Published online Jan 21, 2012. doi: 10.3748/wjg.v18.i3.257
Revised: August 26, 2011
Accepted: September 2, 2011
Published online: January 21, 2012
AIM: To establish a more stable and accurate nude mouse model of pancreatic cancer using cancer cell microencapsulation.
METHODS: The assay is based on microencapsulation technology, wherein human tumor cells are encapsulated in small microcapsules (approximately 420 μm in diameter) constructed of semipermeable membranes. We implemented two kinds of subcutaneous implantation models in nude mice using the injection of single tumor cells and encapsulated pancreatic tumor cells. The size of subcutaneously implanted tumors was observed on a weekly basis using two methods, and growth curves were generated from these data. The growth and metastasis of orthotopically injected single tumor cells and encapsulated pancreatic tumor cells were evaluated at four and eight weeks postimplantation by positron emission tomography-computed tomography scan and necropsy. The pancreatic tumor samples obtained from each method were then sent for pathological examination. We evaluated differences in the rates of tumor incidence and the presence of metastasis and variations in tumor volume and tumor weight in the cancer microcapsules vs single-cell suspensions.
RESULTS: Sequential in vitro observations of the microcapsules showed that the cancer cells in microcapsules proliferated well and formed spheroids at days 4 to 6. Further in vitro culture resulted in bursting of the membrane of the microcapsules and cells deviated outward and continued to grow in flasks. The optimum injection time was found to be 5 d after tumor encapsulation. In the subcutaneous implantation model, there were no significant differences in terms of tumor volume between the encapsulated pancreatic tumor cells and cells alone and rate of tumor incidence. There was a significant difference in the rate of successful implantation between the cancer cell microencapsulation group and the single tumor-cell suspension group (100% vs 71.43%, respectively, P = 0.0489) in the orthotropic implantation model. The former method displayed an obvious advantage in tumor mass (4th wk: 0.0461 ± 0.0399 vs 0.0313 ± 0.021, t = -0.81, P = 0.4379; 8th wk: 0.1284 ± 0.0284 vs 0.0943 ± 0.0571, t = -2.28, respectively, P = 0.0457) compared with the latter in the orthotopic implantation model.
CONCLUSION: Encapsulation of pancreatic tumor cells is a reliable method for establishing a pancreatic tumor animal model.
- Citation: Ma MZ, Cheng DF, Ye JH, Zhou Y, Wang JX, Shi MM, Han BS, Peng CH. Microencapsulated tumor assay: Evaluation of the nude mouse model of pancreatic cancer. World J Gastroenterol 2012; 18(3): 257-267
- URL: https://www.wjgnet.com/1007-9327/full/v18/i3/257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i3.257
Pancreatic adenocarcinoma (PA) is an aggressive malignancy that is more common in elderly persons than in younger persons, and less than 20% of patients present with localized, potentially curable tumors. The overall 5-year survival rate among patients with pancreatic cancer is < 5%[1]. Despite advances in chemoradiation and adjuvant chemotherapy, chemotherapeutic options prolong life only minimally[2]. Therefore, animal models that allow further examination of pancreatic cancer progression are urgently needed.
The majority of in vivo experimental studies of human cancer have been conducted in subcutaneous, orthotopic and heterotopic implantation nude mouse models. Current animal models of PA have low rates of tumor progression and metastases[3]. A variety of techniques for inducing pancreatic cancer growth in immunodeficient mice have been described, and each is associated with potential shortcomings. We hypothesized that the delivery of tumor cells in three-dimensional microcapsules could overcome these shortcomings by providing a contained, growth-enhancing environment in which tumor cells can propagate. Therein, the tumor cells are likely to grow well or even progress to metastatic disease. Matrigel, a widely-used extracellular matrix, has severe technical limitations such as lot-to-lot variability and handling difficulties during the injection of cell suspensions. Since the introduction of alginate-poly-l-lysine-alginate membranes as an immunoisolation device by Chang et al[4] in 1966, further studies have been conducted on the use of microencapsulation in cancer therapies using immunodeficient animals[5]. The first stage of this study compared the growth of tumor cells in microcapsules with the growth of cells alone. Preclinical testing of novel therapeutic strategies in animal models also requires a meticulous assessment of the effects of treatment on local and systemic tumor growth. The second stage of this study evaluated local tumor progression and the systemic spread of tumor cells in microcapsules vs those with single tumor cells.
Laboratory animals: In total, 82 82 nude mice (BALB/c nu/nu), between 4 and 6 wk of age, weighing 18-20 g, half males and half females, were purchased from Shanghai Laboratory Animal Co., Ltd and were kept in a specific pathogen-free laboratory. All the procedures and observations were performed in the laboratory animal center at the Shanghai JiaoTong University School of Medicine. All studies were conducted with the approval and guidance of Shanghai Jiao-Tong University Medical Animal Ethics Committees (approval ID: 2010060).
Cell lines: The undifferentiated human pancreatic cancer cell line MiaPaCa-2 was obtained from the Shanghai Institute of Digestive Surgery at the Ruijin Hospital, which is affiliated with the Shanghai JiaoTong University School of Medicine.
Encapsulation device: A high-frequency pulse microdroplet generator (Figure 1) was provided by the Biological Engineering Institute of the University of Shanghai for Science and Technology.
Animal in-vivo imaging system: An Inveon micro positron emission tomography-computed tomograph (PET-CT) was purchased from Siemens Ltd.
Cell culture: MiaPaCa-2s were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY). Cells were cultured at 37 °C in an incubator with 50 mL/L CO2. MiaPaCa-2 cells in logarithmic growth phase were harvested with 0.25% trypsin, centrifuged at 500 g for 15 min, washed 3 times in phosphate buffered saline (PBS) at 4 °C and the cell number was adjusted to 1 × 107 cells/mL.
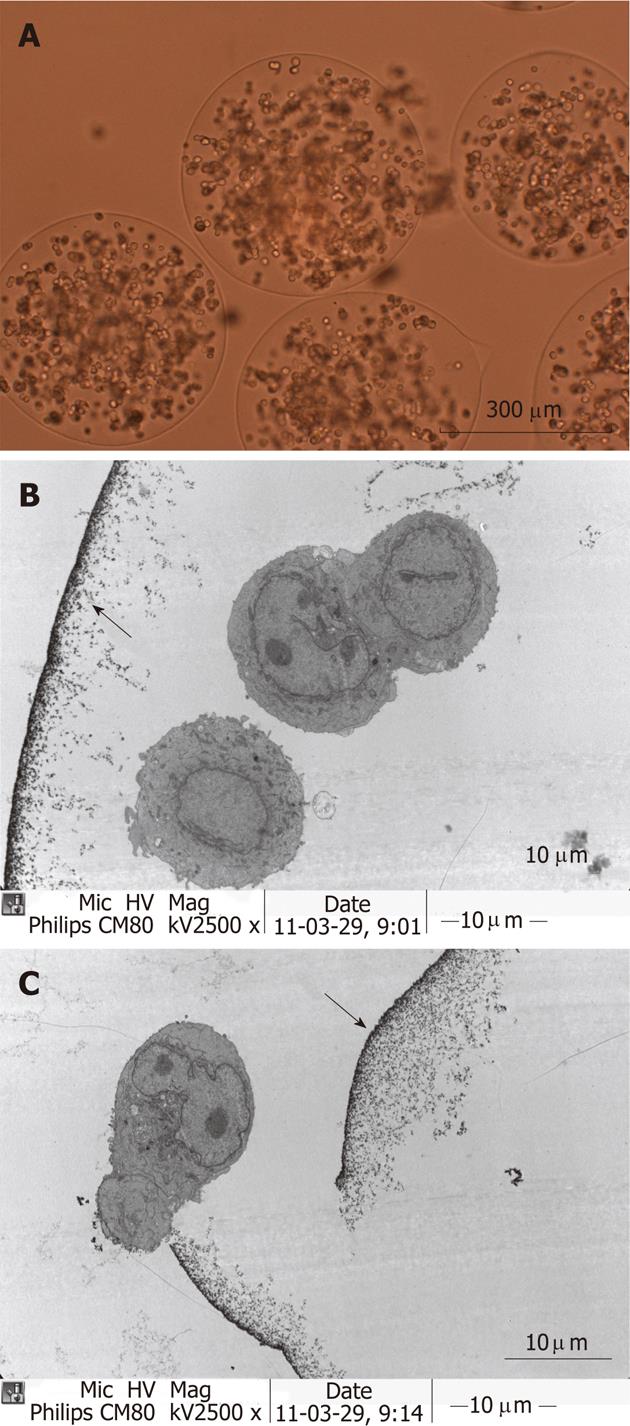
Tumor cell encapsulation: The encapsulation process begun with the preparation of a suspension of tumor cells in 1.8% sodium alginate with Matrigel (BD Bioscience, Bedford, MA) in a 25% (v/v) at a final concentration of 2 × 107 cells/mL. Matrigel can accelerate the proliferation of the cell line. The cell-alginate mixture is then extruded from a 31-gauge needle at 4 mL/min to yield spherical microdroplets using a high frequency pulse microdroplets generator (Mode 1, Voltage 60, Frequency 90, Pulse 06). The microdroplets are formed into discrete insoluble gel spheres by immersion in 1.1% CaCl2 to the neutralize of the negative charge on the surface of the microdroplet, causing gelation. The next step involves the formation of a semipermeable membrane on the surface of the gel beads by washing them with 0.05% (w/v) poly-L-lysine. The gel beads are then overcoated with 0.1% (w/v) alginate. The interior gel is liquefied by contact with a solution of 3% (w/v) sodium citrate. This process results in microcapsules with semipermeable membranes containing viable tumor cells in liquid suspension are made[5-8] (Figure 2A). Cancer microcapsules were incubated in complete medium in vitro at 37 °C with 50 mL/L CO2 to ensure that they were viable at the time of administration to the mice. The complete medium was removed and PBS was added to prepare a 50% (v/v) suspension of microcapsules.
Groups: The test groups were defined as follows, each consisting of 26 nude mice (half males and half females): The single-cell suspension group received single tumor-cell (MiaPaCa-2) suspensions. The cancer-cell microencapsulation group received encapsulated pancreatic tumor cells. The negative control group received saline instead of the tumor cells. As a positive control group, four additional mice were given intraperitoneal injections (IP) of tumor-cell suspensions to determine whether the metastases originated from cell contamination or genuine metastasis.
Establishing a subcutaneous implantation model for each group: Each of twelve nude mice was injected with 100 μL of the encapsulated tumor cell suspensions as described above, into the left flanks. Another twelve nude mice were injected with 100 μL of single tumor cell suspensions as described above, into the right flanks. Twelve nude mice were injected with saline as a negative control group.
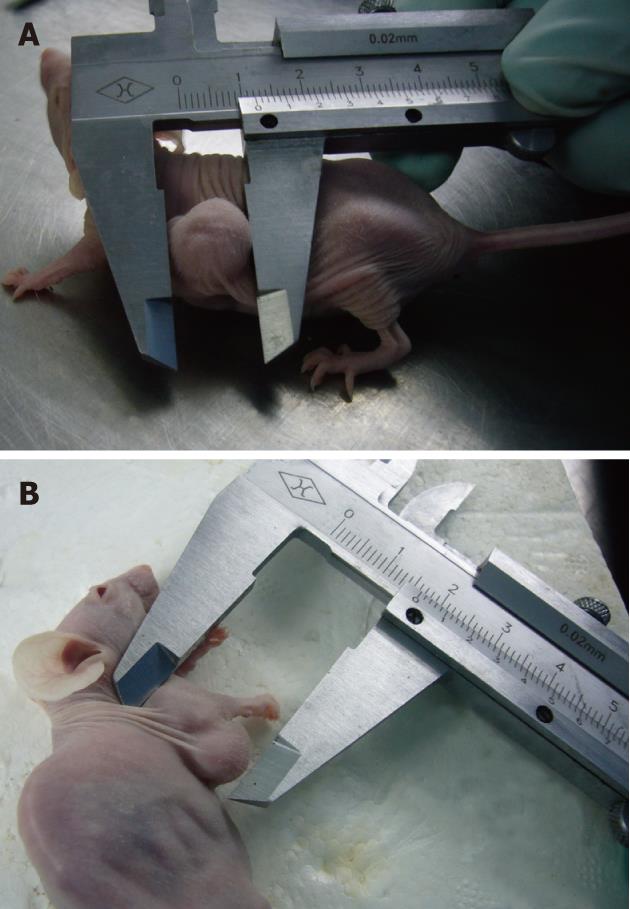
Monitoring subcutaneous xenografts: Subcutaneous xenograft progression was measured with a vernier caliper on a weekly basis. Tumor volume was calculated according with the formula V = length × width × depth/2[9]. The growth curves were constructed accordingly.
Establishing an orthotopic implantation model for each group: Fourteen nude mice were employed for the injection of single tumor cell suspensions. For the tumor induction surgery, they were anesthetized by IP injection of 0.3% chloral hydrate (0.3 g/kg). All procedures were done under aseptic conditions in a laminar airflow workstation. The tail of the pancreas was gently exteriorized through a midline incision into the peritoneal cavity. Mice received a subcapsular injection of 50 μL of single tumor cells. A technically successfully injection was characterized by the formation of a visible bubble within the pancreatic parenchyma. The needle was slowly withdrawn to avoid macroscopic cell leakage from the injection site. The pancreas was closed with 7-0 Prolene. The abdomen was closed using interrupted 6-0 silk sutures closing both skin and muscle simultaneously.
Likewise, fourteen nude mice were injected subcapsularly with encapsulated pancreatic tumor cells 50 μL as described above, and another fourteen nude mice were additionally injected subcapsularly with saline as a negative control group. To determine whether the metastasis was seeding from tumor cell suspensions or were true metastasis originated from primary tumor development, four additional mice were given IP injections of tumor cell suspensions (1 × 107 cells/mL, 100 μL).
PET-CT scan and necropsy: Twenty-eight orthotopically-injected mice, seven from the single tumor-cell suspension group and seven from the tumor-cell microencapsulation groupreceived PET-CT scans and were respectively sacrificed at at 4 and 8 wk post implantation, respectively, to ascertain the extent of metastasis and determine tumor weight. Whole-body imaging was used for primary tumor assessment via18fluorine-fluorinedeoxyglucose; necropsy was used for identification. Isolated tumor nodules with no anatomic connection to the primary tumor were judged as distant metastases.
To evaluate the occurrence of metastasis, organs and other areas, i.e., liver, kidney, spleen, mesentery, peritoneal cavity, injection site and lymph nodes, were carefully examined macroscopically. Any suspicious lesion was removed and subjected to histological analysis.
Ultrastructural observation: Microencapsulated tumor cells were sampled and fixed with 2.5% glutaraldehyde, 1% osmic acid, alcohol and epoxy. The samples were then sent for evaluation by transmission electron microscopy (JEM-1200EX) (Figure 2B and C).
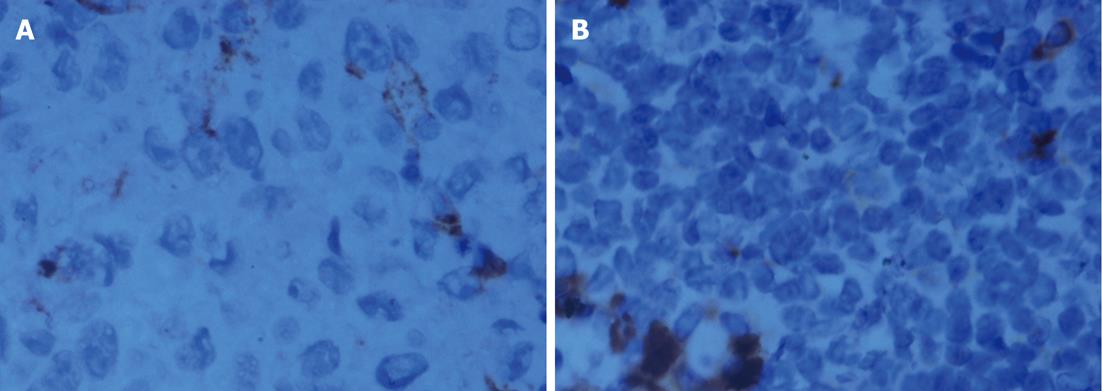
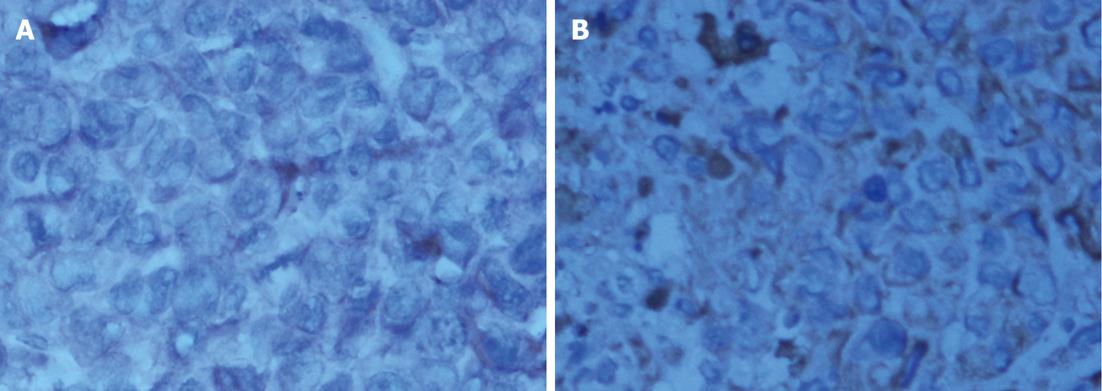
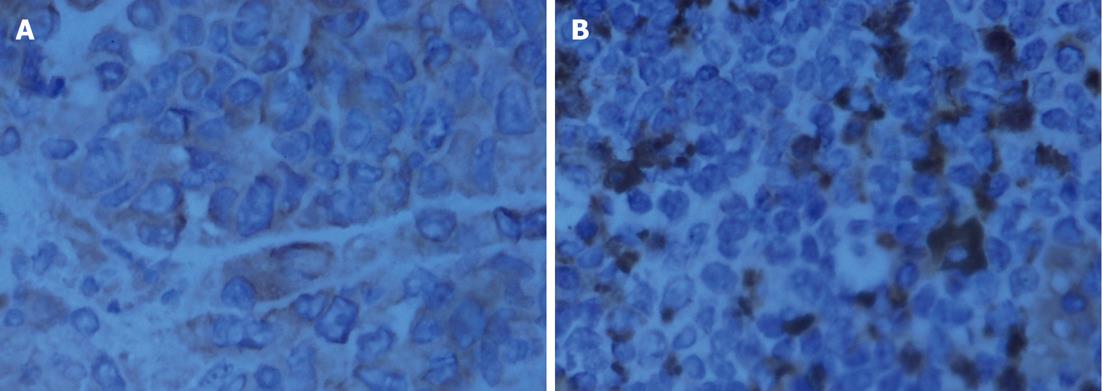
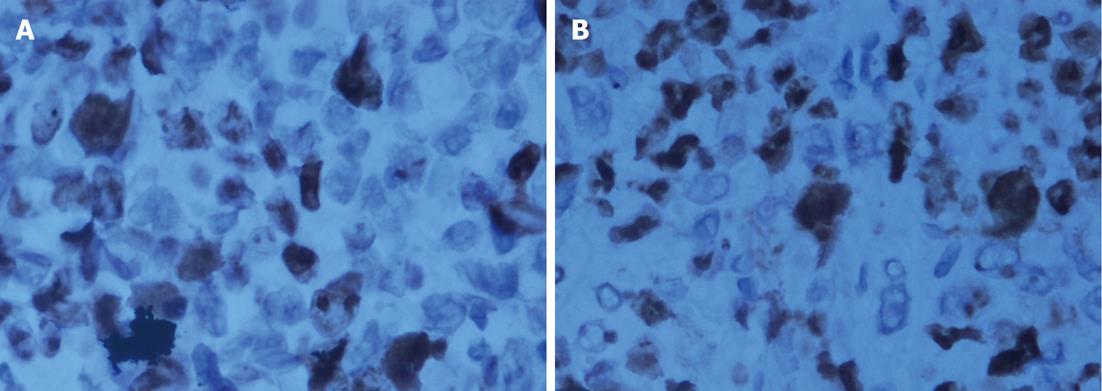
Pathological examination: Specimens were embedded in paraffin, cut into 4-mm serial sections, stained with hematoxylin and eosin and tagged with the following immunohistochemical markers: AE1AE3, carbohydrate antigen 19-9 (CA199), CAM5.2, epidermal growth factor receptor (EGFR), MIB-1 and VIM.
Differences in the rates of tumor incidence and the presence of metastasis between the cancer-cell microencapsulation group and the single-cell suspension group were analyzed using Fisher’s exact test. Variations in tumor volume and tumor weight between the cancer-cell microencapsulation group and the single-cell suspension group were analyzed using student’s t-test. Differences in tumor weight were also analyzed with the Wilcoxon signed rank sum test. P < 0.05 was considered statistically significant. Statistical calculations were done with the SAS 8.0 statistical software package.
The size of cancer microcapsules engineered in this study were uniform in size. The average diameter of 20 randomly sampled microcapsules was 420 μm ± 24 μm.
The microcapsules are completely transparent, and cell growth within them can be readily visualized with the use of an inverted microscope. Sequential in vitro observations of microcapsules showed that the cancer cells within them proliferated well and formed spheroids from days 4 to 6. We observed that all microcapsules containing cancer cells proliferated in a three-dimensional manner. The microcapsule membranes in vitro burst with increasing culture time, and the cells deviated outward and continued to grow in the flasks. The bursting day was defined as the day on which more than 10% of the microcapsules burst. Two or three days before the bursting day was assumed to be the optimal time for injection. Typically, bursting occurred from day 6 to 8. Therefore, the microcapsules were injected at 5 d after tumor cell encapsulation. A total of 100 cancer cell microcapsules were observed in sequence. The proportion of ruptured microcapsules increased to 65% (65 of 100) at 10 d and 100% at 28 d.
Gray nodules growing in round or elliptical patterns were found at the injection site 3 to 5 d after implantation. All mice remained viable during 8 wk of sequential observations; two from the single tumor-cell suspension group (2/12) and one from the encapsulated tumor-cell group (1/12) presented no detectable tumors. None of the negative control group presented detectable tumors. The rate of tumor incidence was 83.3% in the single-cell suspension group and 91.67% in the cancer-cell microencapsulation group. There was no significant difference in the rate of tumor incidence between the cancer-cell microencapsulation group and the single-cell suspension group in the subcutaneous implantation model (91.67% vs 83.3%, respectively, P = 0.5371) (Figure 3A and B).
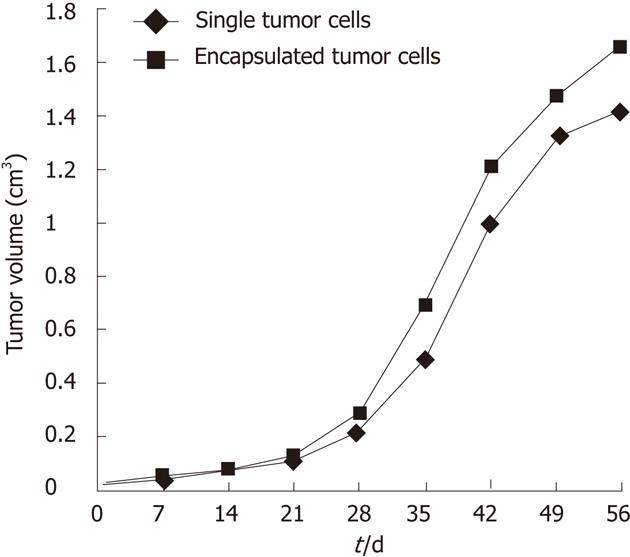
The tumor size was measured with vernier calipers, and the tumor volume was calculated on a weekly basis (Table 1). There was no significant difference in terms of tumor volume between the encapsulated pancreatic tumor-cell group and the single tumor-cell group (6th wk: 1.316 ± 0.927 vs 1.188 ± 0.570, respectively, P = 0.5348, P > 0.05; 7th wk: 2.266 ± 1.307 vs 2.020 ± 0.989, P = 0.5467; 8th wk: 2.794 ± 1.310 vs 2.602 ± 1.100, P = 0.5988). The growth curve for the subcutaneous implantation tumors is shown in Figure 4.
| At day post-implantaion (cm3) | Tumor volume | |||||||
| 4th d | 11th d | 18th d | 25th d | 32th d | 39th d | 46th d | 53th d | |
| Tumor volume of single cells | 0.054 ± 0.038 | 0.092 ± 0.055 | 0.139 ± 0.082 | 0.267 ± 0.155 | 0.596 ± 0.296 | 1.188 ± 0.570 | 2.020 ± 0.989 | 2.602 ± 1.100 |
| Tumor volume of microencapsulated cells | 0.060 ± 0.070 | 0.083 ± 0.097 | 0.145 ± 0.140 | 0.316 ± 0.278 | 0.758 ± 0.655 | 1.316 ± 0.927 | 2.266 ± 1.307 | 2.794 ± 1.310 |
No nude mice orthotopically injected with microencapsulated cancer cells or single cancer-cell suspensions showed any signs of transplantation-related complications. No obvious changes were observed in the first four weeks postimplantation. Six weeks after implantation, the mice displayed marked abdominal distension with palpable abdominal masses. Symptoms such as obvious weight loss and anorexia were observed in nude mice injected with microcapsules or single cancer cells during the 8th wk postimplantation. As expected, no symptoms were observed in the negative control group.
It should be noted that 4 out of 14 mice from the single-cell suspension group (2 of 7 at the 4th wk and 2 of 7 at the 8th wk) in the single cell suspension group had sidewall implants. These implants were located on abdominal wall and appeared as groups of gray nodules around the site of injection; they were not counted as metastases. None of the microcapsule group had sidewall implants. The orthotopic implantation model was successfully established; the rate of successful implantation was 71.43% (10 of 14) in the single-cell suspension group and 100% (14 of 14) in the cancer-cell microencapsulation group. There was a significant difference (100% vs 71.43%, P = 0.0489) in the success rates of the cancer-cell microencapsulation and single-cell suspension groups. As a control, four additional mice were given IP injections of tumor cell suspensions (1 × 107 cells/mL 100 μL) to determine whether the metastasis was caused by seeding or represented true metastasis. These IP-injected cells resulted in tumors attached to the abdominal wall at various locations within the abdomen. However, none of the free IP injections resulted in tumor sites similar to those of the metastases from the orthotopically injected cells, such as the mesentery.
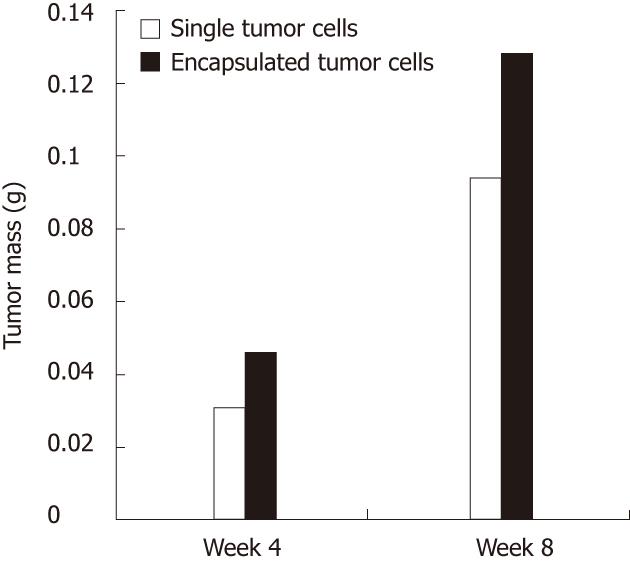
At the 4th wk postimplantation there were no statistical differences between the two groups in terms of tumor mass (0.0461 ± 0.0399 vs 0.0313 ± 0.021, t = -0.81, P = 0.4379). However, the microcapsules had significantly heavier tumors compared to the single tumor cells at eight weeks (0.1284 ± 0.0284 vs 0.0943 ± 0.0571, t = -2.28, P = 0.0457) (Figure 5). Another calculation method showed the same results (Table 2).
| Different stage (wk) | Group | No. of mice | No. of tumor mass > 500 mg | No. of tumor mass > 1000 mg | Z1 | P value1 |
| 4th | Single cells | 5 | 1 | 0 | 1.7618 | 0.0643 |
| Microencapsulation | 7 | 2 | 3 | |||
| 8th | Single cells | 5 | 2 | 1 | 2.1971 | 0.0221 |
| Microencapsulation | 7 | 1 | 6 |
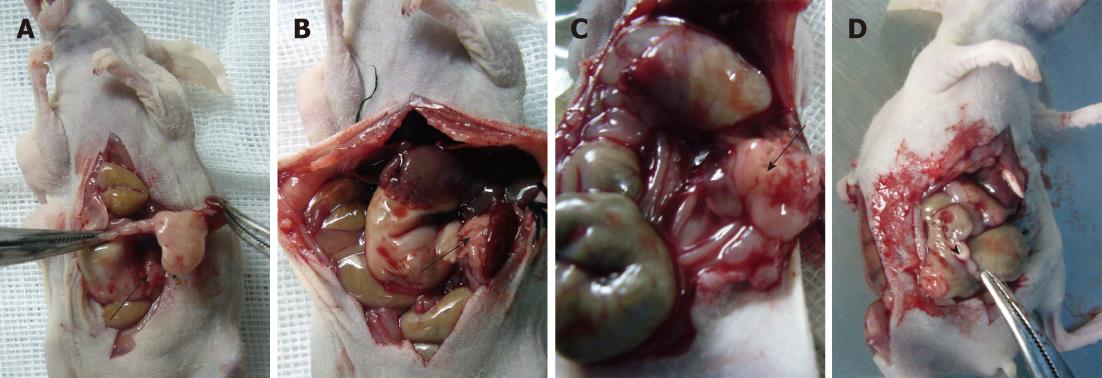
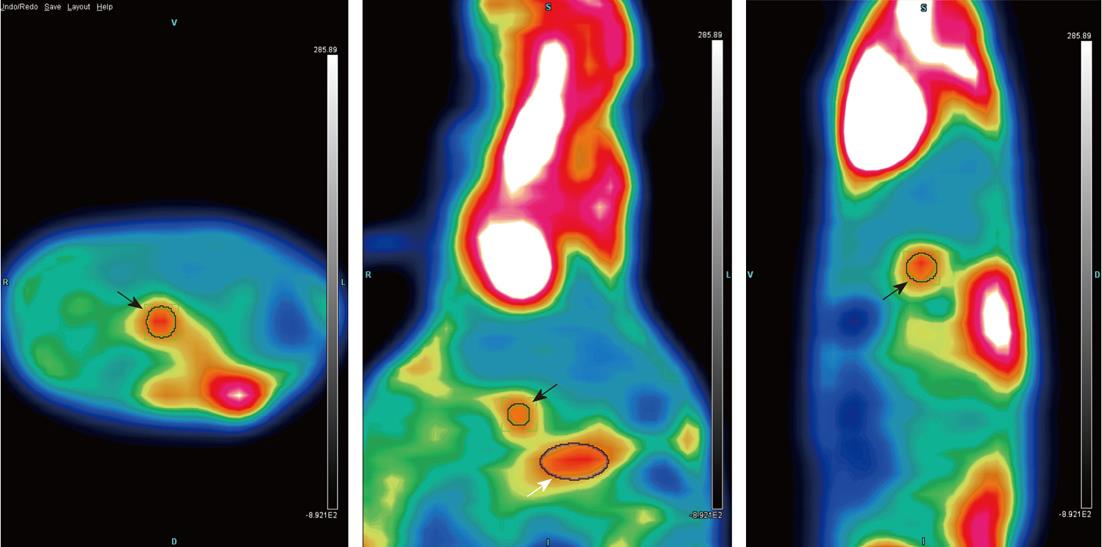
In terms of metastases, one mouse in the microcapsule group exhibited tumor spread to the small bowel mesentery (Figure 6C and D) at the 8th wk, which correlated with the PET-CT (Figure 7). No mice in either group had visible metastatic foci at the 4th week. At the 8th week, two mice in the microencapsulated group displayed metastatic spreading of the tumor to sites distant from the pancreas, which included the mesentery.
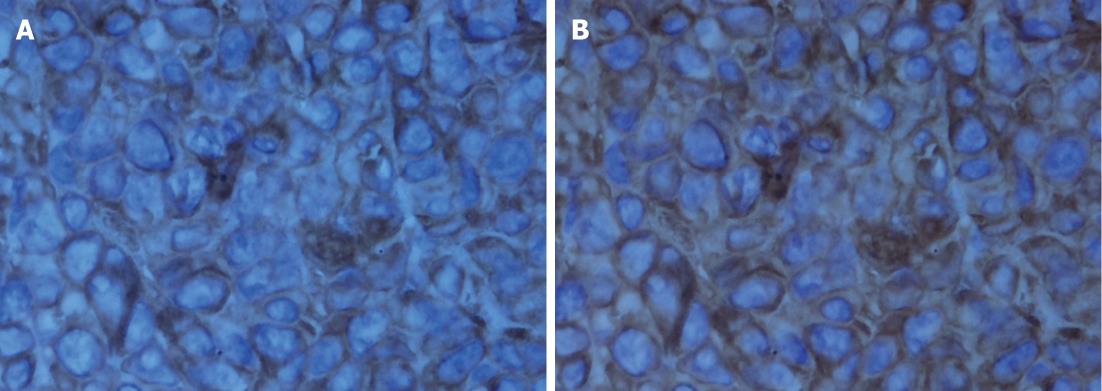
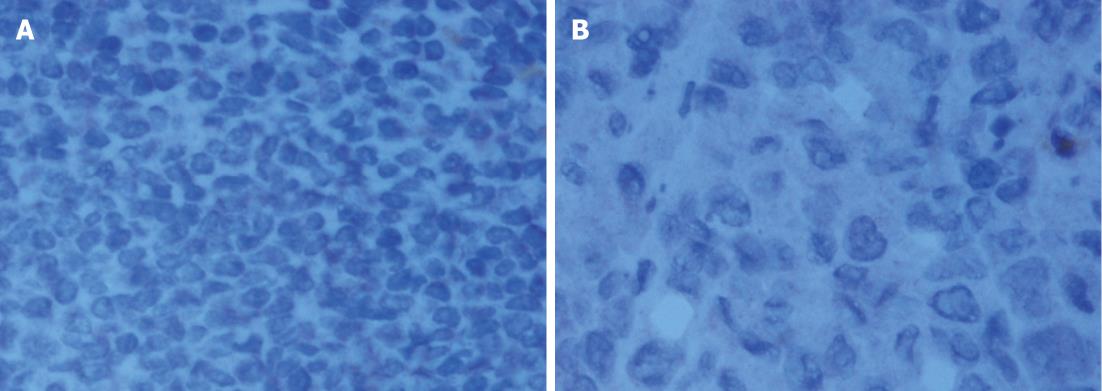
The histological characteristics of the primary tumors in the two groups exhibited similar morphologies at the 4th and 8th wk. Histopathological examinations confirmed that the suspected metastasis spots in the PET-CT truly originated from primary tumors. Immunohistochemical staining showed that tumors arising from both microencapsulated cells and single cells exhibited similar pathological characteristics: AE1AE3 (+), CAM 5.2 (+), EGFR (+), MIB-1 (60% +), CA199 (±), and VIM (-) (Figures 8-14).
The majority of in vivo experimental studies of human cancer have been conducted via subcutaneous, orthotopic and heterotopic implantation nude mouse models. The subcutaneous model has the virtues of simplicity of operation, and ease of tumor assessment. Although subcutaneous xenografts generally grow well and may be phenotypically malignant locally, they tend to form pseudocapsules, resembling encapsulated benign tumors rather than infiltrative malignancies, even when derived from undifferentiated or poorly differentiated aggressive cell lines. Importantly, subcutaneously injected human cancer cells only rarely metastasize to the parenchymatous organs and do not display the signs and symptoms that may arise as a consequence of tumor growth within visceral organs[9-14]. Thus, the subcutaneous model is of limited clinical value.
Orthotopic tumor induction is regarded as being quite suitable for modeling clinical pathology because it mimics the entire process of primary tumor growth, local tumor infiltration, and subsequent distant metastatic spread. Previous studies have shown that injection of cell line suspensions[15-18] and the transplantation of tissue fragments from primary pancreatic tumors[19-21] into the pancreas of nude mice or Syrian golden hamsters generally result in a high degree of tumorigenicity and the establishment of metastases, albeit at a varying rates[13,22]. Implantation of intact tumor fragments yields a higher rate of tumor incidence and avoids the artificial intraabdominal cell seeding associated with direct injection of pancreatic cancer cells[13].
In a clinical setting, patients with positive prognostic factors such as complete resection of the tumor and the absence of lymph node involvement or vascular invasion often succumb to metastatic disease, suggesting that metastasis may occur early in the course of the disease. Therefore, animal models that allow for the examination of cellular mechanisms in the early stage of pancreatic cancer progression are needed. Viable tumor implantation requires the preparation of subcutaneous donor tumors. Thus, implantation is more expensive and time consuming than the injection of cell-line suspensions. Moreover, transplantation of tissue fragments skips the early stage of tumor progression because the primary tumor does not develop orthotopically. Thus, the merits of injecting encapsulated tumor cells manifest themselves in a high rate of tumorigenicity and the establishment of metastases, a low rate of sidewall implants, and the ability to mimic the early progression of tumor cells.
Recently, immunoisolation technology has attracted increasing attention. Microcapsules were originally used for cell transplantation as an immunoisolation device[23]. The cell encapsulation techniques play a vital role in modern science. Microcapsules are spherical, with diameters that can be controlled in the range of 200-1500 μm, and feature a biocompatible semipermeable membrane that allows the bidirectional diffusion of nutrients, oxygen, secreted therapeutic products, and waste but prevents the passage of high-molecular-weight substances into the microcapsule, e.g., antibodies and immunocytes[24]. Immunodeficient mouse hosts were employed in our study; their immune systems were suppressed artificially. The mice did not exclude human tumor cells, which resulted in a higher rate of tumor incidence. Therefore, the immunoisolation function of microcapsules is not the key to this study. Most importantly, microcapsules in our study provide contained environments in which tumor cells grew in a three-dimensional manner and adjusted to the intravital environment of the host so that more viable tumor cells proliferated in the host and deviated to form tumor nodules.
Because nude mice are relatively expensive, delicate, and highly susceptible to infection, breeding and experimental conditions are strict, which means that nude mice cannot be used for large-scale studies. In large-scale studies with conventional mice, large quantities of tumor cells could be protected from immune eradication using tumor-cell encapsulation technology. Cancer cells would be efficiently protected by the outer layer of the microcapsule and could undergo interaction with the host in the process of initial administration and growth. Furthermore, prior studies have confirmed that encapsulated tumor cells are more viable than single cells, and the stability of tumor-associated genes is not affected[5,24].
The coupling of extracellular matrix (Matrigel) to alginate microcapsules enhanced cell proliferation by triggering a cascade of intracellular signaling events through cell-matrix interactions[25-27]. Due to the cell-matrix interactions, more membrane antigens such as AE1AE3 and ERGF may be present in the microencapsulated tumor cell membrane, as illustrated in Figures 9 and 12. Thus, more viable and adaptable cells are expected to proliferate during the primary period before bursting. Under our conditions, the cancer cells finally deviated outward and continued to grow in the flasks, unlike isolated cancer cells derived from a monolayer culture system. Thus, microencapsulated tumor cells are more likely to form tumor nodules.
Our experiments showed that in the subcutaneous implantation model, there were no statistical differences in terms of tumor volume and the rates of tumor incidence between the cancer-cell microencapsulation group and the single-cell suspension group. The former method displayed an obvious advantages in tumor mass (P = 0.0457, P < 0.05) and the rate of model success (P = 0.0489, P < 0.05) compared with the latter in the orthotopic implantation model. Additionally, it is impossible to distinguish sidewall implants from metastases using current pathological examinations. It is also very difficult to distinguish sidewall implants from metastases macroscopically in the advanced stage of pancreatic cancer. The fourth or eighth week postimplantation was not yet the terminal stage of pancreatic cancer according to the results of our experiments. Therefore, it was possible to distinguish seeding from true metastasis by the number and distribution of tumor nodules. Intraperitoneal tumor dissemination was an early event and macroscopically more extensive after injection and was most likely initiated by cells lost during the injection procedure. Thus, sidewall implants usually presented as clusters of gray nodules. For mice without distant tumors spreading to visceral organs such as the liver or mesentery at the 4th to 8th wk postimplantation, the tumor nodules described above can be seen as sidewall implants. During our experiments, we observed sidewall implants in four mice from the single-cell suspension group and did not count them as successful implantations. The primary modes for the spreading of tumors to distant sites are lymphatic and hematogeneous metastasis. Metastases usually appeared as a single nodule or dispersed nodules.
Previous studies using direct cell implantation into the peritoneal cavity, the portal vein, the spleen or the implantation of pancreatic tumor tissue grown subcutaneously in a primary animal have reported higher rates of distant tumor spread, but these models do not replicate the cell signaling and biological changes necessary for a primary tumor to develop viable secondary metastatic cell implantation and growth[3,14,22,28]. Matrigel has been reported to facilitate tumor formation and growth, but it is unknown whether such components exert similar effects on tumor metastasis[26,29]. Our experiments showed that the microencapsulated cancer-cell group was not statistically different from the single-cell suspension group with respect to metastases (2 vs 0, 28.57% vs 0%, χ2 = 2.333, P = 0.2308, P > 0.05), which may be attributed to the small sample size (n < 40) and must be confirmed by further large-scale experiments.
The use of PET-CT in the model allowed for intravital monitoring of tumor growth and improved our evaluation of the extent of disease and metastasis at necropsy. The ability to monitor tumor progression intravitally will facilitate the evaluation of therapeutic interventions.
In summary, we have described a novel orthotopic implantation technique for human pancreatic cancer in a nude mouse model. Compared with the orthotopic implantation approach using direct tumor-cell injection, the implantation of encapsulated tumor cells yielded a comparably higher rate of tumorigenicity and avoided the typically associated artificial intra-abdominal cell seeding; it thus appears to be the favorable technical approach. It is worth attempting to apply tumor-cell encapsulation technology in rats and even in conventional mice to establish an orthotopic model by exerting its advantage as an immunological barrier. By studying the pancreatic cancer model of microencapsulated tumor cells, it is possible to improve our ability to investigate the mechanism of tumor progression and metastasis. This technique provides a novel orthotopic model for anticancer drug development, screening and testing.
The authors wish to thank the staff of the Shanghai Institute of Digestive Surgery for their support in this study.
Pancreatic adenocarcinoma (PA) is an aggressive malignancy; therefore, animal models that allow further examination of pancreatic cancer progression are urgently needed. The majority of in vivo experimental studies of human cancer have been conducted in subcutaneous, orthotopic and heterotopic implantation nude mouse models. Current animal models of PA have low rates of tumor progression and metastasis.Microcapsules are spherical, with diameters that can be controlled in the range of 200-1,500 μm and a biocompatible semipermeable membrane, that allows the bidirectional diffusion of nutrients, oxygen, secreted therapeutic products, and waste but prevents the passage of high-molecular-weight substances such as antibodies and immunocytes into or out of the microcapsule.
Orthotopic tumor induction is regarded as being highly suitable for modeling clinical pathology because it mimics the whole process of primary tumor growth, local tumor infiltration, and subsequent distant metastatic spread. The implantation of intact tumor fragments yields a higher rate of tumor incidence and avoids the artificial intra-abdominal cell seeding associated with the direct injection of pancreatic cancer cells but skips the early stages of tumor progression. Thus, animal models that allow for the examination of cellular mechanisms during the early stages of pancreatic cancer progression are needed.
The microencapsulation technique displays advantages in the rate of successful implantation and tumorigenicity.
It is possible to improve our ability to investigate the mechanism of tumor progression and metastasis using this new assay, which can provide a novel orthotopic model for anticancer drug development, screening and testing.
Microencapsulation: a method of encapsulating cells in small microcapsules with semipermeable membranes.
This study evaluated the effectiveness of a mouse model using an encapsulated pancreatic tumor cell line as a PA assay in comparison with the classical model using the injection of single tumor cells. The authors found no statistically significant differences among the different models used in terms of tumor mass and tumor presence.
Peer reviewers: Juan L Iovanna, MD, PhD, Campus de Luminy, INSERM U624, 163 Av de Luminy, Marseille 13288, France; Marco Del Chiaro, MD, PhD, General and Transplant Surgery, Pisa University Hospital, Via Paradisa, 2-Cisanello, Pisa 56124, Italy
S- Editor Lv S L- Editor Logan S E- Editor Xiong L
| 1. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2204] [Article Influence: 146.9] [Reference Citation Analysis (2)] |
| 2. | Moss RA, Lee C. Current and emerging therapies for the treatment of pancreatic cancer. Onco Targets Ther. 2010;3:111-127. [PubMed] |
| 3. | Grippo PJ, Sandgren EP. Modeling pancreatic cancer in animals to address specific hypotheses. Methods Mol Med. 2005;103:217-243. [PubMed] |
| 4. | Chang TM, MacIntosh FC, Mason SG. Semipermeable aqueous microcapsules. I. Preparation and properties. Can J Physiol Pharmacol. 1966;44:115-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 237] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Gorelik E, Ovejera A, Shoemaker R, Jarvis A, Alley M, Duff R, Mayo J, Herberman R, Boyd M. Microencapsulated tumor assay: new short-term assay for in vivo evaluation of the effects of anticancer drugs on human tumor cell lines. Cancer Res. 1987;47:5739-5747. [PubMed] |
| 6. | Enomoto T, Oda T, Aoyagi Y, Sugiura S, Nakajima M, Satake M, Noguchi M, Ohkohchi N. Consistent liver metastases in a rat model by portal injection of microencapsulated cancer cells. Cancer Res. 2006;66:11131-11139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ma X, Vacek I, Sun A. Generation of alginate-poly-l-lysine-alginate (APA) biomicrocapsules: the relationship between the membrane strength and the reaction conditions. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:43-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Van Raamsdonk JM, Chang PL. Osmotic pressure test: a simple, quantitative method to assess the mechanical stability of alginate microcapsules. J Biomed Mater Res. 2001;54:264-271. [PubMed] |
| 9. | Ikeda Y, Ezaki M, Hayashi I, Yasuda D, Nakayama K, Kono A. Establishment and characterization of human pancreatic cancer cell lines in tissue culture and in nude mice. Jpn J Cancer Res. 1990;81:987-993. [PubMed] |
| 10. | Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin Exp Metastasis. 2004;21:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Fogh J, Orfeo T, Tiso J, Sharkey FE, Fogh JM, Daniels WP. Twenty-three new human tumor lines established in nude mice. Exp Cell Biol. 1980;48:229-239. [PubMed] |
| 12. | Sordat BC, Ueyama Y, Fogh J. Metastasis of tumor xenografts in the nude mouse. The nude mouse in experimental and clinical research. New York: Academic Press 1982; 95-143. |
| 13. | Fidler IJ. Critical factors in the biology of human cancer metastasis. Am Surg. 1995;61:1065-1066. [PubMed] |
| 14. | Loukopoulos P, Kanetaka K, Takamura M, Shibata T, Sakamoto M, Hirohashi S. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Bruns CJ, Shinohara H, Harbison MT, Davis DW, Nelkin G, Killion JJ, McConkey DJ, Dong Z, Fidler IJ. Therapy of human pancreatic carcinoma implants by irinotecan and the oral immunomodulator JBT 3002 is associated with enhanced expression of inducible nitric oxide synthase in tumor-infiltrating macrophages. Cancer Res. 2000;60:2-7. [PubMed] |
| 16. | Mohammad RM, Al-Katib A, Pettit GR, Vaitkevicius VK, Joshi U, Adsay V, Majumdar AP, Sarkar FH. An orthotopic model of human pancreatic cancer in severe combined immunodeficient mice: potential application for preclinical studies. Clin Cancer Res. 1998;4:887-894. [PubMed] |
| 17. | Marincola FM, Da Pozzo LF, Drucker BJ, Holder WD. Adoptive immunotherapy of human pancreatic cancer with lymphokine-activated killer cells and interleukin-2 in a nude mouse model. Surgery. 1990;108:919-929. [PubMed] |
| 18. | Tan MH, Chu TM. Characterization of the tumorigenic and metastatic properties of a human pancreatic tumor cell line (AsPC-1) implanted orthotopically into nude mice. Tumour Biol. 1985;6:89-98. [PubMed] |
| 19. | Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645-5649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 292] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. A novel "patient-like" treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice. Cancer Res. 1993;53:3070-3072. [PubMed] |
| 21. | Morioka CY, Saito S, Ohzawa K, Watanabe A. Homologous orthotopic implantation models of pancreatic ductal cancer in Syrian golden hamsters: which is better for metastasis research--cell implantation or tissue implantation? Pancreas. 2000;20:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Alves F, Contag S, Missbach M, Kaspareit J, Nebendahl K, Borchers U, Heidrich B, Streich R, Hiddemann W. An orthotopic model of ductal adenocarcinoma of the pancreas in severe combined immunodeficient mice representing all steps of the metastatic cascade. Pancreas. 2001;23:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1572] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Wang W, Yu W, Xie Y, Zhang X, Zhang Y, Ma X. Development of an in vitro multicellular tumor spheroid model using microencapsulation and its application in anticancer drug screening and testing. Biotechnol Prog. 2005;21:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Chan G, Mooney DJ. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26:382-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1132] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 27. | Hernández RM, Orive G, Murua A, Pedraz JL. Microcapsules and microcarriers for in situ cell delivery. Adv Drug Deliv Rev. 2010;62:711-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Bao L, Matsumura Y, Baban D, Sun Y, Tarin D. Effects of inoculation site and Matrigel on growth and metastasis of human breast cancer cells. Br J Cancer. 1994;70:228-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |