carcinogenesis[1,2]. Although Virchow first suggested this relationship in the 19th century, clear evidence for it has been obtained only during the last decade[3].The development of hepatocellular carcinoma (HCC) is one of the most extensively investigated inflammation-based carcinogenic processes because more than 90% of HCCs develop in the context of chronic liver damage and inflammation.
HCC is diagnosed in more than half a million people each year and is the third most common cause of cancer mortality worldwide[4]. The short-term prognosis of patients with HCC has improved recently due to advances in early diagnosis and treatment, but long-term prognosis remains unsatisfactory, as indicated by the low overall survival of 22%-35% at 10 years after curative treatment[5,6]. Thus, understanding the molecular carcinogenic mechanisms and the unique pathogenic biology of HCC has become an important issue worldwide.
HCC is a complex and heterogeneous tumor with several genomic mutations, but even the most frequent genetic mutations, such as those in p53 and β-catenin, are seen in 30%-50% of HCC cases at most[7]. On the other hand, as mentioned above, more than 90% of HCC develops based on chronic inflammation, indicating that understanding the mechanism(s) of inflammation-mediated hepatocarcinogenesis is necessary for the treatment and prevention of HCC.
The main cause of HCC is viral hepatitis caused by the hepatitis B virus (HBV) or hepatitis C virus (HCV); other major etiologies include hemochromatosis, alcoholic hepatitis, and non-alcoholic steatohepatitis (NASH)[7]. Most of these diseases are known to cause chronic inflammation in the liver, which plays a critical role in hepatocarcinogenesis. For example, in chronic viral hepatitis, the host immune responses to HBV or HCV are often insufficiently strong to completely clear the infection, resulting in chronic stimulation of an antigen-specific immune response[8]. Virus-infected hepatocytes are killed by host immune cells as well as the intrinsic cytopathic effects of the hepatitis viruses, triggering the production of various cytokines and growth factors and subsequently inducing compensatory hepatocyte regeneration. This persistent cycle of necro-inflammation and hepatocyte regeneration is thought to increase the risk of genetic mutation in hepatocytes, and, furthermore, to promote survival and expansion of initiated cells[9-11]. Additionally, reactive oxygen species (ROS) and nitrogen oxygen species, generated by both initiated cells and inflammatory cells, could accelerate hepatocarcinogenesis through several mechanisms, such as the induction of oxidative DNA damage, DNA methylation, and hepatocyte injury[11].
Multiple signaling pathways are involved in these processes. Among them, recent in vivo studies have shown that several inflammation- and stress-related signaling pathways are key players in hepatocarcinogenesis, including the nuclear factor-κB (NF-κB), signal transducer and activator of transcription (STAT), and stress-activated mitogen-activated protein kinase (MAPK) pathways. Mutations in genes involved in these signaling pathways are currently thought to be rare. Nevertheless, constitutive activation of these pathways is frequently seen in the tumor and surrounding liver tissues, and may be due to the inflammatory microenvironment. Interestingly, these signaling pathways do not act independently, but are linked through extensive crosstalk. This review highlights advances in the understanding of these interesting but complex signaling pathways in hepatocarcinogenesis.
INFLAMMATION-RELATED SIGNALING IN HEPATOCARCINOGENESIS
Role of the I κB kinase/NF-κB pathway in hepatocytes
The NF-κB family of transcription factors consists of five members: p65/RelA, c-Rel, RelB, p50/NF-κB1, and p52/NF-κB2. Two of the five members dimerize and are held in the cytoplasm by the inhibitor of NF-κB (IκB) proteins[12]. In response to many kinds of proinflammatory stimuli, the I κB kinase (IKK) complex, which consists of two catalytic subunits, IKKα and IKKβ, and a regulatory component, IKKγ/I κB kinase (NEMO), phosphorylates IκB and subsequently induces degradation of it. Once activated, NF-κB dimers translocate into the nucleus and stimulate the transcription of various genes, such as those encoding cytokines and anti-apoptotic factors[13]. Mice lacking RelA, IKKβ, or NEMO reveal embryonic lethality with extensive liver apoptosis and degeneration[14-16]. This liver apoptosis is induced by tumor necrosis factor (TNF)-α, and intercrossing with TNF receptor 1 knockout mice prevents liver damage and the lethal phenotype[14,17,18]. Furthermore, hepatocyte-specific IKKβ or NEMO knockout mice are not embryonic-lethal, but are more sensitive to TNF-α-mediated liver injury[19,20]. Thus, NF-κB plays a key role in liver homeostasis by preventing hepatocyte death.
The role of IKKβ in hepatocarcinogenesis has been examined using the diethylnitrosamine (DEN)-induced mouse HCC model[21]. DEN is the most commonly used genotoxic chemical carcinogen to investigate the mechanism of hepatocarcinogenesis, because it is easy to induce HCC and DEN-induced HCC shows histology and gene expression similar to human HCC, especially with a poor prognosis[22,23]. A single dose of DEN given to 2-wk-old male mice is sufficient to induce HCC. However, when DEN is administered to mice older than 4 wk of age, it cannot induce HCC and requires assistance from tumor promoters, such as phenobarbital, because hepatocyte proliferation is rare in adult mice[24]. Thus, some stimulation that induces hepatocyte proliferation is indispensable as a tumor promoter in this model.
Strikingly, DEN-induced HCC was markedly increased in hepatocyte-specific IKKβ knockout mice[21]. Hepatocyte-specific knockout of IKKβ induced a greater extent of hepatocyte death with ROS accumulation after DEN administration, because NF-κB activation is required for the up-regulation of antioxidative genes, such as ferritin heavy chain and manganese-dependent superoxide dismutase. Excess ROS accumulation promotes cell death through various mechanisms, including prolonged c-Jun NH2 terminal kinase (JNK) activation[25]. Cell death is accompanied by an inflammatory reaction, and the elevated hepatocyte death rate enhances compensatory proliferation. Thus, the hepatocyte-specific deletion of IKKβ augments DEN-induced hepatocyte death and cytokine-driven compensatory proliferation, which acts as a tumor promoter and eventually leads to increased HCC development (Figure 1). Similar findings were obtained in mice lacking IKKγ/NEMO, the hepatocyte-specific deletion of which results in spontaneous liver damage, hepatosteatosis, fibrosis, and HCC development[20].
Figure 1 Role of inflammation- and stress-related signaling pathways in hepatocarcinogenesis.
Chronic liver damage induces a persistent cycle of necro-inflammation and hepatocyte regeneration, resulting in genetic mutations in hepatocytes and expansion of initiated cells, eventually leading to hepatocellular carcinoma (HCC) development. As shown in the figure, nuclear factor-κB, signal transducer and activator of transcription 3, and stress-activated mitogen-activated protein kinase pathways play critical roles in these processes. Furthermore, other factors, such as obesity and impaired expression of microRNA, can modify these inflammatory processes and accelerate HCC development. ROS: Reactive oxygen species; TLR: Toll-like receptor; NF-κB: Nuclear factor-κB; TNF: Tumor necrosis factor; IL: Interleukin; JNK: c-Jun NH2 terminal kinase; STAT: Signal transducer and activator of transcription.
Although the experiments described above demonstrate a tumor-suppressive role of NF-κB in the hepatocyte, hepatocyte NF-κB has been also identified to have tumor-promoting roles in other HCC models that depend on chronic inflammation rather than liver damage and death-driven compensatory proliferation. Experiments crossing transgenic mice expressing a non-degradable IκBα mutant in hepatocytes with MDR2 knockout mice, which show low-grade chronic inflammation in the portal area and subsequent cancer development, revealed that the inhibition of NF-κB activation resulted in reduced HCC development[26]. In this model, NF-κB activation in the hepatocyte promoted a low degree of TNF-α production and paracrine TNF-α signaling maintained NF-κB activation in the malignant cells, leading to the expression of anti-apoptotic genes and the survival of malignant cells. Notably, NF-κB activation is more important in the progression of hepatocarcinogenesis than in the initiation step in this model. A similar tumor-promoting role of NF-κB in the hepatocyte has been reported in hepatocyte-specific lymphotoxin αβ transgenic mice[27]. Lymphotoxin αβ transgenic hepatocytes produce chemokines, such as CCL2, CCL7, CXCL1, and CXCL10, in an IKKβ-dependent manner, recruiting inflammatory cells into the liver and inducing spontaneous chronic inflammation and the subsequent development of HCC. These findings suggest that NF-κB activation in the hepatocyte is involved in the production of cytokines and chemokines that maintain the inflammatory environment as a tumor promoter. Thus, the NF-κB pathway in the hepatocyte plays dual roles in hepatocarcinogenesis, according to the disease model and the carcinogenesis stage.
Role of the IKK/NF-κB pathway in myeloid cells
The role of the NF-κB pathway in myeloid cells has also been investigated using a DEN-induced HCC model[21]. In contrast to hepatocyte-specific IKKβ knockout mice, deletion of IKKβ in both hepatocytes and myeloid cells, including Kupffer cells, strongly inhibited DEN-induced HCC development. This phenotype was derived from the markedly reduced production of cytokines, such as TNF-α, interleukin-6 (IL-6), and hepatocyte growth factor, which are secreted by non-parenchymal cells in response to dying hepatocytes and induce compensatory proliferation of residual hepatocytes. Thus, IKKβ/NF-κB in myeloid cells is required for the production of liver growth factors and subsequent hepatocarcinogenesis. Furthermore, IKKβ in myeloid cells, especially in Kupffer cells, has also been implicated in the development of metastatic liver tumors through IL-6 production[28]. Thus, the IKKβ/NF-κB pathway orchestrates inflammatory crosstalk between hepatocytes and myeloid cells in liver cancer development (Figure 1).
Role of inflammatory cytokines in hepatocarcinogenesis
As mentioned above, NF-κB activation-mediated production of inflammatory cytokines plays an important role in the inflammation-carcinogenesis axis of the liver. Various inflammatory cytokines, including TNF-α, IL-1α, IL-1β, IL-6, and IL-8, have been implicated in chronic liver inflammation, among which IL-6 is thought to be one of the most important[8,29,30]. In chronic hepatitis, IL-6 is considered to be produced mainly by activated Kupffer cells and to intensify local inflammatory responses, and then induce compensatory hepatocyte proliferation, facilitating malignant transformation of hepatocytes[30]. Hepatocytes express high amounts of the IL-6 receptor and a signal-transducing element (gp130) that, upon IL-6 binding, activates two signaling pathways, Janus activated kinase (JAK)-STAT and MAPK, which are important in the regulation of cell survival and proliferation[31]. In fact, serum IL-6 levels are elevated in patients with chronic liver diseases, including alcoholic hepatitis, HBV and HCV infections, and NASH[32-34]. Additionally, a higher serum IL-6 level correlates with future HCC development in patients with chronic hepatitis B or C[35,36]. These findings suggest that IL-6 plays a role linking chronic inflammation and hepatocarcinogenesis in humans.
In a mouse HCC model, IL-6 knockout mice showed a marked reduction in DEN-induced HCC development, indicating that IL-6 signaling is directly involved in hepatocarcinogenesis[37]. This study also demonstrated the key role played by the toll-like receptor (TLR) adapter protein MyD88. Necrotic hepatocyte-induced IL-6 production was reduced significantly in MyD88-deficient Kupffer cells. Furthermore, deletion of MyD88 suppresses DEN-induced carcinogenesis, indicating that IL-6 production through the TLR/MyD88/NF-κB pathway in Kupffer cells is essential for HCC development. Another study showed that the DEN-induced acute inflammatory response is triggered by IL-1α release from necrotic hepatocytes, and IL-1α subsequently induces IL-6 production by Kupffer cells[38]. Indeed, IL-1 receptor knockout mice showed significantly reduced DEN-induced IL-6 production and subsequent HCC development[38]. Of note, a clinical study revealed that higher serum IL-6 levels correlated with higher aspartate aminotransferase levels in chronic hepatitis C, suggesting that IL-6 may be produced in response to HCV-induced hepatocyte injury[36].
HCC develops much more frequently in males than in females in almost all populations, with a male-to-female ratio of 2:1-4:1[39]. Interestingly, although this sex disparity is also found in this DEN-induced HCC model, ablation of IL-6 abolished the sex differences in hepatocarcinogenesis[37]. However, ovariectomized female mice revealed enhanced IL-6 production and aggravated HCC development. Furthermore, estrogen administration to Kupffer cells inhibits necrotic hepatocyte-induced IL-6 production. These results suggest that estrogen-mediated down-regulation of IL-6 may partly explain the sex disparity in HCC development. However, more recently, Li et al[40] reported that transcription factors Foxa1 and Foxa2 in the hepatocyte played important roles in the sex disparity in hepatocarcinogenesis through an interaction with the estrogen and androgen receptors, independent of IL-6 signaling. Thus, the sex disparity in hepatocarcinogenesis may have several causes in addition to estrogen-mediated down-regulation of IL-6.
Several epidemiologic studies have shown that obesity and metabolic syndrome increase the risk of HCC[41-43]. Although the mechanism by which obesity and metabolic syndrome promote hepatocarcinogenesis is not fully understood, it seems likely to be mediated, in part, by a state of chronic inflammation. A recent report by Park et al[44] demonstrated that dietary- or genetically induced obesity promoted DEN-induced HCC along with low-grade inflammation, and ablation of IL-6 or the TNF receptor 1 abrogated their tumor-promoting effects, suggesting that IL-6 and TNF-α are required for the promotion of obesity-associated HCC. IL-6 and TNF-α produced by adipose tissue or Kupffer cells activate hepatocyte STAT3 and NF-κB, respectively, promoting cell proliferation and survival of initiated hepatocytes. In fact, recent clinical studies have suggested that visceral fat accumulation, insulin resistance, and dysregulation of adipokines, which induce the activation of the inflammatory response, play important roles in hepatocarcinogenesis[45-47]. As the incidence rate of such obesity-associated hepatocarcinogenesis is likely to increase in the near future, inflammatory cytokines have also attracted considerable attention as a mediator of the association between obesity and hepatocarcinogenesis[48].
Role of JAK-STAT signaling
JAK/STAT signaling pathways are important components of many cytokine receptor systems. Cytokines function by specifically recognizing their receptors, which, as a result of binding to their ligand, undergo conformational changes, resulting in the displacement of JAKs, and subsequently JAKs phosphorylate and activate STATs. The STAT protein family consists of seven members encoded by distinct genes. Among them, STAT3 is the most important IL-6 signaling pathway molecule and is recognized as a key player linking inflammation and cancer[49,50]. In response to IL-6 signaling through gp130/JAK, STAT3 forms homodimers that translocate to the nucleus. STAT3 is constitutively active in many tumor cells, but not in normal cells, and regulates the expression of genes involved in tumor progression, such as cell survival, proliferation, invasion, and angiogenesis[51].
Clinical and experimental evidence suggest the involvement of the STAT3 signaling pathway in hepatocarcinogenesis. Activated nuclear STAT3 is found in 60% of HCC and is more pronounced in aggressive tumors[52]. In contrast, suppressors of this pathway, such as suppressor of cytokine signaling 3 (SOCS3), are down-regulated in HCC[53]. In a mouse model, hepatocyte-specific STAT3 ablation prevented DEN-induced HCC development[52], whereas hepatocyte-specific SOCS3 knockout mice were susceptible to HCC development through the enhanced activation of JAK/STAT and MAPK signaling[53]. Hepatocyte-specific IL-6 and IL-6R transgenic mice spontaneously develop hepatocellular hyperplasia and adenomas, which are considered precancerous lesions in humans, accompanying STAT3 activation[54]. Furthermore, in a human study, gain-of-function mutations in gp130 have been identified in 60% of benign hepatocellular adenomas with an inflammatory phenotype, and when combined with β-catenin-activating mutation, lead to HCC development[55]. Thus, the IL-6-gp130-JAK-STAT3 signaling axis is an important contributor to HCC development, making it an attractive target for the treatment and/or prevention of hepatocarcinogenesis.
Interaction between STAT3 and NF-κB has been reported at several levels in tumors. Some studies showed that STAT3 and NF-κB co-regulate numerous oncogenic and inflammatory genes[50]. Additionally, STAT3 directly interacts with RelA, trapping it in the nucleus, thereby contributing to constitutive NF-κB activation[56]. On the other hand, a recent in vivo study revealed that IKKβ/NF-κB in the hepatocyte negatively regulated STAT3 activation in a DEN-induced HCC model[52]. Inactivation of IKKβ caused ROS accumulation and ROS were found to oxidize protein tyrosine phosphatases, including SHP1 and SHP2, which dephosphorylated JAK2 and STAT3. Oxidation of SHP1 and SHP2 results in the loss of their catalytic activity and the constitutive activation of STAT3. In fact, an inverse correlation between NF-κB and STAT3 has been found in human HCC samples. This crosstalk may be a reason for the aggravation of DEN-induced HCC in hepatocyte-specific IKKβ knockout mice. Furthermore, Bard-Chapeau et al[57] showed that hepatocyte-specific SHP2 knockout mice revealed spontaneous liver inflammation and tumorigenesis, accompanied by STAT3 activation. These mice also showed enhanced DEN-induced HCC, which was decreased significantly by intercrossing with hepatocyte-specific STAT3 knockout mice. These results suggest that the inhibition of STAT3 activation by SHP2 plays an important tumor-suppressing role in hepatocarcinogenesis (Figure 2).
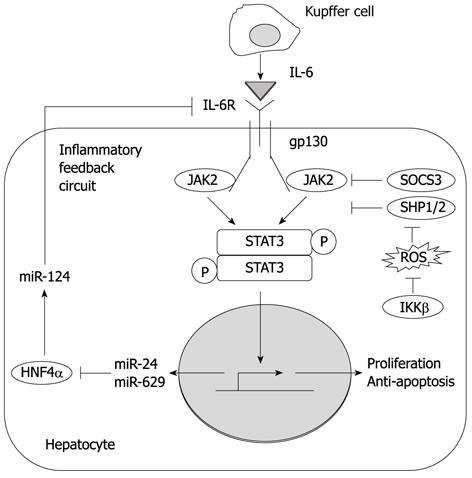
Figure 2 Implications and regulatory system of interleukin-6/gp130/janus activated kinase/signal transducer and activator of transcription 3 signaling in hepatocarcinogenesis.
Interleukin (IL)-6 secreted by Kupffer cells activates signal transducer and activator of transcription (STAT) 3, which promotes the proliferation and survival of initiated hepatocytes. STAT3 activation is suppressed by I κB kinase β through the prevention of reactive oxygen species. However, once STAT3 is activated, STAT3 activation becomes sustained through a microRNA feedback inflammatory loop. JAK: Janus activated kinase; SOCS3: Suppressor of cytokine signaling 3; IKK: I κB kinase; HNF: Hepatocyte nuclear factor; ROS: Reactive oxygen species.
Implications of microRNA in inflammatory signaling
MicroRNAs (miRNAs) are endogenous 20-23-nucleotide RNAs that play important gene-regulatory roles by pairing with the messenger RNAs of protein-coding genes to direct their post-translational repression[58]. Recently, miRNAs have been reported to be implicated in hepatocarcinogenesis through modulating inflammatory signaling pathways. Ji et al[59] found that miR-26 was significantly reduced in human HCC tissues, compared with surrounding non-tumor tissues, and a gene network analysis revealed that miR-26 expression was inversely correlated with the activation of NF-κB and IL-6 signaling pathways. Although a causal relationship between miR-26 and hepatocarcinogenesis could not be evaluated in this study, Kota et al[60] showed that low miR-26 expression played a causal role in hepatocarcinogenesis using a myc-induced mouse HCC model, and induction of miR-26 by gene therapy suppressed HCC development.
More recently, hepatocyte nuclear factor 4α (HNF4α), a transcription factor that is essential for the development of hepatocytes, was reported to play a key role in hepatocarcinogenesis, linking miRNAs and inflammatory signaling pathways[61]. Transient suppression of HNF4α induces decreased miR-124 expression, leading to increased IL-6R expression and subsequent STAT3 activation. STAT3 activation not only plays the tumor-promoting roles described above, but also up-regulates HNF4α-targeting miRNAs, miR-24 and miR-629, resulting in continued suppression of HNF4α. Thus, transient suppression of HNF4α initiates an IL-6/STAT3-mediated hepatocarcinogenesis process through a miRNA feedback loop circuit (Figure 2). The authors also showed that systemic administration of miR-124 prevented hepatocarcinogenesis by inhibiting the feedback loop and inducing tumor-specific apoptosis in mice. Thus, a therapeutic strategy targeting miRNA may be useful for the prevention and treatment of HCC.
STRESS-RELATED SIGNALING IN HEPATOCARCINOGENESIS
Stress-activated MAPK
MAPK cascades are signaling systems that transmit intracellular signals initiated by extracellular stimuli to the nucleus[62,63]. The MAPK family consists of three major MAPK cascades, converging on extracellular signal-regulated kinases (ERKs), JNKs, and p38 MAPKs. Each MAPK signaling system comprises at least three components: MAPK, MAPK kinase (MAPKK), and MAPKK kinase (MAP3K). MAP3K phosphorylates, and thereby activates, MAPKK, and activated MAPKK, in turn, phosphorylates and activates MAPK. Among the three MAPKs, ERKs are activated predominantly by growth factors, whereas JNKs and p38 MAPKs, also called stress-activated MAPKs, are activated by stresses. We discuss the function and regulation of the stress-activated MAPKs in hepatocarcinogenesis in detail below.
Role of JNK signaling
JNK has three isoforms (JNK1, JNK2, JNK3) encoded by three loci. JNK1 and JNK2 are expressed ubiquitously, including in the liver, whereas JNK3 is expressed primarily in the brain[64]. JNK is phosphorylated and activated by two MAPKKs, MKK4 and MKK7, and subsequently phosphorylates transcription factors, such as c-Jun and JunD, which compose the AP-1 complex[65]. Additionally, JNK phosphorylates other proteins, such as Bcl-2 family members, and exerts various kinds of functions, depending on the cell type and stimuli[66]. Furthermore, although JNK1 and JNK2 isoforms play redundant roles in many physiological processes, they also have distinct biological activities in some situations[67,68].
The major functions of JNK in the liver are thought to be the induction of hepatocyte proliferation and cell death. JNK is involved in cell cycle progression, mostly through the activation of c-Jun. In this function, JNK1 is considered to be more important than JNK2, because proliferation of hepatocytes after partial hepatectomy is significantly impaired in JNK1 knockout mice, but not in JNK2 knockout mice[69]. Additionally, hepatocyte death due to TNF-α, lipotoxicity, ER stress, ischemia-reperfusion, and drug toxicity, such as that from acetaminophen, are also considered to be JNK-dependent[19,70-73]. JNK1 and JNK2 are, to some extent, redundant in this function. Although the downstream targets of JNK are not fully understood, most studies have demonstrated that JNK is required for the activation of the mitochondrial apoptotic pathway, through the activation of pro-apoptotic Bcl-2 family members[74,75].
The role of JNK in hepatocarcinogenesis has been investigated using the DEN-induced HCC model[76]. As mentioned above, hepatocyte-specific knockout of IKKβ markedly promotes DEN-induced HCC, through enhanced hepatocyte death and compensatory proliferation[21]. These phenomena can be partly explained by enhanced JNK activation in the setting of IKKβ depletion. Because prolonged JNK activation is closely related to cell death, systems for the regulation of JNK activity are needed for tissue homeostasis. In this regard, NF-κB plays an important role. Although several mechanisms have been proposed in the NF-κB-mediated inhibition of JNK activation, ROS is one of the most important mediators[25,77-79]. ROS accumulation, caused by the reduced expression of NF-κB-dependent antioxidative enzymes, extends JNK activation by inactivating MAPK phosphatases that are essential for the dephosphorylation of activated JNK[25]. In fact, the administration of antioxidants to hepatocyte-specific IKKβ knockout mice decreased sustained JNK activation and hepatocyte death after DEN injection, and furthermore, intercrossing hepatocyte-specific IKKβ knockout mice with JNK1 knockout mice significantly reduced DEN-induced hepatocyte death and compensatory proliferation, eventually suppressing HCC development[21,76]. Additionally, JNK1 knockout mice showed a significant reduction of DEN-induced HCC, compared with wild-type controls. Thus, JNK1 is involved in hepatocarcinogenesis through hepatocyte death and proliferation, which are key components of necro-inflammatory cycles (Figure 1). Furthermore, in addition to the initial phase, JNK1 plays a tumor-promoting role by enhancing cancer cell proliferation and neovascularization through the increased expression of cyclin D1 and vascular endothelial growth factor, respectively[76]. Another study showed that JNK1 promoted HCC cell proliferation in vivo through the up-regulation of c-myc expression and the down-regulation of p21 expression[69]. This study, however, also showed that JNK2 was not involved in hepatocarcinogenesis. In fact, JNK1, but not JNK2, is activated in approximately half of human HCC tissues, compared with adjacent non-tumor tissues[69,80]. These results suggest that JNKs, especially JNK1, play an important role in the development of HCC. Notably, the pharmacological inhibition of JNK suppressed DEN-induced HCC and the growth of xenografted human HCC cells, suggesting that JNK may be a promising therapeutic target for HCC[69].
On the other hand, a recent study using conditional JNK knockout mice showed that the ablation of both JNK isoforms, JNK1 and JNK2, in hepatocytes increased DEN-induced HCC, whereas the ablation of JNK1 and JNK2 in both hepatocytes and myeloid cells reduced hepatic inflammation and the development of HCC, indicating that JNK plays dual roles in hepatocarcinogenesis, depending on cell type and carcinogenesis stage[81].
JNK plays a pivotal role in the development of metabolic syndrome-related disorders, including NASH[41]. Inflammatory cytokines and ROS accumulation in the liver caused by obesity and fatty liver disease induce JNK activation, leading to insulin resistance by increasing inhibitory insulin receptor substrate 1 ser307 phosphorylation[82]. As a clinical study showed that insulin resistance was a major contributor to obesity-mediated hepatocarcinogenesis, JNK may be a candidate therapeutic target in such situations[83]. Furthermore, ROS-mediated JNK activation in the liver is linked not only to liver disease, but also to systemic disorders, such as atherosclerotic cerebrovascular diseases; thus, further elucidation of this process is important[84,85].
Role of p38 signaling
The p38 MAPK family consists of four members: p38α, p38β, p38γ, and p38δ[86]. Among them, p38α is abundant in most cell types, and its function has been investigated in most published studies of p38 MAPKs. p38 is activated through phosphorylation, primarily by MKK3 and MKK6, but phosphorylation by MKK4 and auto-phosphorylation are also involved in some stimuli[87]. p38 can activate not only transcription factors, such as ATF2, p53, and Mitf, but also protein kinases, such as MAPKAP kinase 2 (MK2) and MK5[88]. Although p38 was initially discovered as a regulator of inflammatory cytokine production, recent studies have revealed that it has tumor-suppressing properties. p38 inhibits tumorigenesis by the down-regulation of cyclins, up-regulation of cyclin-dependent kinase inhibitors, and modulation of the tumor suppressor p53, resulting in cell cycle arrest, oncogene-induced senescence, apoptosis induction, and contact inhibition[86].
Although the roles of p38 in the liver have yet to be clarified, compared with JNK, major roles reported to date are the inhibition of hepatocyte death and proliferation. These effects of p38 are partially mediated by negative regulation of the JNK/c-Jun pathway. For example, hepatocyte-specific p38α knockout mice showed much stronger lipopolysaccharide (LPS)-induced JNK activation in the liver, and intercrossing with hepatocyte specific IKKβ knockout mice induced severe liver injury after LPS administration, suggesting that p38α and IKKβ act synergistically to protect the liver from TNF-α-induced hepatocyte death[89].
The role of p38 in hepatocarcinogenesis has also been investigated using the DEN-induced HCC model[38]. Similar to hepatocyte-specific IKKβ knockout mice, hepatocyte-specific p38α knockout mice showed enhancement of DEN-induced ROS accumulation, JNK activation, liver damage, and compensatory hepatocyte proliferation, eventually resulting in enhanced carcinogenesis. Another study showed the tumor-suppressing role of p38 through interaction with the JNK/c-Jun pathway by focusing on the antiproliferative effects in the advanced stage[90]. However, in contrast to IKKβ knockout mice, enhanced activation of the JNK pathway in p38α knockout mice was accompanied by MAPKK activation, suggesting that the targets of p38α may be upstream of JNK, such as MAPKKs and MAP3Ks[38]. Consistent with these animal experiments, in human samples, the activity of the MKK6/p38 pathway is decreased in HCC tissues, compared with adjacent non-tumor tissues, and is significantly lower in larger HCC tissues[91]. These findings suggest that the p38 pathway may play an anti-proliferative role in human HCC.
Regulatory system of stress-activated MAPK signaling by MAP3Ks
The evidence presented above suggests that JNK acts generally as a tumor promoter and p38 acts generally as a tumor suppressor in hepatocarcinogenesis, but some studies have shown opposite roles in HCC and other cancers. For example, JNK plays tumor-suppressing roles in mouse skin cancer and mammalian cancer models[92,93]. Additionally, JNK has been reported to act as a tumor suppressor by inducing cancer cell apoptosis in HCC[94]. As mentioned above, JNK was also reported to play dual roles in hepatocarcinogenesis, depending on cell type and carcinogenesis stage[81]. p38 may also have oncogenic effects, facilitating cell invasion, inflammation, and angiogenesis[95,96]. Furthermore, crosstalk among JNK, p38, and molecules involved in other signaling pathways, such as NF-κB, further complicates their roles[97]. Thus, understanding of the regulatory system of stress-activated MAPK signaling is necessary for the potential use of these molecules as therapeutic targets. Importantly, only two molecules, JNK and p38, are downstream in this pathway, whereas more than 10 molecules have been identified for upstream MAP3Ks[98]. Each MAP3K is activated by several different kinds of stimuli, and integrated into a unique pattern of MAPK activation and substrate phosphorylation, leading to a specific cellular response to the stimulus. Thus, the activities of JNK and p38 are tightly regulated by MAP3Ks. Several recent studies have uncovered roles of MAP3Ks in the regulation of stress-activated MAPK signaling in hepatocarcinogenesis.
Role of apoptosis signal-regulating kinase 1
Apoptosis signal-regulating kinase 1 (ASK1), one of the most important MAP3Ks, selectively activates JNK and p38 signaling in response to a variety of stimuli, including ROS and cytokines[99]. In particular, ASK1 plays a key role in oxidative stress-induced cell death. In the absence of oxidative stress, thioredoxin (Trx), a reduction/oxidation regulatory protein, inhibits ASK1 kinase activity via direct binding to the N-terminal region of ASK1. However, once oxidative stress occurs in the cell, Trx is converted to its oxidized form and dissociates from ASK1, resulting in ASK1 kinase activation[100]. ASK1 is considered to induce cell death through stress-activated MAPK-mediated activation of the mitochondrial cell death pathway[101]. In fact, ASK1 is involved in acetaminophen-induced hepatocyte death, a typical ROS-mediated liver injury, through mechanisms involving Trx-ASK1 dissociation[102]. Furthermore, ASK1 is involved in hepatocyte death mediated by death receptors, such as TNF-R and Fas[75].
ASK1 knockout mice showed significantly increased DEN-induced HCC, suggesting that ASK1 plays tumor-suppressing roles in hepatocarcinogenesis in this model[75]. Activation of JNK and the pro-apoptotic Bcl-2 family member Bim, which are required for death receptor-mediated apoptosis, are attenuated in ASK1 knockout HCC, resulting in decreased cancer cell apoptosis. On the other hand, ASK1 plays a minor role in the tumor-promoting effects of JNK, such as the DEN-induced acute phase reaction, cancer cell proliferation, and neovascularization. Thus, ASK1 is considered to play major roles in the tumor-suppressing part of JNK activity in hepatocarcinogenesis. Furthermore, DNA damage-induced p38 activation and subsequent p21 up-regulation is impaired in ASK1 knockout mice. Thus, ASK1 controls the tumor-suppressing function in stress-activated MAPK signaling through the induction of apoptosis and the DNA damage response.
Another study indicated that ASK1 and Bim are also required for sorafenib-induced apoptosis in HCC cells[103]. Sorafenib is a small-molecule multikinase inhibitor that is currently the sole therapeutic drug effective for the treatment of HCC[104]. Most recently, somatic mutations in the ASK1 gene, which reduce the kinase activity of ASK1, have been identified in melanoma[105]. Thus, it may be important to clarify whether similar mutations are found in HCC, from the point of view of not only the carcinogenesis mechanism, but also possible therapeutic effects of anticancer drugs.
Role of transforming growth factor β-activated kinase 1
Another major MAP3K, activated kinase 1 (TAK1), is activated through TNF receptor, TLR, IL-1 receptor, and transforming growth factor β receptor signaling, and then activates the JNK and NF-κB pathways, which play opposing roles in cell death[106]. Interestingly, hepatocyte-specific TAK1 knockout mice show spontaneous hepatocyte death, and this phenotype is partially rescued by crossing with TNF receptor 1 knockout mice, suggesting that TAK1 knockout hepatocytes are highly sensitive to endogenous TNF-α-induced apoptosis[107]. This spontaneous cell death subsequently causes compensatory hepatocyte proliferation, inflammation, fibrosis, and the eventual development of HCC in aged mice[107,108]. These phenomena resemble the phenotype observed in hepatocyte-specific NF-κB knockout mice. Furthermore, JNK activation is rather enhanced in TAK1 knockout mice, indicating that TAK1 in the hepatocytes acts as a tumor suppressor, mainly by regulating the activation of the NF-κB pathway. However, enhanced JNK activation in TAK1 knockout mice occurs partially through the activation of another MAP3K, TAO2, suggesting that TAK1 may interact with other MAP3Ks[108]. Interestingly, crossing hepatocyte-specific TAK1 knockout mice with NEMO knockout mice attenuated JNK activation and prevented hepatocyte death and the development of HCC, suggesting that NEMO has a tumor-promoting function in the setting of TAK1 deletion[108]. Additionally, this function of NEMO is considered to be independent of NF-κB. Furthermore, a recent study showed that TAK1 inhibits ASK1-mediated apoptosis through a direct interaction between the C-terminal domain of TAK1 and the N-terminal or C-terminal domain of ASK1 in HEK 293 cells[109]. Thus, in the setting of TAK1 deletion, ASK1 may play a tumor-promoting role by accelerating hepatocyte apoptosis and subsequent inflammation. Because crosstalk among MAP3Ks is less well understood, further studies are needed to clarify the whole picture of stress-activated MAPK signaling pathways.