Published online Jun 14, 2012. doi: 10.3748/wjg.v18.i22.2872
Revised: January 9, 2012
Accepted: February 8, 2012
Published online: June 14, 2012
Recently, the development of endoscopic procedures has increased the availability of minimally invasive treatments; however, there have been few case reports of duodenal hemangioma treated by endoscopic mucosal resection. The present report describes a case of duodenal hemangioma that showed various endoscopic changes over time and was treated by endoscopic mucosal resection. An 80-year-old woman presented with tarry stools and a loss of appetite. An examination of her blood revealed severe anemia, and her hemoglobin level was 4.2 g/dL. An emergency upper gastrointestinal endoscopy was performed. A red, protrusive, semipedunculated tumor (approximately 20 mm in diameter) with spontaneous bleeding on its surface was found in the superior duodenal angle. Given the semipedunculated appearance of the tumor, it was suspected to be an epithelial tumor with a differential diagnosis of hyperplastic polyp. The biopsy results suggested a telangiectatic hemangioma. Because this lesion was considered to be responsible for her anemia, endoscopic mucosal resection was performed for diagnostic and treatment purposes after informed consent was obtained. A histopathological examination of the resected specimen revealed dilated and proliferated capillary lumens of various sizes, which confirmed the final diagnosis of duodenal hemangioma. Neither anemia nor tumor recurrence has been observed since the endoscopic mucosal resection (approximately 1 year). Duodenal hemangiomas can be treated endoscopically provided that sufficient consideration is given to all of the possible treatment strategies. Interestingly, duodenal hemangiomas show morphological changes that are influenced by various factors, such as mechanical stimuli.
- Citation: Nishiyama N, Mori H, Kobara H, Fujihara S, Nomura T, Kobayashi M, Masaki T. Bleeding duodenal hemangioma: Morphological changes and endoscopic mucosal resection. World J Gastroenterol 2012; 18(22): 2872-2876
- URL: https://www.wjgnet.com/1007-9327/full/v18/i22/2872.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i22.2872
Vascular lesions of the duodenum, including hemangiomas, are rare causes of gastrointestinal bleeding. Indeed, there have been very few reports of hemangioma in the duodenum. Endoscopically, hemangiomas have various morphological features. The present case is the first report of a duodenal hemangioma that showed various morphological features at the time of endoscopy. Although duodenal hemangioma is treated by surgical duodenectomy, the use of less-invasive procedures, such as endoscopic mucosal resection (EMR), has increased. The present study found that EMR is an effective treatment for hemorrhagic duodenal hemangioma. In addition, the present study summarized previous case reports of duodenal hemangioma.
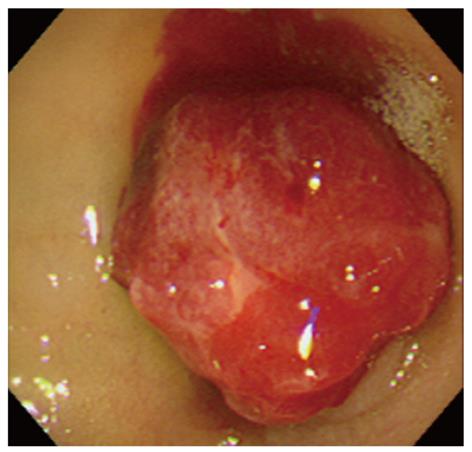
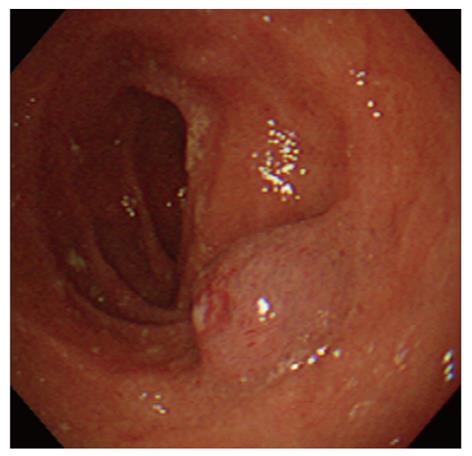
An 80-year-old woman presented with tarry stools and a loss of appetite. She had undergone laparoscopic cholecystectomy for acute cholecystitis when she was in her seventies. She had experienced a loss of appetite and anemic symptoms since approximately March 10, 2008, but she did not seek medical attention. She noticed a tarry stool on March 15 and experienced pallor of the face and astasia on March 18, which was the day she presented at the emergency department of Sakaide municipal hospital. The physical findings upon admission included a body height of 148 cm, a body weight of 45 kg, clear sensorium, a body temperature of 36.6 °C, a blood pressure of 116/56 mmHg, a pulse rate of 84 bpm, and an oxygen saturation of 100%. The palpebral conjunctiva was noted to be anemic. The abdomen was flat and soft with no spontaneous pain or tenderness. An examination of the blood at the time of admission revealed significant anemia with a hemoglobin level of 4.2 g/dL. In addition, her albumin level was decreased to 3.3 g/dL. Platelet counts, coagulation tests, and renal/hepatic function tests were all normal. An emergency upper endoscopy was performed on admission, but no abnormalities were found in the esophagus or the stomach. A red, semipedunculated tumor (20 mm in diameter) with spontaneous bleeding was found in the superior duodenal angle (SDA) (Figure 1). This patient had received an upper endoscopy at another hospital 9 mo earlier and had undergone biopsy after a blue, nonpedunculated, submucosal tumor-like lesion was found in SDA (Figure 2). Because there was no active bleeding during the endoscopic observation, biopsies were obtained from the basal and apical portions of the protruding lesion for diagnostic purposes. Although the pathological examination of the biopsy samples only identified necrotic material in the basal sample, we observed a dense proliferation of capillaries in the apical sample, which resulted in a diagnosis of suspected capillary hemangioma. Contrast-enhanced computed tomography (CT) revealed a slightly enhanced tumor (approximately 20 mm in diameter) in the SDA. Interestingly, we did not observe the presence of thick supplying blood vessels or an extramural extension of the tumor.
Based on the upper endoscopic findings and the results of the pathological examination, a duodenal hemangioma was considered to be the source of the bleeding. With conservative therapy, the hemoglobin level increased to 7 g/dL, and hemostasis was achieved.
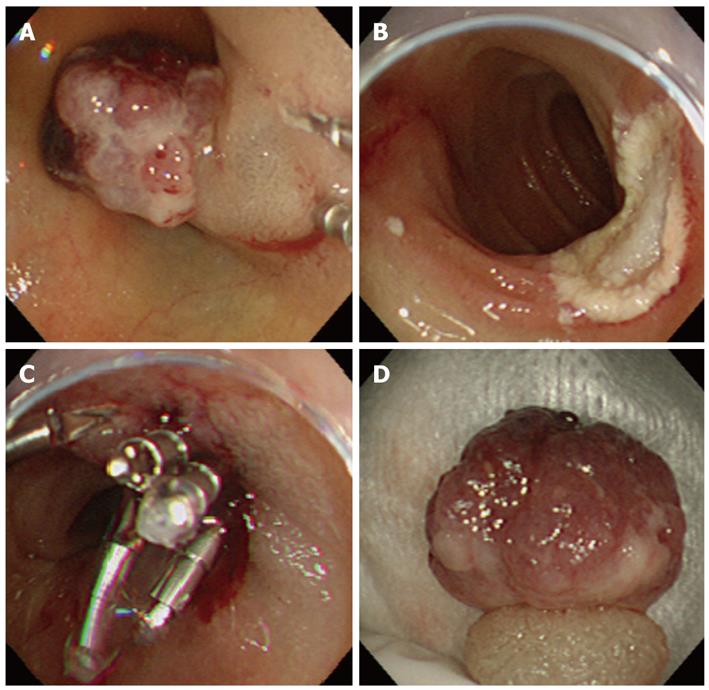
Because of the possibility of recurrent bleeding, EMR was performed to remove the hemangioma (Figure 3A-D). The top of the tumor was semipedunculated and irregular. First, the endothelial tumor was distinguished, but the biopsy showed the presence of a hemangioma, and the CT revealed an absence of thick supplying blood vessels. Additionally, the patient was of advanced aged and suffered from dementia. Also, the patient’s family did not want her to undergo surgery. Moreover, the duodenal mucosa was sufficiently elevated following a local injection of salin solution; thus, EMR was performed. If histology of the resected specimen had revealed malignant potential, then surgery would have been performed.
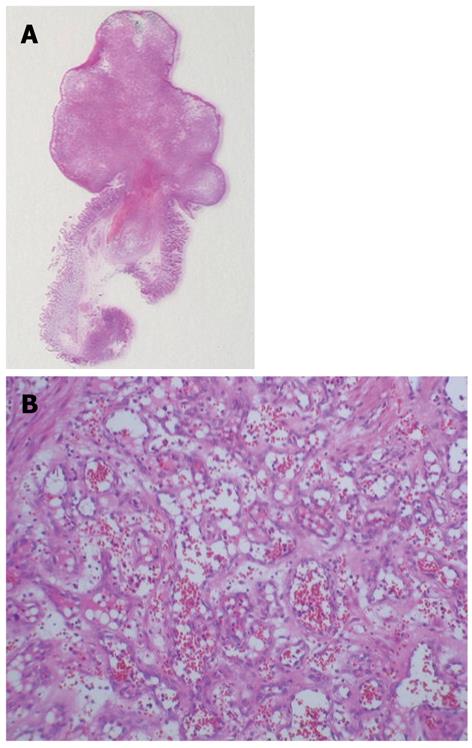
We performed another upper endoscopy 3 d after the EMR, and hemostasis was confirmed. The patient had a favorable postoperative course and was discharged from the hospital without complications 1 wk after the EMR. Histopathological examination revealed the proliferation of endothelial cells lining the lumens of capillaries of various sizes and confirmed the diagnosis of capillary hemangioma (Figure 4A and B).
Hemangiomas only account for approximately 0.3% of all gastrointestinal tumors[1]. A search for Japanese and foreign case reports of duodenal hemangioma between 1978 and 2008 through the Japana Centra Revuo Medicina and MEDLINE only identified 22 reports, including the present case, which indicated a very low incidence of the disease (Table 1)[2-12]. We conducted a clinicopathological review of the identified Japanese case reports, including the present case. The mean age of the patients was 55 years, and the ages ranged from 2 years to 85 years. The chief complaints in the Japanese cases included melena/anemic symptoms in 82% (14 of 17) of the cases and obstructive symptoms in 1 case. Interestingly, there were only 2 asymptomatic cases. Nader et al. reported that only 30% of hemangioma cases were asymptomatic. Gastrointestinal hemorrhage/anemia was observed in 73.2% of the hemangioma cases, and obstructive symptoms were observed in 12.8% of the cases[13]. Tumors were slightly more commonly in the descending portion (8 of 17 cases, 48%) of the duodenum compared with the other portions of the duodenum. In terms of size, cavernous hemangiomas tend to be larger than capillary hemangiomas (11-80 mm vs 8-20 mm, respectively). Interestingly, cavernous hemangiomas accounted for the majority of the reported hemangioma cases. Indeed, cavernous hemangioma was found in 11 cases, capillary hemangioma was observed in 4 cases, and mixed capillary hemangioma and multiple phlebectasia were observed in 1 case.
| Author | Year | Patient age | Chief complaint | Location in duodenum | size (mm) | Morphology | Pathology | Treatment | |
| 1 | Koga | 1978 | 65 | Anemia | 1-2nd | 1 × 1 | Unknown | Multiple hemangiomas | Surgical resection |
| 2 | Ikeda et al[6] | 1980 | 33 | Tarry stool, shock | 2nd | Unknown | SMT-like | Cavernous | Surgical resection |
| 3 | Kawamura | 1982 | 77 | Anemia | 3rd | 80 × 30 | SMT-like | Cavernous | Surgical resection |
| 4 | Furuya | 1987 | 72 | Tarry stool, anemia | 2nd | 19 × 14 | SMT-like | Cavernous | Endoscopic polypectomy |
| 5 | Tadokoro | 1991 | 55 | Massive melena | 2nd | 10 × 14 | SMT-like | Capillary | Endoscopic polypectomy |
| 6 | Amo | 1991 | 20 | Melena | 2nd | Several lesions | SMT-like | Capillary | Local ethanol injection alone |
| 7 | Shin | 1992 | 69 | Hematemesis | 1st | 14 × 9 | SMT-like | Cavernous | Surgical resection |
| 8 | Fujikawa et al[7] | 1996 | 52 | Anemia | 4th | 20 × 10 | SMT-like | Cavernous | Surgical resection |
| 9 | Maeda et al[2] | 2000 | 85 | Anemia | 3rd | 11 × 8 | SMT-like | Capillary/venous | Surgical resection |
| 10 | Terui | 2002 | 7 | Intestinal obstruction | 3rd | 11 × 7 × 3 | SMT-like | Cavernous | Surgical resection |
| 11 | Inoue | 2002 | 40 | Health check-up | 3rd | Unknown | SMT-like | Cavernous | Surgical resection |
| 12 | Oikawa | 2005 | 53 | Melena | 3rd | 30 | SMT-like | Cavernous | Surgical resection |
| 13 | Taniguchi | 2006 | 53 | Melena | 3rd | 30 | SMT-like | Cavernous | Surgical resection |
| 14 | Kakinuma et al[3] | 2007 | 63 | Melena | 4th | 50 | SMT-like | Cavernous | Surgical resection |
| 15 | Yamashita et al[4] | 2008 | 70 | Tarry stool | 3rd | 20 | SMT-like | Cavernous | Unknown |
| 16 | Sakamoto et al[5] | 2008 | 54 | Health check-up | 3rd | 15 | SMT-like | Unknown | Follow-up |
| 17 | Nishiyama (present case) | 2008 | 83 | Tarry stool, shock | 1st | 20 × 12 | Polypous | Capillary | Endoscopic mucosal resection |
| 18 | Bibao et al[8] | 1989 | 61 | Anemia | 2nd | Unknown | SMT-like | Unknown | Embolization (catheter) |
| 19 | Gymermek-klinika | 1991 | 14 | Melena | Unknown | Unknown | Unknown | Unknown | Embolization + sclerotherapy |
| 20 | Lee et al[10] | 1993 | 31 | Melena | 2nd | 40 | Unknown | Cavernous | Surgical resection |
| 21 | Chattopadhyay et al[11] | 2002 | 2 | Vomiting | 4th | 100 | SMT-like | Unknown | Surgical resection |
| 22 | Devadason et al[12] | 2007 | 4 | None | 1-2nd | Unknown | SMT-like | Capillary | Follow-up |
Characteristic endoscopic findings included color ranging from blue to dark red and a structure that was easily deformed by compression. Although duodenal hemangiomas are morphologically classified as submucosal tumorlike, diffusely infiltrating, or polypous lesions, all of the reported cases except for the present case have been classified as submucosal tumor-like lesions. Interestingly, the present case had a polypous appearance.
Because hemangiomas show variable endoscopic findings in terms of size, shape, and color, examinations and pretreatment information are required for the diagnosis. These examinations include contrast-enhanced CT and magnetic resonance imaging to determine the location of the tumor and the blood stream, endoscopic ultrasonography to determine the extent of the lesion, selective angiography, and duodenal X-rays. The final diagnosis is generally made based on the results of the pathological examination of the resected specimens.
Kaijer et al[14] pathologically classified duodenal hemangiomas into 4 types: type I, multiple phlebectasia; type II, cavernous hemangioma of diffuse and localized polypous types; type III, simple capillary hemangioma; and type IV, angiomatosis with a gastrointestinal lesion. In terms of frequency, type II hemangiomas account for the majority (55%) of all cases, but type II and a mixture of types II and III are almost comparable in frequency. The present case was classified as a type II lesion of the localized polypous type.
Although duodenal hemangiomas are conventionally treated surgically (i.e., partial intestinal resection), less invasive treatment strategies have recently been reported, including endoscopic polypectomy, EMR, local ethanol injection therapy, catheter embolization therapy, and endoscopic sclerotherapy. Among the reported cases, surgery was performed in 11 cases, polypectomy was performed in 2 cases, and EMR was performed in 1 case (i.e., the present case). Although no formal criteria have been established for the indications of EMR, patients meeting the following three criteria are considered eligible for EMR: (1) patients with a lesion in the endoscopically accessible SDA or descending portion of the duodenum; (2) patients with a 25-mm or smaller polypous lesion classified as type II, III, or IV according to Yamada’s classification[15]; and (3) patients without a large blood vessel in the lesion (determined using contrast-enhanced abdominal CT or abdominal angiography).
We chose EMR in the present case because the patient had a 20-mm polypous lesion in the SDA, which is an endoscopically accessible region, and we did not detect a large supplying vessel on the contrast-enhanced abdominal CT. Surgical treatment was likely selected in many of the previous cases because the lesions were typically in the descending portion of the duodenum. Because the surgical resection of a duodenal lesion tends to cause excessive surgical stress, endoscopic or catheter-based treatments may be considered as an option after careful consideration. It should be noted, however, that duodenal blood vessels have thinner walls compared with blood vessels in other parts of the intestine. Furthermore, it is difficult to manipulate an endoscope in the duodenum, which has 2 curved sections. In addition, if a resected specimen falls into the anal side of the intestine, it may be difficult to retrieve. Thus, the indication for treatment should be carefully considered.
When the patient from the present study presented at our hospital, the tumor was exposed on the mucosal surface and had increased in size. Hemangiomas can grow into various shapes according to the influence of several factors, such as thrombi, fibrosis, calcification, infection, peristalsis, and mechanical stimuli. Although the literature search did not identify any case reports of duodenal hemangiomas characterized by increasing size, the lesion in the present case appeared to have initially developed as a submucosal tumor. Interestingly, mucosal bleeding and ulceration caused by mechanical stimuli, such as biopsy and contact with food, led to the tumor being exposed to the lumen and resulted in its deformation into a polypous lesion.
We experienced a rare case of duodenal hemangioma in the present patient, and duodenal hemangioma in general can be treated by an appropriately selected, less invasive treatment strategy. The present case provided a valuable experience that enabled us to understand the morphological changes that hemangiomas can display over time.
Peer reviewers: Naoki Ishii, MD, Department of Gastroenterology, St. Luke’s In, 9-1 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan; Dr. Peter Draganov, MD, University of Florida, 1600 SW Archer Rd., Room HD 602, PO Box 100214, Gainesville, FL 32610, United States
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Gentry RW, Dockerty MB, Glagett OT. Vascular malformations and vascular tumors of the gastrointestinal tract. Surg Gynecol Obstet. 1949;88:281-323. [PubMed] |
| 2. | Maeda T, Miyamoto K, Fujisawa T. [Vascular lesions of the intestine: a review focused on localized timorous lesions - a case of duodenal hemangioma found in an anemic patient]. Stom Intest. 2000;36:808-813. |
| 3. | Kakinuma D, Nakamura Y. [A case of cavernous hemangioma in the duodenum accompanied by portal vein thrombosis]. Jpn J Gastroenterol Surg. 2008;41:87-92. |
| 4. | Yamashita M. [A case of duodenal hemangioma effectively diagnosed by contrast-enhanced ultrasonography]. Jpn J Med Ultrasound Technol. 2008;33:190. |
| 5. | Sakamoto S, Ono K. [A case of submucosal tumor in the duodenum found in an asymptomatic patient and suspected to be hemangioma on imaging]. Endosc Forum Dig Dis. 2008;24:149. |
| 6. | Ikeda K, Murayama H, Takano H, Araki S, Ikejiri K. Massive intestinal bleeding in hemangiomatosis of the duodenum. Endoscopy. 1980;12:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Fujikawa T, Kurata M, Takaori K, Matsusue S, Takeda H, Sakai S. Solitary cavernous hemangioma of the duodenum: report of a case. Surg Today. 1996;26:807-809. [PubMed] |
| 8. | Bilbao JI, Longo J, Aquerreta D, San Julián P, Muñoz M. Duodenal angioma: transcatheter embolotherapy. AJR Am J Roentgenol. 1989;152:1128. [PubMed] |
| 9. | Rákóczy G, Szlávy L, Verebély T, Szönyi L, Jellinek K, Székely A. [A case of successfully treated duodenal hemangioma]. Orv Hetil. 1991;132:33-34. [PubMed] |
| 10. | Lee MG, McDonald A, Escoffery C. Cavernous haemangioma of the duodenum. A rare cause of gastrointestinal bleeding. West Indian Med J. 1993;42:34-36. [PubMed] |
| 11. | Chattopadhyay A, Kumar V, Maruliah M, Rao PL. Duodenojejunal obstruction by a hemangioma. Pediatr Surg Int. 2002;18:501-502. [PubMed] |
| 12. | Devadason D, Murphy MS, Brown R, Wilson D, McKiernan PJ. Duodenal capillary hemangiomatous polyps: a novel manifestation of extrahepatic portal hypertension? J Pediatr Gastroenterol Nutr. 2007;45:114-116. [PubMed] [DOI] [Full Text] |
| 13. | Nader PR, Margolin F. Hemangioma causing gastrointestinal bleeding. Case report and review of the literature. Am J Dis Child. 1966;111:215-222. [PubMed] |
| 14. | Kaijser R. Hemangioma of the gastrointestinal tract. Arch Klin Chir. 1936;187:351-388. |
| 15. | Inui M, Horie H. [A case of esophageal hemangioma treated by endoscopic polypectomy]. Gastroenterol Endosc. 1990;32:554-561. |