Published online Jan 21, 2012. doi: 10.3748/wjg.v18.i3.205
Revised: June 15, 2011
Accepted: June 22, 2011
Published online: January 21, 2012
In recent years the advent of programs for enhanced recovery after major surgery (ERAS) has led to modifications of long-standing and well-established perioperative treatments. These programs are used to target factors that have been shown to delay postoperative recovery (pain, gut dysfunction, immobility) and combine a series of interventions to reduce perioperative stress and organ dysfunction. With due differences, the programs of enhanced recovery are generally based on the preoperative amelioration of the patient’s clinical conditions with whom they present for the operation, on the intraoperative and postoperative avoidance of medications that could slow the resumption of physiological activities, and on the promotion of positive habits in the early postoperative period. Most of the studies were conducted on elective patients undergoing colorectal procedures (either laparotomic or laparoscopic surgery). Results showed that ERAS protocols significantly improved the lung function and reduced the time to resumption of oral diet, mobilization and passage of stool, hospital stay and return to normal activities. ERAS’ acceptance is spreading quickly among major centers, as well as district hospitals. With this in mind, is there also a role for ERAS in non-colorectal operations?
- Citation: Gravante G, Elmussareh M. Enhanced recovery for non-colorectal surgery. World J Gastroenterol 2012; 18(3): 205-211
- URL: https://www.wjgnet.com/1007-9327/full/v18/i3/205.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i3.205
In recent years the advent of programs for enhanced recovery after major surgery (ERAS) has led to modifications of long-standing and well-established perioperative treatments. These programs are used to target factors that have been shown to delay postoperative recovery (pain, gut dysfunction, immobility) and combine a series of interventions to reduce the perioperative stress and organ dysfunction[1]. With due differences, the programs of enhanced recovery are generally based on the preoperative amelioration of the patient’s clinical conditions with whom they presents for the operation, on the intraoperative and postoperative avoidance of medications that could slow the resumption of physiological activities, and on the promotion of positive habits in the early postoperative period (Table 1)[2]. Common factors are postoperative pain control through continuous mid-thoracic epidural anaesthesia and avoidance of regular opioids drugs, stimulation of gut motility, no mechanical bowel preparation, early physical reactivation, and limited use of catheters, tubes and drains[1].
| Preoperative | Intraoperative | Postop (first 24 h) | Day 1 | Day 2 | Day 3 | Day 4 | Additional comments | |
| Kahokehr et al[7,8] | Routine nutritional assessment; nutrition supplementation; NBM 2 h preinduction; carbohydrate loading; no bowel preparation; functional assessment and goal setting | Thoracic epidural; short acting anesthetics; intraoperative fluids: 1000 mL of crystalloid and 500 mL of colloid; prophylactic antiemetics at induction (dexamethasone); no drains or NG tubes | All IV fluid stopped before patient discharged to ward; prophylactic antiemetics; early oral feeding; nutritional supplementation; no opioids | Removal of urinary catheter | Removal of epidural | Early mobilization and physiotherapy | ||
| King et al[9-11], Blazeby et al[12], Faiz et al[13] | Optimized pre-morbid health status; functional assessment and goal setting; Meeting with stoma nurse. Nutrition supplementation; bowel preparation (for left colonic, sigmoid and rectal tumours) | Thoracic epidural; intraoperative fluids: 2000 mL of crystalloid; minimal-access surgery; local anaesthetic infiltration to the largest wound; no drains or NG tubes | Free fluid; 1 high-protein/high-calorie drink; patient sat out in chair | All IV fluid stopped; regular paracetamol; 3 high-protein/high-calorie drink; normal diet offered; patient sat out in chair; start walking; removal of urinary catheter for colonic resections; laxatives | Removal of epidural; regular NSAIDS; Morphine for breakthrough | Removal of urinary catheter for rectal resections | Aim for discharge on day 3 for colonic or day 5 for rectal resection; Provision of hospital contact numbers, review on ward if problems within 2 wk; review in outpatient clinic on day 12 | |
| Jottard et al[14] | Functional assessment and goal setting; nutrition supplementation; no bowel preparation | Thoracic epidural; anti-thrombotic and infection prophylaxis; standard anesthetic protocol; prevention of intraoperative hypothermia; no drains or NG tubes | Free fluid | All IV fluid stopped; normal diet offered | Use of anti-emetics; early mobilization; postoperative nutritional care | |||
| Maessen et al[4,5], Nygren et al[3], Hendry et al[6] | Functional assessment and goal setting; nutrition supplementation; no bowel preparation | Thoracic epidural; prevention of intraoperative hypothermia; Transverse/curved incision | Oral analgesia; Patient sat out in chair; nutritional supplements; free fluid > 800 mL | All IV fluid stopped; nutritional supplements > 400 mL; normal diet offered; patient sat out in chair > 6 h | Removal of epidural; removal of urinary catheter | |||
| Soop et al[15] | Nutrition supplementation | Thoracic epidural | Prophylactic antiemetics | Regular paracetamol and NSAIDS; patient sat out in chair for 2 h | Patient sat out in chair for 4 h | Patient sat out in chair for 3 h | Epidural removed (at least) | |
| Raymond et al[16] | Functional assessment and goal setting; nutrition supplementation | Thoracice epidural; Intra-operative targeted fluid management; No NG tube | Early mobilization/resumption of diet | |||||
| Turunen et al[17] | Functional assessment and goal setting; preoperative feeding; bowel preparation | Thoracic epidural; high-oxygen P; prevention of hypothermia; no drains or NG tubes | Removal of urinary catheter | Early mobilization/resumption of diet; no routine opioids, regular; paracetamol and NSAIDS; fluid restriction | ||||
| Senagore et al[18] | No NG tube | PCA; free fluids | Removal of urinary catheter; normal diet offered; regular NSAIDs, gabapentin, hydroxycodone if needed; no drains | |||||
| Wennstrom et al[19] | Functional assessment and goal setting; no bowel preparation; preoperative oral hydration | Thoracic epidural; short acting anaesthetics; no opioids | Free fluid; patient sat out in chair | Epidural removed; urinary catheter removal | ||||
| Mohn et al[20] | Functional assessment and goal setting; nutrition supplementation; bowel preparation. | Thoracic epidural; total intravenous anesthesia; intra-operative targeted fluid management; restricted postoperative intravenous fluids; routing antiemetics postoperatively; short midline incisions; No drains or NG tubes | Patient sat out in chair | Removal of urinary catheter; patient sat out in chair; normal diet offered; regular paracetamol and nsaids, opioids for breakthrough | Epidural removed | Regular laxatives twice daily; anti-thrombotic prophylaxis | ||
| Teeuwen et al[21] | Nutritional supplements; bowel preparation in left-sided resections; thrombotic prophylaxis | Thoracic epidural; transverse incisions except in Crohn’s disease and rectal surgery; intra-operative targeted fluid management (hypotension treated with vasopressors); no drains except in rectal surgery; no NG tubes; prophylactic antiemetics | Free fluids; nutritional supplements; patient sat out in chair | Normal diet offered; intravenous fluid administration; start walking | Epidural removed; urinary catheter removal; regular paracetamol; NSAIDs opioids for breakthrough | |||
| Ahmed et al[22,23] | Functional assessment and goal setting; nutritional supplements; no bowel preparation | High inspired oxygen; concentration; transverse incisions; no drains or NG tubes | Free fluids; soft diet offered; patient sat out in chair | Start walking | Regular paracetamol NSAIDs, opioids for breakthrough | |||
| Kirdak et al[24] | Thrombotic prophylaxis; bowel preparation; nutritional supplements | Thoracic epidural; pelvic drains with rectal dissections; urinary, central venous, and nasogastric catheters were routinely used | Start walking | NG tubes and urinary catheters removed (except pelvic dissection); soft diet offered; start walking; patient sat out in chair | Removal of urinary catheter (low pelvic operations) and drains | Epidural removed; regular paracetamol; central venous catheters removed; normal diet |
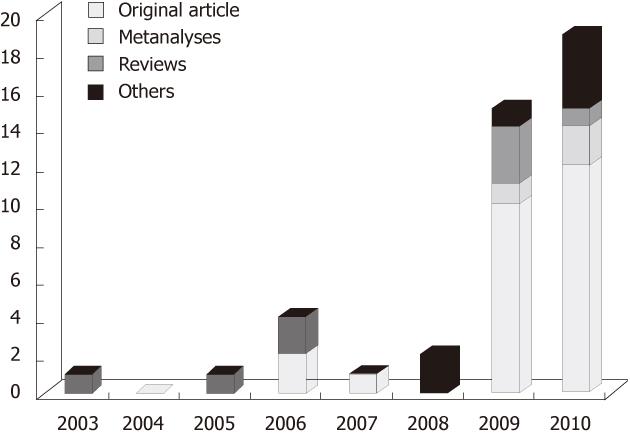
Most of the studies were conducted on elective patients undergoing colorectal procedures (either laparotomic or laparoscopic surgery) and a growing number of articles and reviews have analysed the data produced (Figure 1). Results showed that ERAS protocols significantly improved the lung function and reduced the time to resumption of oral diet, mobilization and passage of stool, hospital stay and return to normal activities[3]. Any delay in hospital discharge or early readmission was due to the development of major complications[4,5]. Higher American Society of Anesthesiologists (ASA) score, advanced age, and rectal surgery were associated with delayed mobilization, morbidity and prolonged stay[6]. ERAS’ acceptance is spreading quickly among major centers as well as district hospitals. With this in mind, is there also a role for ERAS in non-colorectal operations?
Compared to colorectal surgery, fewer studies have investigated ERAS in other operations (Table 1)[3-24].
Radical cystectomy is one of the urological procedures which has the highest rate of complications and longest hospital stay[25]. Overall complication rate is 21%-34%, early reoperation rate 6%-7%, mortality rate 0.4%-2.7%, and the hospital stay is 17.4 ± 4.7 d. The most frequent complications are pelvic lymphoceles (8.1%), wound dehiscence (6%-9%), deep venous thrombosis (4.7%), ileus (3.9%), and pulmonary embolism (2%-4%)[26-28]. Over the years, improvements in the surgical technique, anaesthesia and perioperative care have already resulted in reduced morbidity and shorter hospital stays[28]. Age is not a contraindication, and the operation can be administered even to elderly patients with similar complications rates to younger patients[26,29,30]. More important for the prediction of postoperative complications are the preoperative cardiac history, ASA score and the number of intraoperative blood transfusions. For preoperative mortality, the ASA score, blood transfusions and preoperative nutritional deficiency are important[28,31,32].
Arumainayagam et al[25] developed the only ERAS protocol available for radical cystectomies. The protocol consisted of stopping the use of mechanical bowel preparation before the cystectomy, implementation of early enteral feeding (with nutritional supplements) and mobilization as tolerated. Patients with ileal conduits after radical cystectomy were encouraged self management of the stoma and catheter care on postoperative day 2. The application of the ERAS protocol produced a significant reduction in the length of stay (13 d vs 17 d) but had no effect on the time to first defecation (6 d), morbidity, mortality and readmission rates[25]. Obviously the ERAS protocol does not affect these rates, as it has no influence on risk factors for morbidity, mortality and readmission rates. It would be interesting to evaluate if preoperative nutritional improvement, and not only for early enteral feeding, might decrease the postoperative complications rates in order to better prepare the body for surgical stress.
Liver resections have morbidity rates of 25%-48% and mortality of 1%-7%[33-35]. The length of stay with a traditional perioperative pathway ranges from between 8 and 14 d. The length of stays and intensive therapy unit stays are shorter if resections are conducted with laparoscopic surgery[36,37]. Factors associated with postoperative morbidity are neoadjuvant chemotherapy, vascular clamping, intraoperative blood loss with transfusion[35,38], comorbid conditions, pre-existent liver disease and small remnant liver volume[39]. Factors associated with postoperative mortality are the presence of blood transfusions and extended resections[40]. Age is not associated with an increase of morbidity or mortality[41].
The application of an ERAS protocol to liver surgery was evaluated by van Dam et al[1]. Their protocol was similar to those of colorectal surgery, including: nutritional supplements up to two hours before surgery, thoracic epidural analgesia, short acting anesthetics, avoidance of excessive IV fluids, warm fluids, and one night in the recovery ward before being admitted to the normal surgical ward. Among the criteria for discharge was the normalization or decreasing of serum bilirubin. Results achieved confirmed hospital stays shorter than 2 d and with no significant differences in the rates of morbidity, mortality and readmissions. In fact, as for radical cystectomies, the ERAS protocol did not alter any of the risk factors for these outcomes. A different study performed on liver resections undergoing ERAS evaluated the addition of laxatives to the protocol[42]. Although routing postoperative laxatives resulted in an earlier first passage of stool, the overall rate of recovery remained unaltered[42].
Gastric and oesophageal resections are operations associated with long hospital stays and postoperative morbidities. The average length of hospital stay after oesophagectomy ranges from 11 to 26 d following open surgery, and 7 to 13 d following laparoscopic surgery[43]. Postoperative pulmonary complications have been reported in 15%-30% of cases and are the most common cause of major morbidity and mortality[44]. Risk factors include impairment in lung function, cardiac reserve, preoperative physical activity and body composition[44]. Furthermore, a history of pulmonary disease, age, and preoperative physical activity also significantly predicts postoperative death[45]. For gastric resections, old age does not seem to affect morbidity rates (25%-29%)[46,47] but still influences mortality, which is higher amongst elderly patients (3% vs 10%)[29]. Advanced age, low albumin, ASA score, palliative resections and resection of two or more additional organs were independent risk factors for mortality[47].
ERAS protocols have been applied on both transthoracic oesophagectomies (Ivor-Lewis procedure) and laparoscopic gastric resections[43,48]. The ERAS protocol for oesophagectomies involved extubation in the operating theatre or immediately on arrival in the intensive care unit, early mobilization, negative fluid balance, intense respiratory physiotherapy and epidural analgesia. Patients remained in intensive care for three days, most drains and tubes were removed on day 4, oesophageal radiology studies were performed on day 5 and the nasogastric tube consequently removed. Particular attention was dedicated to early signs of potential complications (pulmonary infections or anastomotic leaks). With this protocol the authors achieved a significant reduction of pulmonary complications (31% vs 38%), mortality (1% vs 5%) and hospital stay (9 d vs 13 d)[43]. Even in this case, as well as for radical cystectomy, no evaluation aimed for an improvement of the preoperative nutritional status, which might decrease the postoperative complications rates in order to better prepare the body for surgical stress.
Patients receiving an ERAS protocol for laparoscopic gastric surgery had their anastomosis tested on the first postoperative day with a water soluble swallow study. If the anastomosis was intact, free fluids were started and an early diet on day 2 consisting of small, frequent low-sugar meals[48]. The urinary catheter was removed on day 1. No epidural analgesia was used for pain relief, nor any abdominal drains or nasogastric tubes. Discharge was planned for day 3. Results showed a short length of stay (4 d, range 2-30) and reduced readmission rate (6.2%). Only two patients developed postoperative complications, namely a wound abscess and an urinary infection[48].
Hysterectomy is a common gynecological procedure that is performed through various routes. Overall morbidity is present in 16% of cases, but their frequency depends on the route adopted to remove the uterus; they are usually rare after open abdominal hysterectomies (hemorrhages: 2.4%, genitourinary disorders: 1.9%, infection: 1.6% and urinary tract infections: 1.6%), even lower with the vaginal route but higher for laparoscopic abdominal hysterectomy[49]. The major causes of morbidity in patients who undergo abdominal hysterectomies are medical rather than surgical and the most important factor associated with them is the presence of comorbidities[50,51]. Readmissions are confined to 5.4%-7.2% of cases.
One article focused on ERAS protocol in gynecological operations. The protocol consisted of intensified information on pre-hospitalization consultations and admission. Intravenous lines, urinary catheters and tampons were removed before the patient left the recovery unit. Mobilization and normal food intake, including per oral analgesics, started a few hours after the operation. Routine postoperative enemas were discontinued[52]. A significant reduction of the length of stay was also confirmed in this study, but no mention was made about the rate of postoperative complications or readmission[52].
Patients undergoing post-bariatric body contouring surgery have a higher risk for postoperative complications (28%)[53,54] and among these the most common are those involving the wound healing process (infections, seromas, hematomas and delayed healing)[55-58]. The causes are multifactorial and include the percentage excess weight loss[53], total tissue resection weight[59], preoperative body mass index[60] and the recently discovered “high-calorie malnutrition”[61]. This syndrome involves the preoperative deficiency of vitamins and minerals that are important for the healing process. Differently from the other risk factors, high-calorie malnutrition syndrome is common to the overweight, obese and post-obese patients, and its perioperative corrective could improve the wound healing in all these subcategories. This is shown in the study by Agha-Mohammadi where the rate of complications in post-bariatric and obese patients was similar to normal-weight patients after perioperative nutritional supplementation of many primary ingredients necessary for wound healing and immune system competency[61]. In this case the length of stay was not evaluated because most plastic surgery procedures can be conducted during short admissions or even day surgery.
The application of ERAS protocols to non-colorectal surgery is more complex due to the paucity of literature available and to the outcomes that might be different according to the peculiarities posed by each discipline and its specific problems. Generally, a principle of ERAS is that the reduction of hospital stay should be balanced against the possibility of increased readmission rates. To achieve this objective, the rate of postoperative complications should be reduced so that patients can be safely sent home earlier with no risk to their health and no need for readmission. These two principles, reduction of the length of stay and of the postoperative morbidity, should both be targeted in a comprehensive ERAS program. However, most of the studies analyzing ERAS protocols in non colorectal-surgery focused mainly on only one factor, the length of hospital stay, which is the most evident in terms of hospital costs and productivity. The analysis conducted on non-colorectal studies showed that most protocols tried to optimize the perioperative administration of drugs, fluids and tubes following the path traced by colorectal studies. These factors obviously impacted on the overall length of stay but did not act on risk factors for postoperative morbidity, with an exception made for the post-bariatric study. Not surprisingly, the incidence of postoperative complications remained the same in most articles except for the post-bariatric study. To further improve the already positive results achieved by most ERAS programs it is advisable to focus more on the clinical conditions with which the patient arrives to the operation, redefining the situation by which the body faces the surgical stress and improving its ability to deal with it. This could not only reduce the length of stay, but also the complication and readmission rates.
Peer reviewers: Fausto Catena, MD, PhD, Department of General, Emergency and Transplant Surgery, St Orsola- Malpighi University Hospital, via Massarenti 9 Bologna 40139, Italy; Paulino Martinez, Department of Colon and Rectal Surgery, Hospital San Jose de Celaya, Celaya 38010, Mexico
S- Editor Tian L L- Editor Rutherford A E- Editor Xiong L
| 1. | van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhaug A, Fearon KC, Garden OJ, Dejong CH. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Association of Surgeons of Great Britain and Ireland. Guidelines for implementation of enhanced recovery protocols. I. Issues in professional practice. 2009;. |
| 3. | Nygren J, Soop M, Thorell A, Hausel J, Ljungqvist O. An enhanced-recovery protocol improves outcome after colorectal resection already during the first year: a single-center experience in 168 consecutive patients. Dis Colon Rectum. 2009;52:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Maessen J, Dejong CH, Hausel J, Nygren J, Lassen K, Andersen J, Kessels AG, Revhaug A, Kehlet H, Ljungqvist O. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 5. | Maessen JM, Dejong CH, Kessels AG, von Meyenfeldt MF. Length of stay: an inappropriate readout of the success of enhanced recovery programs. World J Surg. 2008;32:971-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Hendry PO, Hausel J, Nygren J, Lassen K, Dejong CH, Ljungqvist O, Fearon KC. Determinants of outcome after colorectal resection within an enhanced recovery programme. Br J Surg. 2009;96:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Kahokehr A, Sammour T, Zargar-Shoshtari K, Srinivasa S, Hill AG. Recovery after open and laparoscopic right hemicolectomy: a comparison. J Surg Res. 2010;162:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Kahokehr A, Sammour T, Sahakian V, Zargar-Shoshtari K, Hill AG. Influences on length of stay in an enhanced recovery programme after colonic surgery. Colorectal Dis. 2011;13:594-599. [PubMed] |
| 9. | King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, Kipling RM, Kennedy RH. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006;93:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 279] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | King PM, Blazeby JM, Ewings P, Kennedy RH. Detailed evaluation of functional recovery following laparoscopic or open surgery for colorectal cancer within an enhanced recovery programme. Int J Colorectal Dis. 2008;23:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | King PM, Blazeby JM, Ewings P, Longman RJ, Kipling RM, Franks PJ, Sheffield JP, Evans LB, Soulsby M, Bulley SH. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis. 2006;8:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Blazeby JM, Soulsby M, Winstone K, King PM, Bulley S, Kennedy RH. A qualitative evaluation of patients' experiences of an enhanced recovery programme for colorectal cancer. Colorectal Dis. 2010;12:e236-e242. [PubMed] |
| 13. | Faiz O, Brown T, Colucci G, Kennedy RH. A cohort study of results following elective colonic and rectal resection within an enhanced recovery programme. Colorectal Dis. 2009;11:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Jottard KJ, van Berlo C, Jeuken L, Dejong C. Changes in outcome during implementation of a fast-track colonic surgery project in a university-affiliated general teaching hospital: advantages reached with ERAS (Enhanced Recovery After Surgery project) over a 1-year period. Dig Surg. 2008;25:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Soop M, Carlson GL, Hopkinson J, Clarke S, Thorell A, Nygren J, Ljungqvist O. Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br J Surg. 2004;91:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Raymond TM, Kumar S, Dastur JK, Adamek JP, Khot UP, Stewart MS, Parker MC. Case controlled study of the hospital stay and return to full activity following laparoscopic and open colorectal surgery before and after the introduction of an enhanced recovery programme. Colorectal Dis. 2010;12:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Turunen P, Carpelan-Holmström M, Kairaluoma P, Wikström H, Kruuna O, Pere P, Bachmann M, Sarna S, Scheinin T. Epidural analgesia diminished pain but did not otherwise improve enhanced recovery after laparoscopic sigmoidectomy: a prospective randomized study. Surg Endosc. 2009;23:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Senagore AJ, Emery T, Luchtefeld M, Kim D, Dujovny N, Hoedema R. Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis Colon Rectum. 2009;52:1935-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Wennström B, Stomberg MW, Modin M, Skullman S. Patient symptoms after colonic surgery in the era of enhanced recovery--a long-term follow-up. J Clin Nurs. 2010;19:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Mohn AC, Bernardshaw SV, Ristesund SM, Hovde Hansen PE, Røkke O. Enhanced recovery after colorectal surgery. Results from a prospective observational two-centre study. Scand J Surg. 2009;98:155-159. [PubMed] |
| 21. | Teeuwen PH, Bleichrodt RP, Strik C, Groenewoud JJ, Brinkert W, van Laarhoven CJ, van Goor H, Bremers AJ. Enhanced recovery after surgery (ERAS) versus conventional postoperative care in colorectal surgery. J Gastrointest Surg. 2010;14:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Ahmed J, Khan S, Gatt M, Kallam R, MacFie J. Compliance with enhanced recovery programmes in elective colorectal surgery. Br J Surg. 2010;97:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Ahmed J, Lim M, Khan S, McNaught C, Macfie J. Predictors of length of stay in patients having elective colorectal surgery within an enhanced recovery protocol. Int J Surg. 2010;8:628-632. [PubMed] |
| 24. | Kirdak T, Yilmazlar A, Cavun S, Ercan I, Yilmazlar T. Does single, low-dose preoperative dexamethasone improve outcomes after colorectal surgery based on an enhanced recovery protocol? Double-blind, randomized clinical trial. Am Surg. 2008;74:160-167. [PubMed] |
| 25. | Arumainayagam N, McGrath J, Jefferson KP, Gillatt DA. Introduction of an enhanced recovery protocol for radical cystectomy. BJU Int. 2008;101:698-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Tilki D, Zaak D, Trottmann M, Buchner A, Ekiz Y, Gerwens N, Schlenker B, Karl A, Walther S, Bastian PJ. Radical cystectomy in the elderly patient: a contemporary comparison of perioperative complications in a single institution series. World J Urol. 2010;28:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Novotny V, Hakenberg OW, Wiessner D, Heberling U, Litz RJ, Oehlschlaeger S, Wirth MP. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51:397-401; discussion 401-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Boström PJ, Kössi J, Laato M, Nurmi M. Risk factors for mortality and morbidity related to radical cystectomy. BJU Int. 2009;103:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Donat SM, Siegrist T, Cronin A, Savage C, Milowsky MI, Herr HW. Radical cystectomy in octogenarians--does morbidity outweigh the potential survival benefits? J Urol. 2010;183:2171-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Shariat SF, Milowsky M, Droller MJ. Bladder cancer in the elderly. Urol Oncol. 2009;27:653-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Fisher MB, Svatek RS, Hegarty PK, McGinniss JE, Hightower C, Grossman HB, Kamat AM, Dinney CP, Matin SF. Cardiac history and risk of post-cystectomy cardiac complications. Urology. 2009;74:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Gregg JR, Cookson MS, Phillips S, Salem S, Chang SS, Clark PE, Davis R, Stimson CJ, Aghazadeh M, Smith JA. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 33. | Konopke R, Kersting S, Bunk A, Dietrich J, Denz A, Gastmeier J, Saeger HD. Colorectal liver metastasis surgery: analysis of risk factors predicting postoperative complications in relation to the extent of resection. Int J Colorectal Dis. 2009;24:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Benzoni E, Molaro R, Cedolini C, Favero A, Cojutti A, Lorenzin D, Intini S, Adani GL, Baccarani U, Bresadola F. Liver resection for HCC: analysis of causes and risk factors linked to postoperative complications. Hepatogastroenterology. 2007;54:186-189. [PubMed] |
| 35. | Sun HC, Qin LX, Wang L, Ye QH, Wu ZQ, Fan J, Tang ZY. Risk factors for postoperative complications after liver resection. Hepatobiliary Pancreat Dis Int. 2005;4:370-374. [PubMed] |
| 36. | Abu Hilal M, Di Fabio F, Teng MJ, Lykoudis P, Primrose JN, Pearce NW. Single-centre comparative study of laparoscopic versus open right hepatectomy. J Gastrointest Surg. 2011;15:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Zhou YM, Shao WY, Zhao YF, Xu DH, Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci. 2011;56:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Gruttadauria S, Saint Georges Chaumet M, Pagano D, Marsh JW, Bartoccelli C, Cintorino D, Arcadipane A, Vizzini G, Spada M, Gridelli B. Impact of blood transfusion on early outcome of liver resection for colorectal hepatic metastases. J Surg Oncol. 2011;103:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 40. | Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, Del Gaudio M, Pinna AD. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 41. | Shirabe K, Kajiyama K, Harimoto N, Gion T, Tsujita E, Abe T, Wakiyama S, Nagaie T, Maehara Y. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg. 2009;33:1927-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Hendry PO, van Dam RM, Bukkems SF, McKeown DW, Parks RW, Preston T, Dejong CH, Garden OJ, Fearon KC. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg. 2010;97:1198-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Munitiz V, Martinez-de-Haro LF, Ortiz A, Ruiz-de-Angulo D, Pastor P, Parrilla P. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br J Surg. 2010;97:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Feeney C, Reynolds JV, Hussey J. Preoperative physical activity levels and postoperative pulmonary complications post-esophagectomy. Dis Esophagus. 2011;24:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Liedman BL, Bennegård K, Olbe LC, Lundell LR. Predictors of postoperative morbidity and mortality after surgery for gastro-oesophageal carcinomas. Eur J Surg. 1995;161:173-180. [PubMed] |
| 46. | Kolodziejczyk P, Kulig J, Popiela T, Sierzega M, Jedrys J, Czupryna A, Kubisz A, Szczepanik A, Klek S. Outcome of gastric cancer surgery in elderly patients. Hepatogastroenterology. 2005;52:1911-1915. [PubMed] |
| 47. | Ozer I, Bostanci EB, Koc U, Karaman K, Ercan M, Ulas M, Ozogul YB, Dalgic T, Akoglu M. Surgical treatment for gastric cancer in Turkish patients over age 70: early postoperative results and risk factors for mortality. Langenbecks Arch Surg. 2010;395:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Grantcharov TP, Kehlet H. Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg. 2010;97:1547-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Hill DJ, Maher PJ, Wood CE, Lolatgis N, Lawrence A, Dowling B, Lawrence M. Complications of laparoscopic hysterectomy. J Am Assoc Gynecol Laparosc. 1994;1:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Boyd ME, Groome PA. The morbidity of abdominal hysterectomy. Can J Surg. 1993;36:155-159. [PubMed] |
| 51. | Spilsbury K, Hammond I, Bulsara M, Semmens JB. Morbidity outcomes of 78,577 hysterectomies for benign reasons over 23 years. BJOG. 2008;115:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Sjetne IS, Krogstad U, Ødegård S, Engh ME. Improving quality by introducing enhanced recovery after surgery in a gynaecological department: consequences for ward nursing practice. Qual Saf Health Care. 2009;18:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | van der Beek ES, van der Molen AM, van Ramshorst B. Complications after body contouring surgery in post-bariatric patients: the importance of a stable weight close to normal. Obes Facts. 2011;4:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Araco A, Gravante G, Araco F, Delogu D, Filingeri V, Cervelli V. Body contouring after weight loss: the plastic-bariatric surgery symbiosis. Aesthetic Plast Surg. 2006;30:374-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Gravante G, Araco A, Sorge R, Araco F, Delogu D, Cervelli V. Wound infections in post-bariatric patients undergoing body contouring abdominoplasty: the role of smoking. Obes Surg. 2007;17:1325-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Gravante G, Araco A, Sorge R, Araco F, Delogu D, Cervelli V. Wound infections in body contouring mastopexy with breast reduction after laparoscopic adjustable gastric bandings: the role of smoking. Obes Surg. 2008;18:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Araco A, Sorge R, Overton J, Araco F, Gravante G. Postbariatric patients undergoing body-contouring abdominoplasty: two techniques to raise the flap and their influence on postoperative complications. Ann Plast Surg. 2009;62:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Araco A, Gravante G, Araco F, Sorge R, Cervelli V. Postoperative seromas after abdominoplasty: a retrospective analysis of 494 patients and possible risk factors. Plast Reconstr Surg. 2009;123:158e-159e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Gravante G, Araco A, Sorge R, Araco F, Nicoli F, Caruso R, Langiano N, Cervelli V. Pulmonary embolism after combined abdominoplasty and flank liposuction: a correlation with the amount of fat removed. Ann Plast Surg. 2008;60:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | de Kerviler S, Hüsler R, Banic A, Constantinescu MA. Body contouring surgery following bariatric surgery and dietetically induced massive weight reduction: a risk analysis. Obes Surg. 2009;19:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Agha-Mohammadi S, Hurwitz DJ. Enhanced recovery after body-contouring surgery: reducing surgical complication rates by optimizing nutrition. Aesthetic Plast Surg. 2010;34:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |