Published online Jun 14, 2012. doi: 10.3748/wjg.v18.i22.2798
Revised: September 4, 2011
Accepted: April 12, 2012
Published online: June 14, 2012
AIM: To investigate plasma microRNA (miRNA) profiles indicative of hepatotoxicity in the setting of lethal acetaminophen (APAP) toxicity in mice.
METHODS: Using plasma from APAP poisoned mice, either lethally (500 mg/kg) or sublethally (150 mg/kg) dosed, we screened commercially available murine microRNA libraries (SABiosciences, Qiagen Sciences, MD) to evaluate for unique miRNA profiles between these two dosing parameters.
RESULTS: We distinguished numerous, unique plasma miRNAs both up- and downregulated in lethally compared to sublethally dosed mice. Of note, many of the greatest up- and downregulated miRNAs, namely 574-5p, 466g, 466f-3p, 375, 29c, and 148a, have been shown to be associated with asthma in prior studies. Interestingly, a relationship between APAP and asthma has been previously well described in the literature, with an as yet unknown mechanism of pathology. There was a statistically significant increase in alanine aminotransferase levels in the lethal compared to sublethal APAP dosing groups at the 12 h time point (P < 0.001). There was 90% mortality in the lethally compared to sublethally dosed mice at the 48 h time point (P = 0.011).
CONCLUSION: We identified unique plasma miRNAs both up- and downregulated in APAP poisoning which are correlated to asthma development.
- Citation: Ward J, Bala S, Petrasek J, Szabo G. Plasma microRNA profiles distinguish lethal injury in acetaminophen toxicity: A research study. World J Gastroenterol 2012; 18(22): 2798-2804
- URL: https://www.wjgnet.com/1007-9327/full/v18/i22/2798.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i22.2798
Acetaminophen (APAP) continues to be an important cause of acute liver failure in the developed world, second only to infectious etiologies worldwide[1]. It is also the most common cause of death due to analgesic ingestion in the United States[2]. Numerous emergency department patients in the setting of acetaminophen overdose are admitted to hospital for N-acetylcysteine (NAC) treatment. This necessary treatment modality places these patients at risk for health-care associated disease. It also places likely unnecessary additional financial strain on an already burdened healthcare system. In addition, the estimated United States cost of treating intentional acetaminophen overdose is $86.9 million per year[3]. However, in over 30 years of research it is still unclear how the exact mechanism of acetaminophen toxicity occurs[4].
Current literature implicates N-acetyl-para-benzoquinone-imine, or N-acetyl-p-benzoquinoneimine (NAPQI), as the primary metabolite responsible for hepatotoxicity. An estimated 90% of APAP is metabolized in the liver via either glucuronidation or sulfation. Another approximate 5% is urinated unaltered. However, approximately 5% of APAP is metabolized by the cytochrome P450 2E1 pathway into NAPQI. In the scenario of APAP overdose, the normal route of metabolism becomes overburdened, so an overabundance of NAPQI is produced, causing hepatotoxicity. Glutathione can rescue this process, and is the reason NAC is used as a treatment modality. However, it is presumed only a portion of hepatotoxicity occurs via this mechanism. Lipid peroxidation via free radical formation, and mitochondrial dysfunction via increased permeability of the mitochondrial permeability transition, are also postulated as causes of APAP-associated hepatotoxicity[5,6].
Clearly, a better understanding of how APAP specifically causes hepatic toxicity from a pathophysiologic perspective still needs to be determined. In addition, standard clinical laboratory testing may not reveal evidence of hepatic injury for up to 24 h following APAP ingestion. A considerable proportion of APAP-exposed individuals therefore receive unnecessary empiric treatment with an antidote before hepatic injury can be ruled out. To overcome this clinical problem, early diagnostic indicators of hepatic injury have been sought.
MicroRNA fragments (miRNAs), are short, chemically stable biomolecules, noncoding posttranslational regulators that bind to untranslated mRNA sequences to produce gene silencing[7-10]. Moreover, each miRNA targets several different mRNAs; the same target gene may be regulated by several different miRNAs in different biological situations, a process that allows enormous complexity and flexibility in their regulatory potential. miRNAs have been characterized as regulators of protein expression in diverse disease processes, including acute hepatic injury[9,10]. Importantly, miRNAs have already been successfully utilized as early biomarkers for esophageal squamous cell carcinoma detection in serum[7], identifying Parkinson’s disease onset and disease progression[8], and diagnosis of hepatocarcinoma[9,10], demonstrating miRNAs as an ideal area of research to determine other early biomarkers for disease states, notably APAP-associated hepatotoxicity[11]. In addition, miRNA fragments do not require the post-translational modifications necessary in protein production; with fewer human miRNAs to evaluate (an estimated 1000 human miRNAs compared to approximately 20 000 proteins), there is an improved likelihood of identifying unique APAP-associated miRNA profiles[11].
Recent work has also shown the medical utility of miRNA[12,13]. Interestingly, literature also supports its association specifically in the setting of acetaminophen toxicity. For instance, Wang et al[11] (2009) showed increased levels of miR-122 and miR-192 in the plasma of acetaminophen overdosed mice, yet decreased levels of these miRNAs in the liver tissue. In addition, these determined markers changed with time and dosing corresponding to histologic liver damage. Of note, these profiles were not evaluated at the lethal APAP dosing of 500 mg/kg, a parameter requiring further investigation to specifically compare lethal and sublethal miRNA profiles. Furthermore, additional literature has described miRNA involvement in acetaminophen toxicity, as well as the utility of miRNAs as biomarkers useful in the setting of other hepatotoxic disorders, such as hepatitis B and C, alcoholic liver disease, non-alcoholic fatty liver disease, and primary biliary cirrhosis[14].
However, these miRNAs could be a potential marker for liver toxicity in the human patient as well, specifically in the setting of acetaminophen overdose. The identification of early plasma markers of acetaminophen toxicity is necessary and paramount. Early identification of acetaminophen toxicity would identify those requiring more expeditious treatment, potentially improving morbidity and mortality of these individuals. It would also possibly abrogate the need for patient admission, mitigating the resultant financial system burden and iatrogenic risk of hospital-acquired infections.
C57Bl/6 wild-type mice were purchased from Jackson Laboratory (Bar Harbor, ME) and received proper care in agreement with animal protocols approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School.
For all experiments, 6-wk-old female C57/BL6 mice, with food deprivation 12 h prior to experimentation, were used for intraperitoneal (ip) injections. For lethal dosing APAP experiments, 20 C57/BL6 mice were each injected with acetaminophen 500 mg/kg (0.9% normal saline suspension) ip at time zero. For the sublethal dosing APAP experiments, 20 C57/BL6 mice were injected with acetaminophen 150 mg/kg acetaminophen (0.9% normal saline suspension) ip at time zero. At times 0.5 h, 2 h, 12 h, 24 h, and 48 h, 5 mice per group (both lethal and sublethal) were sacrificed via cervical dislocation. Just prior to sacrifice, 400 mL of cheek blood was obtained from each mouse. The whole blood was then centrifuged at 14 000 g for 10 min at room temperature. The plasma was removed, aliquoted, and stored at -80 °C. After sacrifice, livers were snap frozen in liquid nitrogen for protein, stored in RNA stabilization reagent (RNAlater, Qiagen, Hilden, Germany) for RNA extraction, or fixed in 10% neutral-buffered formalin for histopathologic analysis. Five mice were injected with saline only ip and sacrificed at time 48 h as controls.
Alanine aminotransferase (ALT) was quantified by biochemical assay (D-Tek Analytical Laboratories Inc, San Diego, CA).
Ten mice were injected with APAP 500 mg/mL ip and an additional 10 mice were injected with APAP 150 mg/mL, all at time zero. Mice were monitored for 48 h and the mortality for each recorded and plotted using Kaplan-Meier survival statistics (GraphPad Prism Software, LaJolla, CA). All mice were housed, watered and fed under the same conditions throughout the experimental protocol.
Sections of formalin-fixed, paraffin-embedded livers after sublethal and lethal APAP dosing were stained with hematoxylin and eosin and assessed for inflammatory infiltrate calculated with Microsuite (Olympus Soft Imaging Solution GmbH, Munster, Germany) image analysis software in 20 X objective.
MicroRNA was purified from plasma using the MiRNeasy Mini kit (Qiagen Sciences, MD). The cDNA was prepared using (reverse transcriptase2) RT2 First Strand cDNA kit (SABiosciences, Qiagen Sciences, MD). The libraries were screened using pooled plasma samples from the 12 h time point for each APAP dosing parameter using saline injected mice as controls (5 mice/group). The screening libraries utilized were the RT2 miRNA PCR arrays for mouse whole genome, per the manufacturer’s protocol (SABiosciences, Qiagen Sciences, MD). Real-time quantitative polymerase chain reaction (QPCR) was performed using RT2 QPCR SYBR green MasterMix (SABiosciences, Qiagen Sciences, MD) and the iCycler iQ Cycler (Bio-Rad Laboratories, Inc, Hercules, CA).
QPCR data were analyzed using manufacturer software (http://pcrdataanalysis.sabiosciences. com/pcr/arrayanalysis.php).
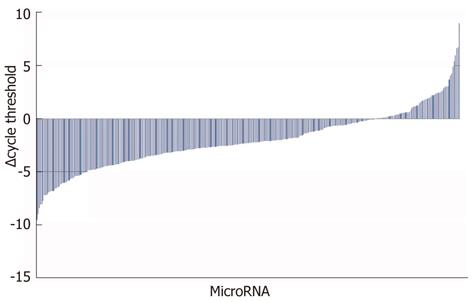
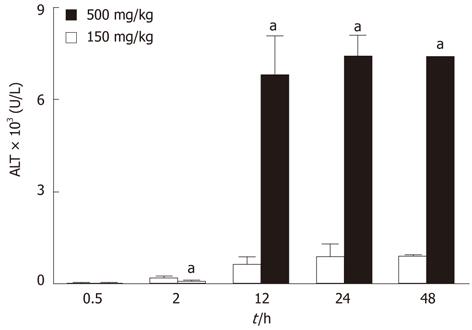
Numerous plasma miRNAs were both upregulated and downregulated, respectively, for the lethally compared to sublethally dosed APAP mice (Figure 1). Increase in serum levels of ALT is a well characterized marker of liver injury in the clinical setting as well as in animal models. Hepatotoxicity induced by APAP administration in our experiments was confirmed utilizing an ALT assay. An ALT average for the sublethal APAP dosing (150 mg/kg) peaked at 883 IU/L and for the lethal APAP dosing (500 mg/kg) peaked at 7396 IU/L, both at 24 h (Figure 2). There was a statistically significant increase in ALT levels in the lethal dosing compared to the sublethal dosing groups (642 IU/L compared to 6796 IU/L, respectively) starting at the 12 h time point (P < 0.001). There remained statistically significant increased ALT levels for both the sublethal and lethal dosed groups at the 48 h time point compared to the 0.5 h and 2 h time points (Figure 2).
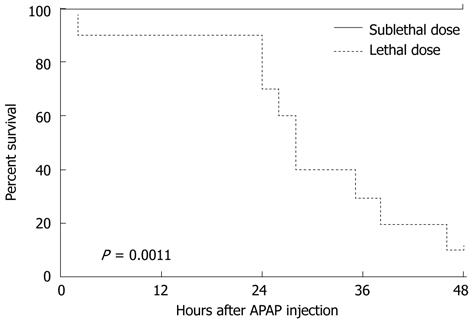
With administration of a high dose APAP (500 mg/kg), we found that there was 90% lethality compared to no lethality with the sublethal dosing (150 mg/kg) at the 48 h time point as demonstrated by the nonparametric maximum likelihood estimate of a Kaplan-Meier survival curve (Figure 3). This difference was statistically significant at P = 0.011, with ten mice utilized per dosing parameter analyzed (Graphpad Prism Software; LaJolla, CA).
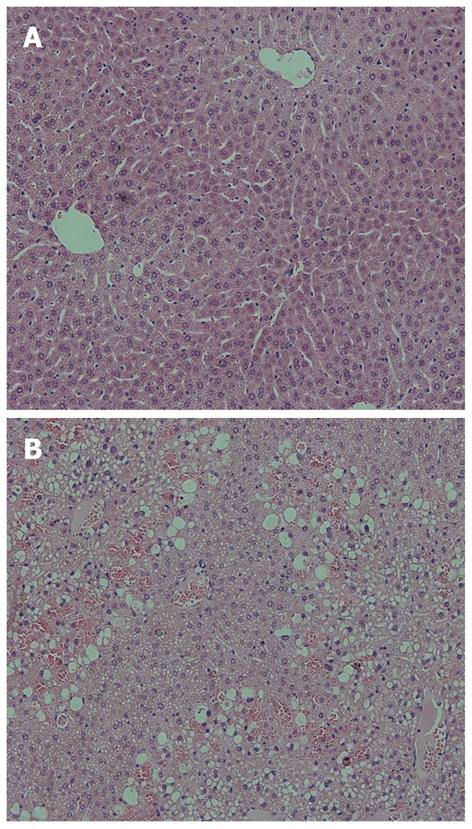
For the sublethally dosed mice, the portal and periportal regions appear normal, with no signed of centrilobular necrosis or inflammation (Figure 4A). However, for the lethally dosed mice, obvious extensive centrilobular necrosis and inflammation were present, with distinctively enlarged hepatocytes and highly vacuolated cytoplasm. The lethal dosed liver also demonstrated pyknotic nuclei with extensive ballooning vacuolar degeneration (Figure 4B).
In an attempt to evaluate plasma miRNAs as potential indicators of APAP-induced liver damage, we screened plasma of mice after administration of a lethal or sublethal dose of APAP. Out of the 528 murine miRNAs analyzed, there were more than 40 potential miRNAs that were both greater than 2-fold up- and downregulated in the lethal (500 mg/kg) compared to sublethal (150 mg/kg) dosing (Table 1). The miRNAs listed were effectively detected suggesting the actual fold-change value is as large as the calculated and reported fold-change result (SABiosciences, Qiagen Sciences, MD). The small nucleolar RNA, C/D Box 68 (Snord68), was used as the internal control for each library evaluated.
| MicroRNA | Fold increase | MicroRNA | Fold decrease |
| 574-5p | 203.7 | 342-3p | 0.0005 |
| 135a* | 173.6 | 195 | 0.0041 |
| 466g | 110.7 | 375 | 0.0085 |
| 1196 | 82.7 | 29c | 0.0134 |
| 466f-3p | 71 | 148a | 0.0152 |
| 877 | 64.4 | 652 | 0.0199 |
| 139-3p | 59.7 | 202-5p | 0.0317 |
| 686 | 48.8 | 200a | 0.039 |
| 346 | 47.5 | 320 | 0.0422 |
| 149 | 34.9 | 374* | 0.0508 |
| 485 | 34.5 | 9* | 0.0556 |
| 409-3p | 30.7 | 342-5p | 0.0629 |
| 202-3p | 28.1 | 192 | 0.0656 |
| 298 | 27.4 | 412 | 0.0713 |
| 15a | 27.1 | 1 | 0.0775 |
| 341 | 26.2 | 199b | 0.0775 |
| 296-3p | 24.3 | 741 | 0.0902 |
| 466i | 22.1 | 100 | 0.1145 |
| 1186 | 21.4 | 18b | 0.1604 |
| 200a | 20.5 | 122 | 0.2348 |
Finally, we performed an extensive literature search to gather information on the known and putative targets of 12 miRNAs that were uniquely changed in the plasma after administration of the lethal dose of APAP (Table 2). Interestingly, we found 6 out of the top 12 miRNAs with the greatest fold change (both up- and downregulated) in the lethally compared to sublethally dosed APAP mice were associated with asthma. The other miRNAs found both highly up- and downregulated were found to be associated with hypoxia-inducible factor (HIF)-1[15], follicle stimulating hormone[16], type 1 diabetes[17], procollagen type III[18], colon cancer[19], gastric carcinoma[20], and elongation factor 2 tumor suppression[21] (Table 2).
| MicroRNA | Function |
| 574-5p | Acute and chronic asthma (Garbacki et al[23]); Procollagen type III A1 (Sterling[18]) |
| 135a | Hypoxia-inducible factor-1 and nuclear factor-κB production (Gonsalves et al[15]) |
| 466g | Acute and chronic asthma (Garbacki et al[23]) |
| 1196 | Follicle-stimulating hormone regulation (Yao et al[16]) |
| 466f-3p | Acute and chronic asthma (Garbacki et al[23]); Procollagen type III A1 (Sterling[18]) |
| 877 | Human type 1 diabetes (Zhou et al[17]) |
| 139-3p | Colon and rectal cancer (Slattery et al[19]) |
| 342-3p | HBV infection and HBV-positive hepatocarcinoma biomarker (Li et al[10]) |
| 195 | E2F tumor suppressor (Xu et al[21]) |
| 375 | Acute and chronic asthma (Garbacki et al[23]); Pyruvate dehydrogenase kinase inhibition in gastric carcinomas (Tsukamoto et al[20]) |
| 29c | Acute and chronic asthma (Garbacki et al[23]) |
| 148a | HLA-G and risk asthma (Tan et al[22]) |
Our study reveals numerous miRNAs, notably 574-5p, 135a*, 466g, 1196, 466f-3p, and 877, are upregulated in the setting of lethal compared to sublethal APAP-associated hepatotoxicity, whereas miRNAs 342-3p, 195, 375, 29c, 148a and 652 are markedly downregulated. We demonstrate elevated ALT levels as well as histologic evidence supporting worsened hepatotoxicity in the setting of lethally dosed mice compared to non-lethally dosed mice (Figures 2 and 4). With 90% lethality in the lethally dosed mice in relation to no lethality in sublethally dosed mice (P = 0.0011), this supports the premise that unique plasma miRNA profiles may correlate with non- vs life-threatening APAP dosing (Figure 3). The fold-change of a variety of miRNAs in the setting of lethally dosed mice compared to sublethal doses is of interest. Of note, more than 40 were both up- and downregulated, with the greatest fold miRNA changes reported (Table 1).
A literature search to investigate possible functions of the miRNAs both up- and downregulated was undertaken. Intriguingly, many of those most up- and downregulated in the lethally compared to sublethally dosed mice, namely 574-5p, 466g, 466f-3p, 375, 29c, and 148a, have also been implicated in the development of asthma[22,23]. For instance, 574-5p may be involved with asthma pathogenesis, with decreased miRNA 574-5p in chronic compared to acute asthma in a mouse model sensitized with ovalbumin[23]. In another study, a potential relationship between histocompatibility antigen-G, chronic asthma, and miRNA 29c was determined[22]. This consequently suggests a pathophysiologic relationship between APAP toxicity and asthma[22,23].
Interestingly, prior research has shown an association between APAP use and asthma although the exact association is still unclear[24,25]. For instance, an adult case control study described a relationship between acetaminophen use and asthma[26]. Additional literature revealed an increased risk of wheeze in children whose mothers used prenatal APAP[27]. The etiology still remains unclear. However, one theory is that decreased glutathione (due to depletion secondary to APAP toxicity) provides the opportunity for unchecked reactive oxygen species to promote asthma development[24]. Additional theories include increased prostaglandin E2 production secondary to elevated cyclooxygenase-2 activity in the presence of APAP promoting a T2 allergic response[25]. A third cause could be direct lung damage from NAPQI, a byproduct of APAP metabolism[28]. Clearly, more information is needed to further elucidate the relationship between APAP and risk of asthma.
The previous literature describing the function of the other miRNAs up- and downregulated in our study is more varied. For example, some literature reveals a miRNA 135a association with HIF 1-α[15]. Interestingly, previous literature has demonstrated HIF 1-α induction prior to APAP toxicity in the setting of lethal APAP dosing, with toxicity prevented by the presence of cyclosporine A, a HIF 1-α inhibitor which prevents mitochondrial permeability transition and oxidative stress[29]. Additional studies have also shown elevation of HIF 1-α in the setting of APAP toxicity, with increased HIF 1-α causing increased glucose transporter-1 expression[30]. Of note, miRNAs 195 and 342-3p have been shown as involved with hepato cytopathology in the setting of tumor suppression in hepatocellular carcinoma models[21] and hepatitis B virus hepatocarcinoma diagnosis[10], respectively.
However, how these miRNAs in total affect hepatotoxicity in the setting of APAP poisoning still needs to be elucidated. Prior studies reveal upregulation of plasma miR-122 in the setting of APAP-associated liver toxicity, while our data suggest downregulation of plasma miR-122 at the 12 h time point. This discrepancy could be potentially explained by examination of upregulation of miR-122 at 1 h, 3 h and 24 h time points, not at a 12 h time point in previous literature. In addition, different APAP dosing levels were used in each study (300 mg/kg compared to 500 mg/kg), again demonstrating the dynamic nature of miRNA regulation across both time and clinical setting[11]. Of note, we found upregulation of plasma miR-298 and miR-370, whereas other researchers found downregulation of these miRNAs in the setting of APAP-associated hepatotoxicity[31]. Again, this may be due to evaluation at different time points (6 h compared to 12 h) and differing APAP dosing parameters (1000 mg/kg compared to 500 mg/kg)[31]. Together, these results demonstrate the need for further identification of additional plasma microRNA profiles at various time points and dosing levels.
Our ultimate goal would be to eventually have a miRNA APAP nomogram to be used for human patient care, similar to the previous effective Rumack-Matthew nomogram[32,33]. The problem with this current nomogram, however, is that it relies on knowing when a patient initially ingested APAP. This is often difficult, if patients are poor historians or have ingested mind-altering substances such as alcohol at the time of evaluation. In addition, patients may have been taking APAP chronically, not acutely, making use of the Rumack-Matthew nomogram pointless. The importance of our work is to establish a novel miRNA nomogram that would be used for patients who have taken APAP at an unknown time or with chronic ingestions. In turn, this would avoid investigation of patients who have taken it chronically, thus preventing unnecessary treatment and iatrogenic ingestions. Additional studies in this field, with additional time points and dosing levels, however, are clearly still necessary.
The specificity of this profile also requires further improvement. For instance, in a recent report miRNA-122 was shown to be increased in the setting of APAP toxicity, although this increase was under the detection cut-off of our study[11]. Of note, miRNA-122 is also upregulated in hepatitis C settings which, by itself, is evidently not specific enough to uniquely identify APAP toxicity[14]. However, with additional future studies and data analysis, this may possible. This approach may also be used as a model to develop profiles for additional disease processes, namely non-alcoholic fatty liver disease and hepatocellular carcinoma, since microRNA profiles are already being used as early biomarkers for numerous pathologic states[9,10]. Together, this may improve the diagnostic accuracy of hepatopathology, namely early APAP-induced hepatotoxicity. In turn, this may allow clinicians to better and more rapidly distinguish which patients who have ingested APAP will actually mandate therapy. Subsequently, this may result in decreasing the number of patients who receive unnecessary, expensive empiric treatment.
In conclusion, lethal dosing of APAP in a murine model is consistent with hepatotoxicity and up- and downregulation of a unique pattern of circulating plasma miRNAs, which is different from the plasma miRNA profile associated with sublethal APAP dosing. These differences may be useful in the future to distinguish lethal and sublethal APAP toxicity in humans.
Acetaminophen (APAP) continues to be an important cause of acute liver failure in the developed world, being the most common cause of death due to analgesic ingestion in the United States. The authors report unique microRNA (miRNA) profiles associated with lethal acetaminophen poisoning. Determining which specific miRNAs are associated with lethal acetaminophen toxicity may prove helpful in the future for prognosticating which patients will require N-acetylcysteine treatment.
The importance of our work is to establish a novel miRNA nomogram that would be used for patients who have taken APAP at an unknown time or with chronic ingestions. This would be the first miRNA profile that could prognosticate patients who will require treatment for hepatotoxicity due to acetaminophen poisoning.
The ultimate goal is to eventually have a miRNA APAP nomogram to be used for human patient care. The limitation of the current Rumack-Matthew nomogram for APAP toxicity is that it relies on knowing the time of APAP ingestion. This is often difficult if patients are poor historians or have ingested mind-altering substances at the time of evaluation, or have been chronically ingesting APAP.
Recent work has also shown the medical utility of miRNA. miRNAs, are short, chemically stable biomolecules that produce gene silencing. Furthermore, additional literature has described miRNA involvement in acetaminophen toxicity, as well as the utility of miRNAs as biomarkers.
The manuscript provides some interesting information on the potential use of miRNAs for the early diagnosis of APAP associated liver toxicity. The scientific goal of the study has significant clinical application but the interpretation of the data needs to be revised.
Peer reviewer: Oliver Grundmann, PhD, Clinical Assistant Professor, Department of Medicinal Chemistry, College of Pharmacy, University of Florida, 1600 SW Archer RD, Room P6-20, Gainesville, FL 32610-0484, United States
S- Editor Cheng JX L- Editor Logan S E- Editor Zhang DN
| 1. | Craig DG, Lee A, Hayes PC, Simpson KJ. Review article: the current management of acute liver failure. Aliment Pharmacol Ther. 2010;31:345-358. [PubMed] [DOI] [Full Text] |
| 2. | Bronstein AC, Spyker DA, Cantilena LR, Green JL, Rumack BH, Giffin SL. 2008 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (. Phila). 2009;47:911-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Bond GR, Novak JE. The human and economic cost of paracetamol (acetaminophen) overdose. Pharmacoeconomics. 1995;8:177-181. [PubMed] [DOI] [Full Text] |
| 4. | Salhanick SD, Shannon MW. Acetaminophen. Haddad Winchester’s Clinical Management of Poisoning and Drug Overdose. 4th ed. Philadelphia: Saunders Elsevier 2009; 825-834. |
| 5. | Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11:604-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, Dai J, Hu Z, Zhou X, Chen L. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56:1871-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Margis R, Margis R, Rieder CR. Identification of blood microRNAs associated to Parkinsonĭs disease. J Biotechnol. 2011;152:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798-9807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402-4407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Bala S, Marcos M, Szabo G. Emerging role of microRNAs in liver diseases. World J Gastroenterol. 2009;15:5633-5640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Gonsalves CS, Kalra VK. Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p. J Immunol. 2010;184:3878-3888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Yao N, Yang BQ, Liu Y, Tan XY, Lu CL, Yuan XH, Ma X. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells. Endocrine. 2010;38:158-166. [PubMed] [DOI] [Full Text] |
| 17. | Zhou L, He H, Mi JX, Li C, Lee B, Mi QS. MicroRNA genes. Ann N Y Acad Sci. 2008;1150:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Sterling KM. The procollagen type III, alpha 1 (COL3A1) gene first intron expresses poly-A+ RNA corresponding to multiple ESTs and putative miRNAs. J Cell Biochem. 2011;112:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Slattery ML, Wolff E, Hoffman MD, Pellatt DF, Milash B, Wolff RK. MicroRNAs and colon and rectal cancer: differential expression by tumor location and subtype. Genes Chromosomes Cancer. 2011;50:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010;70:2339-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP, Zhuang SM. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology. 2009;50:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Tan Z, Randall G, Fan J, Camoretti-Mercado B, Brockman-Schneider R, Pan L, Solway J, Gern JE, Lemanske RF, Nicolae D. Allele-specific targeting of microRNAs to HLA-G and risk of asthma. Am J Hum Genet. 2007;81:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Garbacki N, Di Valentin E, Huynh-Thu VA, Geurts P, Irrthum A, Crahay C, Arnould T, Deroanne C, Piette J, Cataldo D. MicroRNAs profiling in murine models of acute and chronic asthma: a relationship with mRNAs targets. PLoS One. 2011;6:e16509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Persky VW. Acetaminophen and asthma. Thorax. 2010;65:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Allmers H, Skudlik C, John SM. Acetaminophen use: a risk for asthma? Curr Allergy Asthma Rep. 2009;9:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Shaheen S, Potts J, Gnatiuc L, Makowska J, Kowalski ML, Joos G, van Zele T, van Durme Y, De Rudder I, Wöhrl S. The relation between paracetamol use and asthma: a GA2LEN European case-control study. Eur Respir J. 2008;32:1231-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Perzanowski MS, Miller RL, Tang D, Ali D, Garfinkel RS, Chew GL, Goldstein IF, Perera FP, Barr RG. Prenatal acetaminophen exposure and risk of wheeze at age 5 years in an urban low-income cohort. Thorax. 2010;65:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Eneli I, Sadri K, Camargo C, Barr RG. Acetaminophen and the risk of asthma: the epidemiologic and pathophysiologic evidence. Chest. 2005;127:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | James LP, Donahower B, Burke AS, McCullough S, Hinson JA. Induction of the nuclear factor HIF-1alpha in acetaminophen toxicity: evidence for oxidative stress. Biochem Biophys Res Commun. 2006;343:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Salhanick SD, Belikoff B, Orlow D, Holt D, Reenstra W, Buras JA. Hyperbaric oxygen reduces acetaminophen toxicity and increases HIF-1alpha expression. Acad Emerg Med. 2006;13:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Fukushima T, Hamada Y, Yamada H, Horii I. Changes of micro-RNA expression in rat liver treated by acetaminophen or carbon tetrachloride--regulating role of micro-RNA for RNA expression. J Toxicol Sci. 2007;32:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Rumack BH, Peterson RG. Acetaminophen overdose: incidence, diagnosis, and management in 416 patients. Pediatrics. 1978;62:898-903. [PubMed] |
| 33. | Green TJ, Sivilotti ML, Langmann C, Yarema M, Juurlink D, Burns MJ, Johnson DW. When do the aminotransferases rise after acute acetaminophen overdose? Clin Toxicol (. Phila). 2010;48:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |