Published online Jun 14, 2012. doi: 10.3748/wjg.v18.i22.2775
Revised: February 28, 2012
Accepted: March 20, 2012
Published online: June 14, 2012
AIM: To define the rational extent of regional lymphadenectomy for gallbladder cancer and to clarify its effect on long-term survival.
METHODS: A total of 152 patients with gallbladder cancer who underwent a minimum of “extended” portal lymph node dissection (defined as en bloc removal of the first- and second-echelon nodes) from 1982 to 2010 were retrospectively analyzed. Based on previous studies, regional lymph nodes of the gallbladder were divided into first-echelon nodes (cystic duct or pericholedochal nodes), second-echelon nodes (node groups posterosuperior to the head of the pancreas or around the hepatic vessels), and more distant nodes.
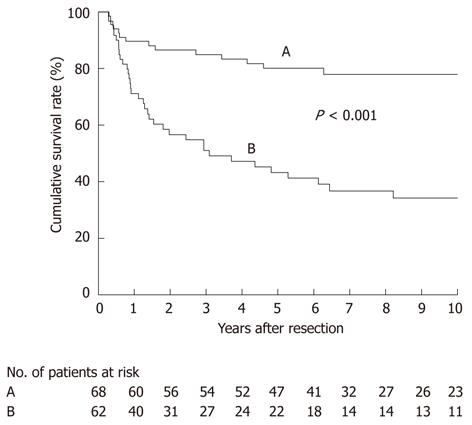
RESULTS: Among the 152 patients (total of 3352 lymph nodes retrieved, median of 19 per patient), 79 patients (52%) had 356 positive nodes. Among node-positive patients, the prevalence of nodal metastasis was highest in the pericholedochal (54%) and cystic duct (38%) nodes, followed by the second-echelon node groups (29% to 19%), while more distant node groups were only rarely (5% or less) involved. Disease-specific survival after R0 resection differed according to the nodal status (P < 0.001): most node-negative patients achieved long-term survival (median, not reached; 5-year survival, 80%), whereas among node-positive patients, 22 survived for more than 5 years (median, 37 mo; 5-year survival, 43%).
CONCLUSION: The rational extent of lymphadenectomy for gallbladder cancer should include the first- and second-echelon nodes. A considerable proportion of node-positive patients benefit from such aggressive lymphadenectomy.
- Citation: Shirai Y, Wakai T, Sakata J, Hatakeyama K. Regional lymphadenectomy for gallbladder cancer: Rational extent, technical details, and patient outcomes. World J Gastroenterol 2012; 18(22): 2775-2783
- URL: https://www.wjgnet.com/1007-9327/full/v18/i22/2775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i22.2775
Oncological resection for gastrointestinal cancers conventionally involves en bloc resection of the primary tumor and the regional lymphatic basin. However, in cases of gallbladder cancer, most surgeons have developed a negative attitude toward regional lymphadenectomy because of reported poor outcomes after radical resection for node-positive disease[1-5]. In contrast, some surgeons including us advocate aggressive lymphadenectomy[6-10]. Thus, the role of regional lymphadenectomy in treating gallbladder cancer remains controversial.
The National Comprehensive Cancer Network (NCCN) guidelines[11] recommend portal lymph node dissection for pathologic T1b (pT1b) or more advanced gallbladder cancer, where the extent of lymphadenectomy is described as “porta hepatis, gastrohepatic ligament, retroduodenal.” The guidelines also state, “Patients with nodal disease outside this area are unable to undergo resection”[11]. In contrast, some Japanese and Western groups advocate more extensive lymphadenectomy including some peripancreatic (head only) node groups[6,12-14]. Thus, the extent of regional lymphadenectomy for gallbladder cancer remains non-standardized worldwide.
Previously we explored the routes and directions of gallbladder lymphatic drainage in a dye injection study[6]; lymph flow originating from the gallbladder descends around the bile duct, and via the first-echelon nodes (cystic duct or pericholedochal nodes) into the second-echelon nodes located posterosuperior to the head of the pancreas or around the portal vein/hepatic arteries, before finally flowing into the paraaortic nodes[6]. Thereafter, we adopted en bloc removal of the first- and second-echelon nodes (designated as “extended” portal lymph node dissection) as a standard procedure for pT2 or more advanced gallbladder cancer[6,10,15].
This retrospective study sought to define a rational extent of regional lymphadenectomy for gallbladder cancer and to clarify outcomes after such a procedure in 152 patients subjected to radical resection. The study goal was to establish the role of regional lymphadenectomy in treating gallbladder cancer.
From May 1982 through December 2010, a total of 173 consecutive Japanese patients with gallbladder cancer underwent a radical resection at the Niigata University Medical and Dental Hospital. A radical resection was defined as removing both the primary tumor and the regional lymph nodes of the gallbladder. Cancer arising in the cystic duct was included as gallbladder cancer[16]. We excluded 13 patients due to an invasive primary malignancy in other organs and 8 patients who did not undergo removal of the second-echelon nodes, leaving 152 patients in this study. They included 96 women and 56 men ranging in age from 37 years to 86 years (median, 68 years).
A variety of radical resection procedures were performed for gallbladder cancer in the patients analyzed; the choice of procedure for each patient depended on the extent of tumor spread (Table 1). An “extended” radical cholecystectomy, which was instituted at our department in 1982[6,10,15], was the most common operation used among our study cohort; it involved a cholecystectomy, wedge resection of the gallbladder fossa with a rim of non-neoplastic liver tissue (about 2 cm in thickness or more), resection of a suprapancreatic segment of the extrahepatic bile duct (bile duct resection), and “extended” portal lymph node dissection in an en bloc fashion. This “extended” radical cholecystectomy is a modification of the “radical cholecystectomy” (Glenn operation) as proposed by Glenn et al[17,18] in 1954; we now also call it the “modified Glenn operation”[15].
| Procedure | No. of patients |
| Extended cholecystectomy (n = 93) | |
| C + WR + BD + N1 | 54 |
| C + WR + N | 21 |
| C2 + BD + N | 12 |
| C2 + N | 6 |
| More extensive resection (n = 59) | |
| C + ERH + BD + N | 27 |
| C + Central hepatectomy3 + BD + N | 3 |
| C + Extended left hepatectomy4 + BD + N | 1 |
| C + Right trisectionectomy + BD + N | 1 |
| C + WR + PD + N | 15 |
| C + ERH + PD + N | 7 |
| C + ERH + PPPD + N | 3 |
| C2 + PD + N | 2 |
Some patients with early-stage disease, comorbid disease(s), or advanced age had a less aggressive resection, by omitting the bile duct resection and/or wedge hepatectomy (Table 1). Although pT1 tumors do not warrant radical resection[19], 14 patients with pT1 tumors underwent a radical resection because pT2 or more advanced tumor was not ruled out preoperatively. In contrast, patients with late-stage disease often required more extensive resections such as major hepatectomy (removal of two sections or more extended hepatectomy), pancreaticoduodenectomy (Whipple procedure or pylorus-preserving procedure), or combined major hepatectomy and pancreaticoduodenectomy (Table 1)[12,20].
Despite an aggressive attitude toward lymphadenectomy, we maintain a rather modest attitude toward hepatectomy for gallbladder cancer, and consequently, the most common hepatectomy procedure in our series was (nonanatomic) wedge resection of the gallbladder fossa. However, cases of deep hepatic invasion through the gallbladder fossa and/or invasion of the right portal pedicle (i.e., extension into the triangle of Calot) required a right hemihepatectomy extended to an inferior part of Couinaud segment 4 (designated as extended right hepatectomy in this study) for tumor clearance. Five patients underwent other hepatectomy procedures (Table 1).
There were also 25 patients in this study who underwent a combined resection of contiguous tissues comprising the transverse colon (n = 12), portal vein (n = 9), duodenum (n = 5), stomach (n = 2), and inferior vena cava (n = 2). Of the 152 patients, 127 underwent an initial radical resection and 25 underwent a radical second resection after a prior simple cholecystectomy for presumed benign disease.
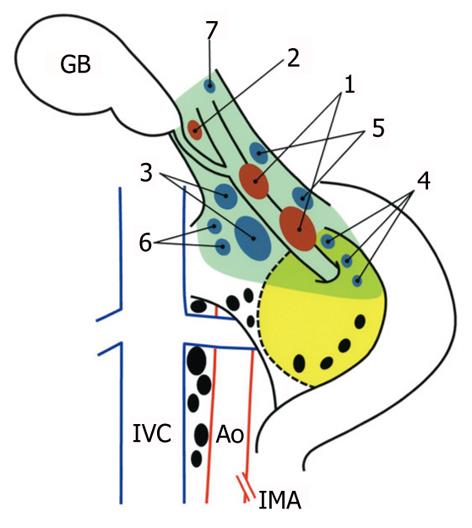
According to earlier studies[6,8,15,21,22], we classified the regional lymph nodes of the gallbladder into three categories as detailed in Table 2: first-echelon nodes, second-echelon nodes, and more distant nodes. The topographical distribution of the first- and second-echelon node groups is illustrated in Figure 1. Briefly, the first-echelon nodes are located along the cystic duct or the common bile duct, and the second-echelon nodes are located posterosuperior to the head of the pancreas or around the portal vein/hepatic arteries. Although the hilar (porta hepatis) nodes were not regarded as significant regional nodes[6,21,22], we categorized them as second-echelon nodes for convenience (Table 2) because these nodes are located within the hepatoduodenal ligament, and thus are usually harvested during regional lymphadenectomy[11]. Perigastric nodes are not regarded as regional, but they were analyzed in this study since they were included in the Whipple procedure collection. Paraaortic lymph nodes were considered the terminal station for the regional lymphatic system of the gallbladder[6,21,22].
| Node group | No. of patients with node group evaluated | No. of lymphnodes evaluated | No. of patients with positive nodes | No. ofpositive nodes | ||
| Range per patient (median) | Total | Range per patient (median) | Total | |||
| First-echelon node groups | ||||||
| Pericholedochal1 | 152 | 0-9 (2) | 410 | 43 | 1-9 (1) | 75 |
| Cystic duct | 152 | 0-2 (1) | 109 | 30 | 1-2 (1) | 33 |
| Second-echelon node groups | ||||||
| Retroportal | 152 | 0-9 (3) | 458 | 23 | 1-5 (2) | 47 |
| Posterior superior pancreaticoduodenal2 | 150 | 0-9 (2) | 341 | 20 | 1-7 (1) | 37 |
| Hepatic artery | 148 | 0-12 (3) | 536 | 20 | 1-10 (1) | 50 |
| Right celiac3 | 128 | 0-8 (2) | 320 | 15 | 1-4 (1) | 28 |
| Hilar (porta hepatis) | 121 | 0-5 (0) | 37 | 0 | NA | 0 |
| More distant node groups | ||||||
| Superior mesenteric | 50 | 0-14 (2) | 171 | 4 | 1-5 (2) | 10 |
| Posterior inferior pancreaticoduodenal | 38 | 0-7 (1) | 56 | 3 | 1-5 (2) | 8 |
| Anterior superior pancreaticoduodenal | 29 | 0-5 (0) | 19 | 1 | 1-1 (1) | 1 |
| Anterior inferior pancreaticoduodenal | 27 | 0-4 (0) | 15 | 2 | 1-3 (2) | 4 |
| Perigastric | 52 | 0-23 (2) | 205 | 4 | 1-1 (1) | 4 |
| Paraaortic node groups | 934 | 1-28 (6) | 675 | 15 | 1-16 (2) | 59 |
In the current study, regional lymphadenectomy for gallbladder cancer was divided into two categories according to the extent of nodal dissection: “extended” portal lymph node dissection and “peripancreatic (head only) lymph node dissection with pancreaticoduodenectomy”[15]. The former was defined as en bloc removal of the first- and second-echelon node groups (without pancreaticoduodenectomy), while the latter was defined as en bloc removal of more distant node groups with pancreaticoduodenectomy in addition to the first- and second-echelon node groups. The extent of an “extended” portal lymphadenectomy is illustrated in Figure 1.
Of all the study patients, 125 underwent an extended portal lymph node dissection (without pancreaticoduodenectomy), while the remaining 27 patients underwent a peripancreatic (head only) lymph node dissection with pancreaticoduodenectomy (Table 1). In addition, 55 of the total number of patients who showed suspected (or confirmed) regional nodal disease also underwent a paraaortic (mainly interaortocaval) lymph node dissection, provided that they were fit enough.
It should be noted that despite our consistent policy of aggressive lymphadenectomy, the degree of regional lymphadenectomy varied somewhat in individual patients at the discretion of the individual surgeons, primarily due to the current study spanning a long period of time. For example, elderly patients or those with comorbid diseases tended to undergo a less aggressive lymphadenectomy.
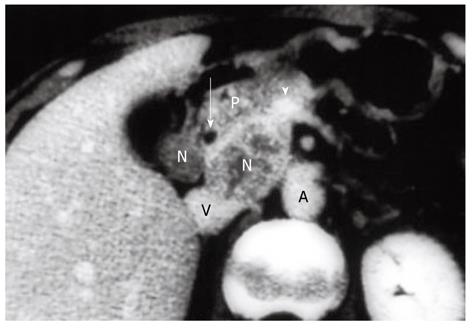
The following describes the technical details of our “extended” portal lymph node dissection (combined with both wedge hepatectomy of the gallbladder fossa and bile duct resection) for gallbladder cancer. After laparotomy, scrutinizing distant metastases by meticulous inspection and palpation is mandatory. Any suspicious nodules in the liver or on the peritoneal surface should be excised and submitted to frozen section examination, while any plan of radical resection should be abandoned if distant metastases are confirmed histologically. If no distant metastases are found, a full Kocher maneuver should be conducted (Figure 1) to enable the peripancreatic nodal status to be appropriately assessed. We favor ex-tended portal lymphadenectomy in the absence of clinically evident peripancreatic (head only) nodal disease, while pancreaticoduodenectomy is often required for peripancreatic (head only) nodal disease adherent to or invading the pancreatic parenchyma (Figure 2)[15].
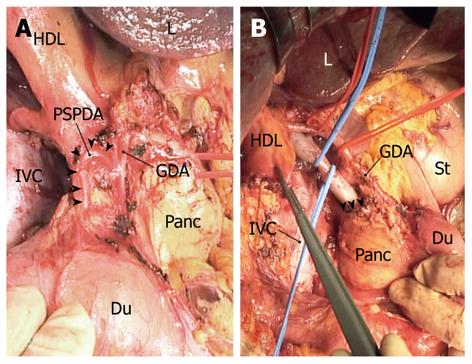
“Extended” portal lymph node dissection usually starts by dissecting the posterior superior pancreaticoduodenal nodes, where a layer of node-bearing adipose tissue is carefully peeled from the posterosuperior surface of the head of the pancreas. Several superior duodenal vessels are then divided to expose the superior aspect of the head of the pancreas. After the lesser omentum is incised, the common hepatic artery is secured with tape and skeletonized with dissecting nodes around the artery; the hepatic-gastroduodenal artery junction is also exposed. The posterior superior pancreaticoduodenal artery (PSPDA), usually the first branch of the gastroduodenal artery, which traverses the common bile duct, can now be identified (Figure 3A). This ensures that dissection of the posterior superior pancreaticoduodenal nodes is complete. We often sacrifice PSPDA, because its tiny branches often cause unexpected bleeding due to injury during the nodal dissection. Transection of the common bile duct, just above or at the level of PSPDA, ensues.
The peritoneum covering the hepatoduodenal ligament is incised longitudinally along the proper hepatic artery, which is then skeletonized. The right hepatic artery is also skeletonized by dividing its ductal branch. The portal vein is then secured with tape and skeletonized by dissecting the retroportal nodes, which are then dissected from the superior border of the uncinate process. Next, the right celiac nodes are dissected from the right of the celiac artery. At this stage, the whole node-bearing tissues dissected en bloc are freed from the hepatic vessels and the head of the pancreas (Figure 3B); exposure of the superior border of the uncinate process ensures that dissection of the retroportal nodes has been completed (Figure 3B). A wedge hepatectomy of the gallbladder fossa ensues. Following parenchymal division, the cystic plate is exposed as a dense fibrous plate connecting with the portal pedicle of the right hemiliver, and then is divided at its base. The adipose tissue within the triangle of Calot, underneath the cystic plate, contains a cystic node(s), and is dissected downward by dividing the cystic artery at its origin. The extended portal lymph node dissection is now complete. Finally, the common hepatic duct is transected just below the confluence of the right and left hepatic ducts, and the surgical specimen including the gallbladder, gallbladder fossa, bile duct, and node-bearing tissues is harvested en bloc.
Immediately after resection, the surgeon(s) retrieved lymph nodes from the node-bearing adipose tissues of the fresh surgical specimen, which were then divided by the surgeon(s) into individual node groups according to their locations.
Pathological findings were documented using the American Joint Committee on Cancer (AJCC) cancer staging manual (7th edition)[16]. The primary tumor was pT1 in 14 patients, pT2 in 60, pT3 in 54, and pT4 in 24. Resection margin status was judged as no residual tumor (R0) or microscopic/macroscopic residual tumor (R1-2). The lymph nodes retrieved were examined histologically for metastases using a representative 3-µm thick section cut from each node.
The number of positive lymph nodes as well as the total lymph node count (TLNC) was recorded for each patient. Here, TLNC represented the sum of regional and paraaortic (if any) nodes evaluated in the patient; the number of positive lymph nodes represented the sum of positive regional and paraaortic (if any) nodes in the patient.
Of 152 patients, 6 died during their hospital stay for the definitive resection, giving an in-hospital mortality rate of 4%. Patients discharged to home were followed regularly in outpatient clinics every 1-6 mo for at least five years, with a median follow-up time of 142 mo (range, 0.5 mo to 351 mo). Adjuvant chemotherapy was administered to 53 patients at the discretion of the individual surgeons.
Medical records and survival data were obtained for all 152 patients. Continuous variables were compared with the Mann-Whitney U test. Only deaths from tumor recurrence were treated as failure cases in the analysis of disease-specific survival (DSS), whereas those from other causes were recorded as censored cases. The survival time in each patient was defined as the interval between the date of definitive resection and the date of last follow-up or death. Survival curves were constructed using the Kaplan-Meier method, and differences in survival were evaluated with the log rank test. The IBM SPSS Statistics 19 software (IBM Japan, Inc., Tokyo, Japan) was used for all statistical evaluations. All tests were two-tailed and P < 0.05 was used to indicate statistical significance.
A total of 3352 lymph nodes taken from the 152 study patients were evaluated. TLNC ranged from 3 to 78 (median, 19) per patient and varied according to the type of regional lymphadenectomy: 27 patients who underwent a peripancreatic (head only) lymph node dissection with pancreaticoduodenectomy had a greater TLNC (median, 36; range, 7 to 78) compared with 125 patients who underwent an extended portal lymph node dissection (median, 17; range, 3 to 53) (P < 0.001).
The topographical distribution of the analyzed lymph nodes included 519 first-echelon nodes and 1692 second-echelon nodes (Table 2). There were significantly more second-echelon nodes per patient (median, 10; range, 2 to 29) than first-echelon nodes (median, 3; range, 0 to 10) (P < 0.001).
Of the 152 study patients, 79 (52%) had a total of 356 positive lymph nodes. The number of positive nodes per patient ranged from 1 to 41 (median, 2). None of the 14 patients with pT1 tumor had nodal disease, whereas 79 (57%) of 138 patients with pT2 or more advanced tumor had nodal disease.
The topographical distribution of all positive lymph nodes is shown in Table 2. Among the 79 node-positive patients, the prevalence of nodal disease was highest in the pericholedochal (43 of 79; 54%) or cystic duct (30 of 79; 38%) node group, followed by the retroportal (29%), posterior superior pancreaticoduodenal (25%), hepatic artery (25%), and right celiac (19%) node groups. The hilar nodes were not involved in any of our patients. The prevalence of nodal disease was 5% or less in more distant node groups. Of note, paraaortic nodal involvement was found in 15 (19%) patients.
Of 27 patients with a single positive node, 20 (74%) had nodal disease in either the pericholedochal or cystic duct node group, suggesting that initial nodal involvement occurred primarily in these node groups (Table 3).
| Node group involved | No. of patients |
| Cystic duct | 11 |
| Pericholedochal | 9 |
| Posterior superior pancreaticoduodenal | 3 |
| Retroportal | 2 |
| Hepatic artery | 1 |
| Paraaortic | 1 |
Analysis of the topographical distribution of positive lymph nodes (Tables 2 and 3) can be used to derive the route of lymphatic spread from gallbladder cancer. In our study patients, gallbladder cancer primarily spread to the first-echelon nodes, then to the second-echelon nodes (other than the hilar nodes), and finally to the paraaortic nodes, while other distant node groups were only rarely involved.
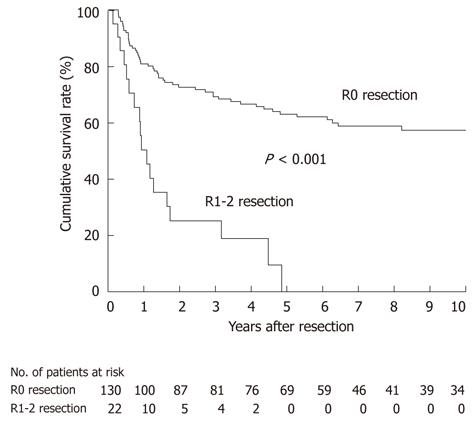
For all 152 patients, DSS was significantly worse in 22 patients undergoing an R1-2 resection than in 130 patients undergoing an R0 resection (P < 0.001; Figure 4); all of the patients undergoing an R1-2 resection died of recurrence within 5 years of resection. This indicates that an R0 resection is a prerequisite for long-term survival after radical resection.
We then focused on a subgroup of 130 patients who had undergone an R0 resection for survival analysis; they comprised 68 node-negative and 62 node-positive patients. DSS after R0 resection differed according to the nodal status (P < 0.001; Figure 5), with most node-negative patients achieving long-term survival (median survival time, not reached; 5-year survival rate, 80%). Of the 62 node-positive patients, 22 survived for more than 5 years after an R0 resection, 37 expired within 5 years, and the remaining 3 had survived within 5 years at the time of last follow-up (median survival time, 37 mo; 5-year survival rate, 43%) (Figure 5). These findings suggested that regional lymphadenectomy could achieve an acceptable rate of long-term survival even in patients with nodal metastasis, provided that an R0 resection is feasible.
The 22 patients with node-positive disease who survived for more than 5 years (Figure 5) comprised 13 with a single positive node, 5 with 2-3 positive nodes, and 4 with ≥ 4 positive nodes (Table 4). Of the 5-year survivors with ≥ 4 positive nodes, three underwent a peripancreatic (head only) lymph node dissection with pancreaticoduodenectomy for marked peripancreatic nodal disease (Figure 2), suggesting that the addition of pancreaticoduodenectomy is effective for selected patients with peripancreatic nodal disease.
| No. of positive nodes | No. of 5-year survivors | ||
| Extended portal LND | Peripancreatic (head only) LND with PD | Total | |
| 1 | 12 | 1 | 13 |
| 2-3 | 4 | 1 | 5 |
| ≥ 4 | 1 | 3 | 4 |
The high propensity for lymphatic spread in gallbladder cancer[6,8,10,15,23] renders adequate lymphadenectomy indispensable for improving patient outcomes after resection. However, what constitutes adequate lymph node dissection for these patients remains unresolved and prompted the current study. Since the 1980s, we have routinely harvested at least the first- and second-echelon nodes (Figure 1) during curative-intent resection for pT2 or more advanced gallbladder cancer. As a result, and to the best of our knowledge, we report herein the largest single-institutional series of 5-year survivors (22 patients) with nodal disease ever analyzed. The study findings also indicated that a considerable proportion of node-positive patients could benefit from regional lymphadenectomy, providing that an R0 resection is feasible, and would seem to justify continuing our policy of aggressive lymphadenectomy for gallbladder cancer.
In this study cohort, initial nodal involvement occurred primarily in the cystic duct or pericholedochal nodes (Table 3). Although these node groups are widely accepted as first-echelon nodes of the gallbladder, further possible routes of lymphatic spread have been poorly defined[6,21-23]. In 1991, an autopsy study by Ito and colleagues[21] implicated the cholecystoretropancreatic pathway, which descends along the common bile duct into the retroportal nodes, as the main lymphatic pathway of the gallbladder. Soon after, we identified the same pathway in a dye injection study and showed the second-echelon nodes located posterosuperior to the head of the pancreas or around the hepatic vessels; the dye solution finally drained into the interaortocaval nodes near the left renal vein[6]. In 1996, Uesaka et al[22] reported similar findings using vital staining. These studies therefore uniformly confirmed that lymph from the gallbladder first flows in a hepatofugal direction around the common bile duct and into the first-echelon nodes, before reaching the second-echelon nodes (other than the hilar nodes), and finally the paraaortic nodes[6,21,22]. In addition, the prevalence of nodal disease in our series was high in both the first-echelon and second-echelon node groups (other than the hilar nodes), while the other node groups were only rarely involved (Table 2). Thus, the rational extent of regional lymphadenectomy for gallbladder cancer should include at least the first- and second-echelon node groups as defined in the current study (Figure 1).
Despite a number of 5-year survivors with nodal disease reported in the Japanese literature[8,10,12,13], such survivors are exceptionally rare in the Western literature[1,2,5,24]. Since the first proposal by Glenn et al[17] in 1954, portal lymph node dissection has been regarded as the standard lymphadenectomy procedure for localized gallbladder cancer throughout the world[15]. However, the scope of portal lymph node dissection differs considerably among institutions. In 2005, Dixon et al[24] from the University of Toronto described “a complete portal lymph node dissection, with thorough skeletonization of the portal structures, down to and including the suprapyloric lymph node overlying the hepatic-gastroduodenal artery junction,” while Ito and colleagues from the Memorial Sloan-Kettering Cancer Center (MSKCC) in 2011[5] reported a portal lymphadenectomy including “the lymph nodes in the hepatoduodenal ligament and those along with the common hepatic artery.” The NCCN guidelines[11] described the extent of portal lymphadenectomy as “porta hepatis, gastrohepatic ligament, retroduodenal.” From the above descriptions, we thus assume that the portal lymphadenectomy performed in North America may leave behind some of the second-echelon nodes, particularly the retroportal, posterior superior pancreaticoduodenal, and right celiac node groups, which were frequently involved in the current series (Table 2). This may explain, in part, why portal lymph node dissection only rarely achieves 5-year survival in cases of node-positive gallbladder cancer[1,2,5,24].
Although the AJCC staging manual (6th edition)[25] recommended “analysis of a minimum of three lymph nodes” for accurate staging of gallbladder cancer, a recent population-based study by Coburn et al[26] disclosed that among patients in the United States with resectable T1-T3 disease, only 5.3% had retrieval of three or more lymph nodes. In addition, a recent report from MSKCC of 122 patients who underwent a portal lymph node dissection cited a median TLNC of only 3[5]. In contrast, TLNC was much greater (median, 19) in the current series. Insufficient lymph node retrieval with portal lymphadenectomy may also explain, in part, the poor survival of node-positive patients in the Western literature[5,26].
Thorough dissection of the second-echelon nodes is challenging even in the hands of expert hepatobiliary surgeons. In particular, complete removal of the posterior superior pancreaticoduodenal, retroportal, and right celiac node groups mandates a meticulous technique. As described in the Materials and Methods section, there are some measures that can be taken to facilitate adequate dissection of the second-echelon nodes: first, a full Kocher maneuver (Figure 1) is essential for assessing the peripancreatic nodal status; second, identification of PSPDA (Figure 3A) ensures that dissection of the posterior superior pancreaticoduodenal nodes has been completed; and third, exposure of the superior border of the uncinate process (Figure 3B) ensures complete dissection of the retroportal nodes.
In our institution, we prefer to perform bile duct resection in fit patients with pT2 or more advanced gallbladder cancer; indeed, 66 (71%) of the 93 patients who underwent an extended cholecystectomy had a bile duct resection (Table 1). Although the presence of ductal involvement is an absolute indication for such an approach, controversy exists regarding bile duct resection for tumors without clinically evident ductal involvement[27]. Our rationale for bile duct resection for tumors with no evident ductal involvement is to facilitate regional lymphadenectomy, to remove the pericholedochal lymphatic vessels and nodes simultaneously, and to remove the possible presence of microscopic ductal (periductal) involvement as suggested by Shimizu et al[28]. Another justification is to avoid the occurrence of ischemic biliary stricture after aggressive periductal nodal dissection[29]. The suprapancreatic segment of the extrahepatic bile duct gets its arterial blood supply mainly from ductal branches of both PSPDA (“retroduodenal artery” by Northover et al[30]) and the right hepatic artery[30]. As described in the Materials and Methods section, during our extended portal lymph node dissection, the PSPDA (Figure 3A) was often sacrificed with division of the ductal branch of the right hepatic artery. In addition, skeletonization of the bile duct may inadvertently injure the periductal arterial plexus with “the 3 o’clock and 9 o’clock arteries”[30]. Thus, we believe that simultaneous bile duct resection is a safer option if such aggressive periductal nodal dissection is required[29].
Indications for pancreaticoduodenectomy for gallbladder cancer include direct invasion of the pancreaticoduodenal region and evident peripancreatic (head only) nodal disease (Figure 2)[10,12,15]. While the former indication is widely accepted, it seems that most Western surgeons hesitate to undertake this procedure for the purpose of lymph node dissection because they believe that peripancreatic nodal disease is beyond the scope of resection[1,11,16,27]. Shirai et al[12] in 1997 and Sasaki et al[13] in 2006 independently reported the effectiveness of pancreaticoduodenectomy for selected cases of peripancreatic nodal disease. This was also suggested by the current study. In 2002, Doty et al[14] also suggested that the addition of pancreaticoduodenectomy could result in an R0 resection by removing extensive peripancreatic nodal disease in a select group of patients. Western surgeons should therefore be more open than ever to performing pancreaticoduodenectomy for gallbladder cancer.
The main limitations of the current study revolved around the retrospective nature of the analysis and considerable variability in the degree of nodal dissection among individual patients. However, the unique nature of this study has more clearly defined the role of regional lymphadenectomy for gallbladder cancer than earlier studies, although the survival of node-positive patients remains unsatisfactory (Figure 5). Since 2009, we have therefore routinely administered adjuvant chemotherapy (using gemcitabine and/or S-1 for 6-12 mo) to patients with nodal disease (especially those with multiple positive nodes) to improve survival. In addition, we now use “extended” portal lymphadenectomy for both gallbladder and bile duct cancers, with the latter also showing some success (unpublished data). Thus, it seems that “extended” portal lymph node dissection is applicable to a wide range of biliary tract malignancies.
In conclusion, gallbladder cancer first spreads to the first-echelon nodes (cystic duct or pericholedochal nodes), then to the second-echelon nodes located posterosuperior to the head of the pancreas or around the portal vein/hepatic arteries, and finally to the paraaortic nodes. The rational extent of regional lymphadenectomy for pT2 or more advanced tumors should include the first- and second-echelon node groups. Such aggressive lymphadenectomy can achieve an acceptable rate of long-term survival even in patients with nodal metastasis, provided that a potentially curative (R0) resection is feasible. The addition of pancreaticoduodenectomy may also be beneficial in selected patients with peripancreatic (head only) nodal disease. This study confirmed that regional lymphadenectomy plays a key role in radical surgery for gallbladder cancer.
The extent of regional lymphadenectomy for gallbladder cancer has not been standardized worldwide. Since 1982, the authors have consistently adopted an aggressive lymphadenectomy strategy. What constitutes adequate lymph node dissection for gallbladder cancer remains unresolved and prompted the current study.
The study aims to define the rational extent of regional lymphadenectomy for gallbladder cancer and to clarify its effect on long-term survival.
The authors clearly define the rational extent of regional lymphadenectomy for pT2 or more advanced gallbladder cancer as at least the first- and second-echelon node groups. They also demonstrate that such aggressive lymphadenectomy can achieve an acceptable rate of long-term survival even in node-positive patients.
The authors imply that “extended” portal lymph node dissection is applicable to a wide range of biliary tract malignancies (both gallbladder and bile duct cancers).
First-echelon nodes mean lymph nodes located along the cystic duct or the common bile duct; second-echelon nodes mean lymph nodes located posterosuperior to the head of the pancreas or around the portal vein/hepatic arteries; “extended” portal lymph node dissection mean en bloc removal of the first- and second-echelon node groups.
This paper provides detailed description of their surgical technique for gallbladder cancer, “extended” portal lymph node dissection, of which this group has been conducting over the years. Documentation of detailed surgical technique is important not only as a material and method of a study, but even more as a tool to compare studies, and as guidance to the next generation of surgeons. They boast the largest single-institutional number of 5-year survivors with nodal disease (22 cases), suggesting the effectiveness of such aggressive lymphadenectomy.
Peer reviewers: Kazuaki Takabe, MD, PhD, Surgical Oncology, Virginia Commonwealth University, West Hospital 7-402, 1200 East Broad Street, Richmond, VA 23298-0011, United States; Toru Ishikawa, MD, PhD, Department of Gastroenterology, Saiseikai Niigata Second Hospital, 280-7 Teraji, Niigata City 950-1104, Japan
S- Editor Gou SX L- Editor Webster JR E- Editor Zhang DN
| 1. | Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 270] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998;175:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Behari A, Sikora SS, Wagholikar GD, Kumar A, Saxena R, Kapoor VK. Longterm survival after extended resections in patients with gallbladder cancer. J Am Coll Surg. 2003;196:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ito H, Ito K, D'Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Shirai Y, Yoshida K, Tsukada K, Ohtani T, Muto T. Identification of the regional lymphatic system of the gallbladder by vital staining. Br J Surg. 1992;79:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 7. | Chijiiwa K, Tanaka M. Carcinoma of the gallbladder: an appraisal of surgical resection. Surgery. 1994;115:751-756. [PubMed] |
| 8. | Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997;79:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Wang JD, Liu YB, Quan ZW, Li SG, Wang XF, Shen J. Role of regional lymphadenectomy in different stage of gallbladder carcinoma. Hepatogastroenterology. 2009;56:593-596. [PubMed] |
| 10. | Sakata J, Shirai Y, Wakai T, Ajioka Y, Hatakeyama K. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Ann Surg Oncol. 2010;17:1831-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica MI, Davila R. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350-391. [PubMed] |
| 12. | Shirai Y, Ohtani T, Tsukada K, Hatakeyama K. Combined pancreaticoduodenectomy and hepatectomy for patients with locally advanced gallbladder carcinoma: long term results. Cancer. 1997;80:1904-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Sasaki R, Itabashi H, Fujita T, Takeda Y, Hoshikawa K, Takahashi M, Funato O, Nitta H, Kanno S, Saito K. Significance of extensive surgery including resection of the pancreas head for the treatment of gallbladder cancer--from the perspective of mode of lymph node involvement and surgical outcome. World J Surg. 2006;30:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Doty JR, Cameron JL, Yeo CJ, Campbell K, Coleman J, Hruban RH. Cholecystectomy, liver resection, and pylorus-preserving pancreaticoduodenectomy for gallbladder cancer: report of five cases. J Gastrointest Surg. 2002;6:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am. 2007;16:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Edge SB, Byrd DR, Compton CC Fritz AG, Greene FL, Trotti A 3rd, editors . AJCC Cancer Staging Manual. 7th ed. New York: Springer 2010; 211-217. |
| 17. | Glenn F, Hays DM. The scope of radical surgery in the treatment of malignant tumors of the extrahepatic biliary tract. Surg Gynecol Obstet. 1954;99:529-541. [PubMed] |
| 18. | Glenn F. Radical cholecystectomy for carcinoma of the gallbladder. New York: The Macmillan Company 1963; 86-88. |
| 19. | Wakai T, Shirai Y, Yokoyama N, Nagakura S, Watanabe H, Hatakeyama K. Early gallbladder carcinoma does not warrant radical resection. Br J Surg. 2001;88:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 20. | Wakai T, Shirai Y, Tsuchiya Y, Nomura T, Akazawa K, Hatakeyama K. Combined major hepatectomy and pancreaticoduodenectomy for locally advanced biliary carcinoma: long-term results. World J Surg. 2008;32:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Ito M, Mishima Y, Sato T. An anatomical study of the lymphatic drainage of the gallbladder. Surg Radiol Anat. 1991;13:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Uesaka K, Yasui K, Morimoto T, Torii A, Yamamura Y, Kodera Y, Hirai T, Kato T, Kito T. Visualization of routes of lymphatic drainage of the gallbladder with a carbon particle suspension. J Am Coll Surg. 1996;183:345-350. [PubMed] |
| 23. | Fahim RB, McDonald JR, Richards JC, Ferris DO. Carcinoma of the gallbladder: a study of its modes of spread. Ann Surg. 1962;156:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Dixon E, Vollmer CM, Sahajpal A, Cattral M, Grant D, Doig C, Hemming A, Taylor B, Langer B, Greig P. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005;241:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M, editors . AJCC Cancer Staging Manual. 6th ed. New York: Springer 2002; 139-144. |
| 26. | Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008;207:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Pitt HA, Nakeeb A. Operative approach to gallbladder cancer. Curr Gastroenterol Rep. 2006;8:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Kato A, Miyazaki M. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136:1012-1017; discussion 1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Ishizuka D, Shirai Y, Hatakeyama K. Ischemic biliary stricture due to lymph node dissection in the hepatoduodenal ligament. Hepatogastroenterology. 1998;45:2048-2050. [PubMed] |
| 30. | Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 286] [Article Influence: 6.2] [Reference Citation Analysis (0)] |