Published online Mar 28, 2012. doi: 10.3748/wjg.v18.i12.1348
Revised: September 22, 2011
Accepted: January 22, 2012
Published online: March 28, 2012
AIM: To investigate all patients referred to our center with non-responsive celiac disease (NRCD), to establish a cause for their continued symptoms.
METHODS: We assessed all patients referred to our center with non-responsive celiac disease over an 18-mo period. These individuals were investigated to establish the eitiology of their continued symptoms. The patients were first seen in clinic where a thorough history and examination were performed with routine blood work including tissue transglutaminase antibody measurement. They were also referred to a specialist gastroenterology dietician to try to identift any lapses in the diet and sources of hidden gluten ingestion. A repeat small intestinal biopsy was also performed and compared to biopsies from the referring hospital where possible. Colonoscopy, lactulose hydrogen breath testing, pancreolauryl testing and computed tomography scan of the abdomen were undertaken if the symptoms persisted. Their clinical progress was followed over a minimum of 2 years.
RESULTS: One hundred and twelve consecutive patients were referred with NRCD. Twelve were found not to have celiac disease (CD). Of the remaining 100 patients, 45% were not adequately adhering to a strict gluten-free diet, with 24 (53%) found to be inadvertently ingesting gluten, and 21 (47%) admitting non-compliance. Microscopic colitis was diagnosed in 12% and small bowel bacterial overgrowth in 9%. Refractory CD was diagnosed in 9%. Three of these were diagnosed with intestinal lymphoma. After 2 years, 78 patients remained well, eight had continuing symptoms, and four had died.
CONCLUSION: In individuals with NRCD, a remediable cause can be found in 90%: with continued gluten ingestion as the leading cause. We propose an algorithm for investigation.
- Citation: Dewar DH, Donnelly SC, McLaughlin SD, Johnson MW, Ellis HJ, Ciclitira PJ. Celiac disease: Management of persistent symptoms in patients on a gluten-free diet. World J Gastroenterol 2012; 18(12): 1348-1356
- URL: https://www.wjgnet.com/1007-9327/full/v18/i12/1348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i12.1348
Celiac disease (CD) is induced by ingestion of gluten and related proteins with consequent intestinal injury and varied clinical manifestations. The defining feature is the expectation that the intestinal lesion improves with strict exclusion of gluten from the diet. However, a proportion of individuals do not respond to a gluten-free diet (GFD), in terms of clinical or histological recovery. Early analysis has indicated that as many as 30% of individuals prescribed a GFD do not experience symptomatic improvement[1]. Non-responsive CD (NRCD) is defined as continued symptoms (including lethargy, abdominal pain and diarrhea) in patients on a GFD. There have been no recent studies to provide robust epidemiological data to assess the incidence of NRCD, although in clinical practice it is a common occurrence, based on the authors’ experience and several publications[2-5]. The investigation of NRCD has been reported[6], however, there are no data on the management and longer term follow-up of these subjects. Most patients with CD experience a rapid symptomatic recovery with a strict GFD. In 30% of cases there may be a protracted (≥ 12 mo) or incomplete phase of mucosal recovery[7]. An arbitrary period of 6-12 mo on a GFD before reassessment has been suggested but the urgency of further investigation is often dictated by the severity of continued symptoms or clinical manifestations. In this context, we define NRCD as failure of expected symptomatic response to a GFD. Accordingly, NRCD is not intended to be a diagnostic term but rather a clinical description to allow a pragmatic and systematic approach to be followed to evaluate and investigate these patients. The practical management of NRCD depends on establishing a cause for continued symptoms. The commonest reason for persistent symptoms in a previous study of 55 patients was failure to comply with a GFD[6]. Imposition of a strict gluten-free dietary regimen appears to abolish symptoms in the majority of CD patients with continued symptoms[8].
Refractory CD (RCD) describes a distinct clinical entity and represents a subset of non-responsive patients. RCD is defined by symptomatic and persistent villous atrophy in patients despite a strict GFD[8]. RCD can be diagnosed after primary failure of GFD or occur as a secondary phenomenon in previously treated CD. It can be subdivided into types I and II. This clinical definition has been refined by the discovery that 80% of individuals with true RCD possess an abnormal population of intraepithelial lymphocytes (IELs) detectable in their small intestinal mucosa (CD103+, intracellular CD3+, CD4-, CD8-, surface CD3-)[8]. These IELs may demonstrate a monoclonal T cell receptor (TCR)-γ gene rearrangement, detectable by polymerase chain reaction (PCR) analysis of biopsy specimens. The presence of this aberrant T cell phenotype has been termed type II RCD (as opposed to type I RCD in which this anomaly is not present). Studies have shown that type II RCD is associated with a significantly greater mortality than type I RCD; 41% vs 14% at 2 years[9]; 42% vs 4%[10] and 56% vs 7% 5-year mortality[11], with the major cause of death attributed to the development of enteropathy-associated T cell lymphoma (EATL). This is characterized by malignant lymphoid tissue with the same immunophenotype as described in type II RCD. It has been postulated that the presence of this type II RCD T cell phenotype may represent a cryptic T cell lymphoma. In 41 patients with RCD, over 50% developed EATL during a mean of 2 years follow-up[9]. Survival from EATL remains abysmal. Thus, there are compelling clinical reasons to investigate CD patients with continued symptoms despite a GFD, in order to establish a treatable cause or identify cases of RCD or intestinal lymphoma. NRCD and RCD may both be present with weight loss, diarrhea, or malabsorption; all of which warrant expeditious investigation.
We maintain a prospective database of patients diagnosed with CD. We selected patients who were referred to our institution with a diagnosis of NRCD (defined as failure of expected symptomatic response to a GFD) between April 2002 and October 2003.
Initial evaluation included an appraisal of the original diagnosis of CD, history of symptoms (including lethargy, increased bowel frequency and weight loss), clinical examination, routine blood tests and assessment of dietary intake and GFD compliance. Patients were then investigated according to our usual clinical practice and subsequent findings; thus, some patients were investigated differently to others, however, all patients were followed for a minimum of 2 years; those who developed further symptoms were reinvestigated. Unless an obvious cause was immediately apparent, we undertook a further small bowel biopsy, which was performed by the authors to ensure a standard quality of biopsy specimen. Jumbo endoscopy forceps were used to obtain four samples that were carefully placed, mucosal surface upwards, onto paper to ensure optimal orientation.
Following standard preparation, histological examination was performed by our histopathology department, although in borderline or ambiguous cases, we often elected additionally to examine the slides within our department. An excess above 20 IELs per 100 enterocytes defined a pathological increase and villous atrophy was defined as being unequivocally present if the villous height to crypt depth ratio was below 2[12]. Direct visual comparison was made with any previous small intestinal specimens for the same patient. If there were any concerns regarding the validity of the diagnosis of CD, a gluten challenge was carried out. This involved ingestion of 10 g gluten (equivalent to four slices of white bread daily) for a minimum of 2 wk before repeat duodenal biopsy[13]. If colonoscopy was performed, random colonic biopsies were taken. In the diagnosis of microscopic colitis, we defined this condition as > 20 lymphocytes per 100 epithelial cells in the superficial colonic mucosa in patients with diarrhea[14].
Tests for small bowel bacterial overgrowth (SBBO) involved a lactulose hydrogen breath test. A positive test was indicated by an early rise in breath hydrogen > 20 ppm from baseline after ingestion of 10 g lactulose. We note the low sensitivity and specificity of breath tests for bacterial overgrowth, including hydrogen and labeled carbon tests[15]. In order to validate a diagnosis of SBBO, we additionally required that symptoms resolved following rotating antibiotic treatment (ciprofloxacin 250 mg bd for 2 wk followed by metronidazole 200 mg tds fortnightly for 4 mo).
Lactose intolerance was diagnosed on dietary exclusion alone as tests are also unreliable. Exclusion of dairy products carries no risk and, if symptoms resolve, is reliable in establishing a confident diagnosis of lactose intolerance. Non-invasive testing of pancreatic function was performed in a number of patients (pancreolauryl test). False positives may occur in CD[16], so the diagnosis could only be confirmed with symptomatic improvement with oral pancreatic supplements.
RCD was suspected in those with severe, symptomatic NRCD with demonstrated villous atrophy, particularly those with pronounced weight loss. Urgent and extensive investigation was arranged in these individuals. This included computed tomography scanning of the abdomen and pelvis, colonoscopy and small bowel imaging. Video capsule endoscopy was not routinely performed at the outset of this study, although this now forms part of our assessment of suspected RCD. If appropriate, serological testing for anti-enterocyte antibody was performed to exclude autoimmune enteropathy.
Additionally, tissue analysis for IEL immunophenotyping and PCR reaction amplification for TCR clonality were undertaken; DNA was analyzed by a series of multiplex PCR assays, which amplified TCRβ and γ gene rearrangements. PCR primer sequences were those used by the Biomed-2 consortium and have been shown to detect clonal signals in approximately 95% of all T cell clonal cases[17,18].
The presenting symptoms, investigation process, results and outcome of subsequent management were recorded. We followed up patients for a minimum of 2 years and observed if patients remained symptom-free or suffered further relapses or related adverse events such as death or identification of malignancy. Any other tests deemed necessary, based on clinical history and examination, were performed, which resulted in a number of other diagnoses.
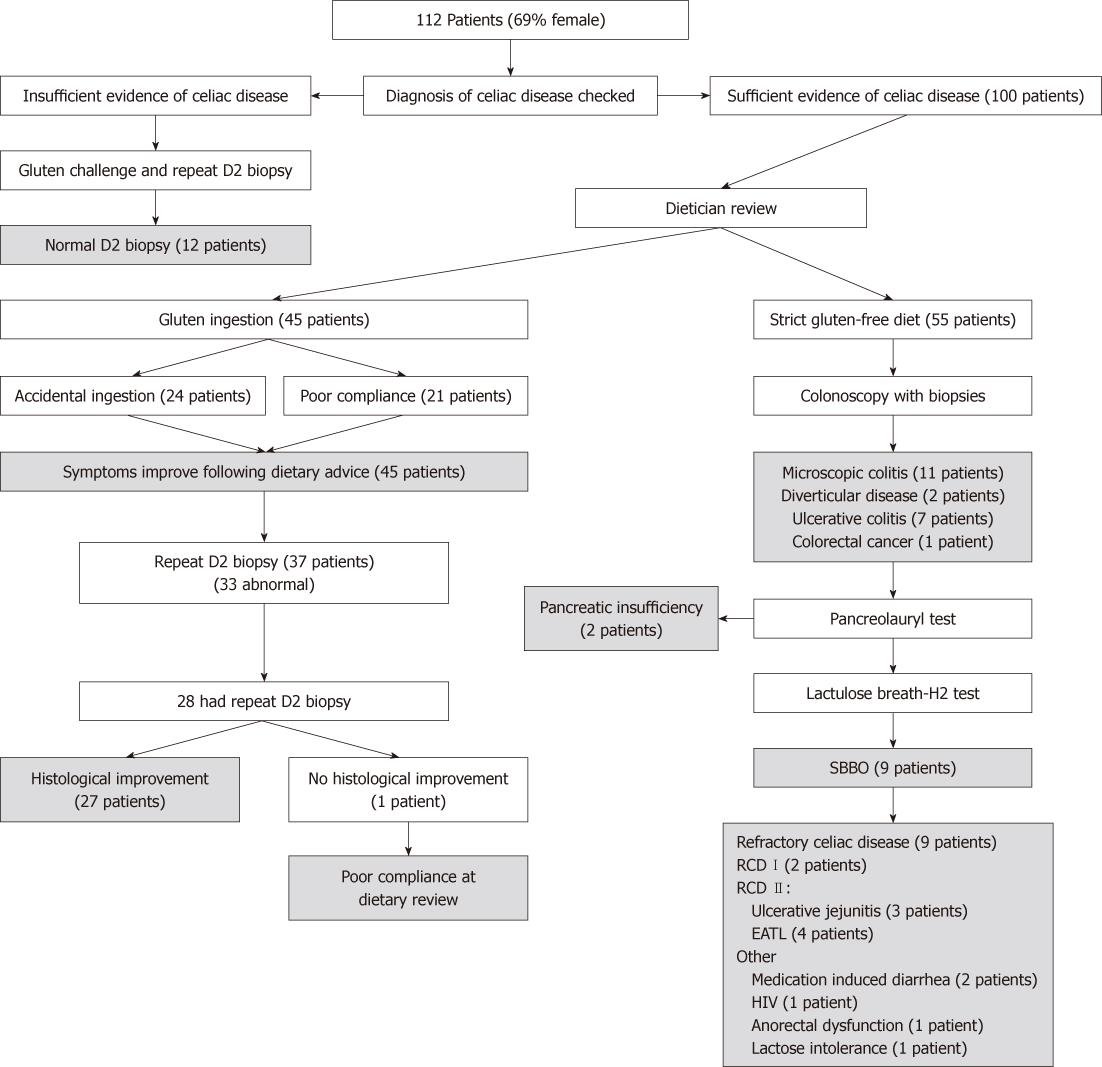
One hundred and twelve patients were referred to our center and underwent assessment for NRCD. The mean age of this group was 48.5 years (range 19-72 years) and 69% were female; CD had been diagnosed at a mean age of 31 years. The commonest presenting symptoms were diarrhea (65%), lethargy (43%), abdominal pain (27%) and weight loss (23%). The demographic details and clinical symptoms are shown in Table 1. The results are summarised in Figure 1
| Male | 31 |
| Female | 69 |
| Mean age (yr) | 48.5 |
| Primary non-responsive | 72 |
| Secondary non-responsive | 28 |
| Mean years since diagnosis of CD (yr) | 3 (range 1-12) |
| Diarrhea | 65 |
| Lethargy/fatigue | 43 |
| Abdominal pain | 37 |
| Weight loss | 23 |
| Nausea and vomiting | 10 |
| Symptoms of anemia | 10 |
| Two symptoms | 49 |
| Three symptoms | 20 |
Twelve out of the 112 patients had been wrongly diagnosed with CD. Due to the doubt over the diagnosis, these 12 patients underwent gluten challenge and repeat biopsy which was normal in all cases. Additionally, anti-endomysial antibody (EMA) tests were all negative, although four patients had anti-gliadin antibodies detected. In four cases, initial duodenal biopsy had not been performed previously and diagnosis had been made based on dramatic reduction in symptoms with initial wheat exclusion. In the remaining eight, we were able to examine the original histology in five patients. Four of these were sufficiently normal to exclude CD in tandem with the subsequent negative gluten challenge. One patient did have villous atrophy on their original biopsy, which was felt to have been due to bacterial overgrowth, which had subsequently improved with antibiotic treatment. We were not able to examine previous specimens from three patients but the negative gluten challenge was deemed sufficient to exclude a diagnosis of CD. In total, six out of 12 patients had been previously shown to have supportive positive serology for CD in other institutions (mainly anti-gliadin antibody). Seven patients were diagnosed with irritable bowel syndrome; three with primary SBBO; and one each with anorexia nervosa and IgE-mediated wheat allergy. These individuals were subsequently removed from the analysis.
Forty-five of the remaining 100 patients were found to be ingesting sufficient gluten to cause their symptoms. Of these, 24 were discovered to be consuming gluten accidentally, and 21 admitted poor compliance with aspects of their prescribed diet. In total, 37 (23/24 accidental group and 14/21 poor compliance group) patients underwent repeat duodenal biopsy in order to establish this information. Of these specimens, 33/37 were abnormal (Marsh IIIa-c) which assisted in correlating the continued ingestion with the persisting histological abnormalities.
The majority (28/37) proceeded to have a further duodenal sample taken that showed comparative improvement in all but one case. In this case, further gluten ingestion was admitted on further questioning. In summary, all 45 patients in this group reported symptomatic improvement on a strict GFD, with 27/45 having demonstrable histological improvement.
Eleven patients were treated successfully for microscopic colitis. Diagnosis was made based on the presence of diarrhea and typical colonic histological features. All of these patients underwent simultaneous small bowel biopsy which was abnormal in 7/11 (64%) cases, mainly with an isolated intra-epithelial lymphocytosis. No alternative cause was established on enquiry or testing. These individuals were treated with a combination of mesalazine, loperamide, prednisolone and azathioprine (1-2.5 mg/kg). Five out of 11 required azathioprine for resolution of symptoms. Three patients suffered a relapse of diarrhea within 2 years; again treated successfully with oral steroids. When abnormal, patients had comparative improvement in their duodenal histology following resolution of symptoms. We performed a total of 75 colonoscopies in NRCD patients with diarrhea and found significant lymphocytic infiltration in 15. This included four patients defined as having RCD who did not show histological or clinical improvement with immunosuppressive treatment.
Nine patients were successfully diagnosed and treated for bacterial overgrowth with sustained resolution of symptoms. There have been two relapses both in the same patient within 2 years; responding on each occasion to further courses of antibiotic treatment (metronidazole and ciprofloxacin). Interestingly, one patient was found to have combined variable immunodeficiency as an underlying cause for bacterial overgrowth and was referred for immunoglobulin infusions as part of further management.
Ten patients had normal investigations (all had duodenal biopsy and colonoscopy). This group was reassured and treated symptomatically for irritable bowel syndrome. At review after 2 years, 5/10 had continued functional symptoms with no new positive investigations. One patient had been diagnosed empirically with lactose intolerance. The remaining four patients were symptom free.
Lactose intolerance was diagnosed in six individuals; all of whom had dramatic symptomatic resolution when a lactose-free diet was commenced. All of these patients had primary NRCD.
We identified seven patients with coexisting inflammatory bowel disease (IBD); all of whom were suffering from ulcerative colitis. The predominant pattern was proctitis in five patients, and two had sigmoid colitis. Six responded to 5-ASA therapies, and one required azathioprine to control their IBD. All remained well and no surgical intervention has been required at 2 years follow-up.
After initial assessment and duodenal biopsy, 20 patients were considered to have a high suspicion of RCD. All of these patients had weight loss and diarrhea and a history of positive correlative celiac serology. After exhaustive investigation and assessment according to the United European Gastroenterology Week guidelines[11] (median duration 5 mo), a firm diagnosis of RCD was made in 9/20 patients; all of whom had a raised IEL count (> 20 per 100 enterocytes). Furthermore, all had marked villous atrophy (Marsh IIIa-c). None of this group was found to have a positive anti-enterocyte antibody. An alternative and remediable explanation for symptoms was identified in 11 patients (seven continued gluten ingestion; three with bacterial overgrowth; and one with microscopic colitis). RCD may be divided into those without aberrant T cells (type I) and those with aberrant T cells or ulcerative jejunitis (type II)[11]. Of the nine refractory patients, seven had type II RCD with positive clonality by γ TCR PCR. Three had ulcerative jejunitis; four were found to have or developed an enteropathy-associated intestinal lymphoma, two of whom have subsequently died, one from proven EATL and the other from suspected EATL (a post-mortem was refused by the relatives); both survived less than 1 year from diagnosis. The other two patients remain alive; one is on immunosuppressive medication and the other has been successfully treated with surgery. The remaining patients have continued to have symptoms over the follow-up period of 2 years (median 33 mo).
Of the two patients with type I RCD, one has died but we have no information available as to the precise cause of death, and the other patient has continued to have symptoms over the follow-up period of 2 years. In summary 3/9 (33%) patients diagnosed with RCD in our study have died.
Other diagnoses that were established are listed in Table 2. A diagnosis was only included if the symptoms were clearly attributable and symptomatic improvement occurred with appropriate treatment. Ten patients had more than one diagnosis established during the study period (median 33 mo). This was largely as a result of ongoing investigation for additional symptoms during the study period.
| Diagnosis | n |
| Continued dietary gluten | 45 |
| Microscopic colitis | 11 |
| Bacterial overgrowth | 9 |
| Lactose intolerance | 7 |
| Inflammatory colitis | 7 |
| Irritable bowel syndrome | 10 |
| Refractory celiac disease | 9 |
| Type I RCD | 2 |
| Type II RCD | 7 |
| Anorexia nervosa | 2 |
| Pancreatic insufficiency | 2 |
| Diverticular disease | 2 |
| Medication-induced diarrhea | 2 |
| Combined variable immunodeficiency | 1 |
| Human immunodeficiency virus | 1 |
| Colorectal cancer | 1 |
| Anorectal dysfunction | 1 |
| Incorrect diagnosis of celiac disease | 12 |
Further assessment of patients’ symptoms was conducted 2 years after their initial evaluation. Overall, four patients had died, with one from an unrelated cause. The vast majority (78%) reported being symptom-free at 2 years. A total of eight patients reported continued symptoms, with four describing them as moderate or severe. Those with continued symptoms included four diagnosed with RCD, two with irritable bowel syndrome and two with microscopic colitis. Ten patients could not be contacted.
In the 100 patients with NRCD, 73% had detectable anti-tissue transglutaminase (tTG) antibodies at varying titers. There was no statistical correlation between presence of antibodies, antibody titer and the established cause of NRCD. However, it was noted that 9/20 patients with RCD had positive celiac serological tests.
Evaluation of patients referred to us with continued symptoms on a GFD concluded that 12 out of 112 patients did not actually have CD. The diagnosis of CD might appear straightforward but this indicates that errors are still made in clinical practice. The main difficulties appear to be basing the diagnosis on serology alone; where available tTG and EMA should be tested because these are most sensitive and specific[19,20]. DQ2/8 HLA typing may be useful to exclude CD in patients when tTG is negative but villous atrophy is present, and there is doubt over the diagnosis. In this study, DQ2/8 was not performed given difficulty in availability; furthermore, it adds little in patients who have a positive tTG and villous atrophy. In experienced hands, serology testing is highly specific but, there can be discordant results between different laboratories. The limitations of celiac serology have previously been reported[21]. Accordingly, duodenal biopsy remains mandatory for a clear diagnosis of CD to be made and this is supported by current recommendations. We feel it is important to reassess the initial biopsy, as failure to orientate the small intestinal mucosal biopsy can result in a false interpretation of villous atrophy.
When the diagnosis of CD is secure, investigation of continued symptoms yields a remediable cause in 90% of cases, with continued gluten ingestion as the leading diagnosis. This parallels the findings of a previous study in an NRCD group[6]. In our study, the commonest culprit for inadvertent intake was malted breakfast cereals, although beer, cooking sauces, pizza, and biscuits - the latter two of which were clearly labeled as containing gluten - were also identified as sources of continued gluten ingestion. The diagnosis of continued gluten ingestion was only accepted, if after dietary modification, the patients’ symptoms were reported to have resolved at a later follow-up appointment. It is of interest that nearly half of those failing to adhere to a GFD were aware that their compliance was suboptimal but withheld this information at initial assessment. It appears that some CD patients are reluctant to acknowledge that a minor intake of gluten could account for their continued symptoms. It is therefore important that appropriate dietary advice is provided at the outset to avoid unnecessary investigation at a later date. Celiac societies have a useful role in advising on GFD. However, some patients in our study were following a recommended GFD but improved when certain “safe” foods were removed from their diet. There has been considerable debate as to the acceptable safe threshold for gluten in foods, with 200 ppm being initially recommended. Some individuals do appear to suffer ongoing symptoms with persistent duodenal injury, even with trace quantities of gluten ingested in certain foods. Therefore, a lower limit of 20 mg/kg (20 ppm) has been accepted for labeling of gluten-free foods with 100 mg/kg labeled as gluten-reduced. These regulations will be introduced in 2012[22]. We advise patients that appear to be exquisitely sensitive to traces of gluten to adhere to a wheat-free GFD. This involves avoidance of products that are made by extraction of wheat proteins from flour because this process is usually incomplete to some degree, with traces of residual gluten remaining.
The association of microscopic colitis has been reported in CD patients[6,13]. It has been postulated that the lymphocytic infiltrate is part of the same autoimmune pathogenesis that is seen in the small bowel and that this infiltrate improves with a GFD. Similarly, microscopic colitis appears to be linked with RCD, which again suggests an aberrant immunological process. In our study, we suggest that there may have been an overlap between the groups diagnosed with microscopic colitis and RCD. It may be difficult to differentiate the two conditions, especially when duodenal abnormalities are marked. We based our diagnosis on the predominant abnormality between colonic and duodenal histology, severity of clinical manifestations, and the response to treatment. In practice, once lymphoma has been exhaustively excluded, the management of resistant symptoms may be largely similar with recourse to immunosuppressive therapy. Treatment of microscopic colitis is currently suboptimal but overall, the natural history is benign. We do not attempt here to discuss the validity of treatments for microscopic colitis in CD; only that sustained symptomatic improvement was achieved in these cases. In our experience, a trial of oral mesalazine may prove sufficient, although this is frequently ineffective. Following this, moderate-dose oral systemic steroids (20 mg/d prednisolone) usually provides rapid complete symptomatic response. The dose should be tapered gradually, although in a few cases it may be necessary to maintain 5-7.5 mg/d; in such instances, azathioprine as a steroid-sparing agent should be considered.
SBBO is associated with CD and is probably under-diagnosed[23]. The mucosal abnormalities may theoretically disrupt the innate defenses of the small intestine and predispose to this condition. In our study, patients all responded to antibiotic therapy but relapse was common. A second longer course of rotating antibiotic therapy was prescribed, which appeared to eradicate symptoms in the long term. If suspected, the diagnosis can be difficult to confirm, either by duodenal aspiration or breath test because these tests have a low sensitivity and specificity[15]. The duodenal histology may be normal, abnormal or exhibit patchy changes that are difficult to detect[24]. Treatment may be reasonably advised empirically if this diagnosis is suspected[23]. Although there is minimal data from clinical trials, it is our practice to treat patients with ciprofloxacin 250 mg twice daily, rotating fortnightly with metronidazole 200 mg three times daily for 3 mo. Symptomatic response is assessed to determine success of treatment. Duration of treatment depends on the conviction that SBBO is the underlying course. In our experience, patients may require treatment with alternating antibiotics for up to 4 mo if symptoms are resistant or recur after discontinuation of a short course of empirical antibiotics.
Acquired lactose intolerance is widely recognized to be a potential problem in CD. Exclusion of dairy produce is often recommended in the first 3-6 mo of GFD to allow disruption of the brush-border disaccharidase activity to recover. IBD can coexist with CD, because both are common and not mutually exclusive. One study has previously reported an increased incidence of IBD in patients with CD compared with the general population[25]. Two patients in our cohort had evidence of concomitant pancreatic insufficiency. Abnormal exocrine function, as tested with fecal elastase, was demonstrated in 13 (42%) subjects in one series of 31 CD patients, although only in three was this clinically significant[26]. A trial of treatment with pancreatic supplements may be advisable in those in whom pancreatic insufficiency is suspected.
Continued symptoms in CD patients may be functional because the symptoms are often indistinguishable[27]. In 10% of our NRCD patients, further investigations, including duodenal biopsy, were normal and the symptom pattern was consistent with standard criteria for irritable bowel syndrome. It is possible that the original symptoms at presentation were functional and that the CD was an incidental diagnosis. Additionally, a GFD frequently fails to provide adequate fiber intake that may exacerbate constipation and symptoms of irritable bowel syndrome, for which we advise supplementary fiber with either an ispaghula or psyllium seed husk preparation. Clearly, the GFD should be continued if the diagnosis of CD has been confirmed. Patients with CD may also suffer from a range of other conditions that affect the general population and they should be investigated accordingly. It is not satisfactory to attribute any subsequent symptoms to a previous diagnosis of CD, particularly in cases in which symptoms initially responded to a GFD.
In our study of NRCD, nine patients were characterized as having RCD. Three were diagnosed with intestinal lymphoma, but one survived following treatment. At 2 years, 3/9 had died (33%), which is comparable to pre-existing cohorts[9,28]. There are no controlled trials but there are reports of symptomatic improvement with use of oral steroids and azathioprine. It is our practice to manage RCD and ulcerative jejunitis with moderate-dose prednisolone (20 mg/d), with initiation of azathioprine as a steroid-sparing agent (2-2.5 mg/kg). The steroid dose is tapered according to symptomatic response. We continue to monitor for the development of EATL. It is our practice to repeat duodenal biopsy after 4-6 mo to assess the small bowel inflammation and correlate this to ongoing symptoms and treatment.
We test for serum IgA EMA and tTG antibodies in all patients with suspected CD, because these are the most sensitive and specific. We also test for IgA deficiency because this is over-represented in CD patients and can lead to a false-negative EMA result. We no longer recommend using anti-gliadin antibody testing because of poor specificity[19]. Initial reports have suggested that celiac serology is a good indicator of response to GFD[29]. However, a further study has indicated that serology correlates poorly with histological recovery[30]. In our experience of NRCD, there was a high rate of low titer positive serology and this disappointingly failed to correlate with specific causes. Although celiac antibody testing should be performed routinely in symptomatic CD, we do not feel that this should deter further investigation of the non-responsive patient.
We have followed up this group of NRCD patients to provide information on longer-term outcome of NRCD. Only eight patients reported continued symptoms after 2 years, which included patients with RCD, as one might expect, and microscopic colitis. This is reassuring in that NRCD has a good prognosis if evaluated and managed appropriately.
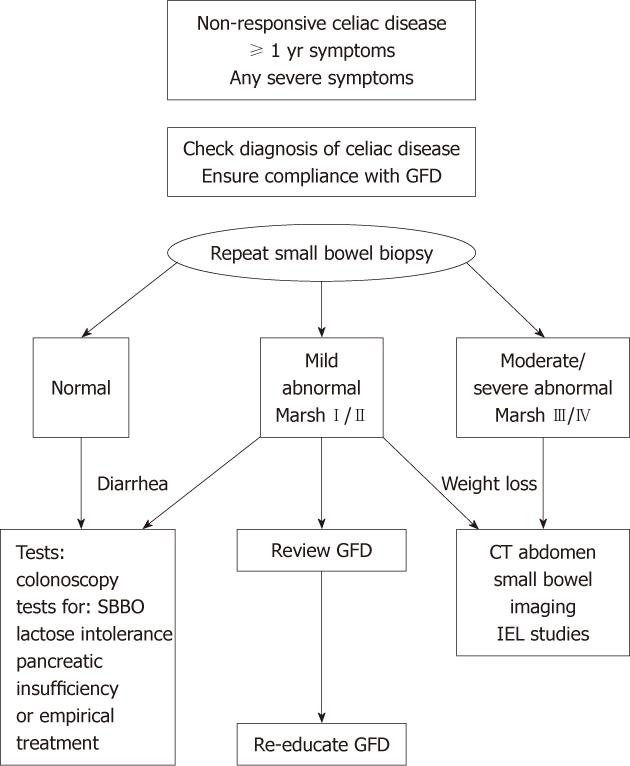
This algorithm (Figure 2) has been used as a basic guide in the investigation of patients referred to our institution with NRCD. It reflects the pivotal role of repeat duodenal biopsy. It recognizes that mild histological abnormalities are more likely to indicate continued trace gluten intake or be present in the context of a secondary diagnosis. More severe histological changes or significant weight loss warrant more urgent investigation for RCD or intestinal lymphoma. In our study, all nine patients with RCD had significant weight loss and severe histological abnormalities on duodenal biopsy.
The management of NRCD depends on confirming the diagnosis of CD and establishing a cause for the symptoms, which should be possible in 90% of cases. We suggest that those with RCD should be evaluated for lymphoma and subsequently managed with immunosuppressive therapy. Alternative strategies involving treatment with cyclosporine[31], cladribine[32], or fluadribine and melaphan, stem cell transplantation for type II RCD[33] have been reported, although their use is not generally accepted. Continued gluten ingestion accounts for 45% of persistent symptoms in patients with CD and a thorough and honest dietary assessment should be encouraged. Microscopic colitis and SBBO are important causes of persistent ongoing symptoms that should respond to treatment. The longer-term prognosis of NRCD is good, with a 90% prospect of sustained symptom resolution.
Celiac disease (CD) is a common disease that affects approximately 1% of Northern Europeans and North Americans. It is an inflammatory condition predominantly involving the proximal small bowel in genetically susceptible individuals. Treatment involves a life-long gluten-free diet (GFD) with avoidance of dietary gluten present in wheat, rye and barley. Thirty percent of CD patients fail to improve or may relapse while on a GFD, which is termed non-responsive CD (NRCD).
The investigators report that the commonest cause of NRCD is continued gluten ingestion, either deliberately or by accidental ingestion. This cause is easily remediable by simple dietary measures. The authors also describe how other diagnoses can also contribute to the persistent symptoms: this includes microscopic colitis, a disease that causes diarrhea with a normal visual examination of the large bowel, and small bowel bacterial overgrowth; both of which occur more commonly in CD than previously reported.
This article helps investigation of NRCD through provision of an investigative algorithm for physicians to investigate the persistent symptoms in individuals with CD who have been prescribed a GFD. It also highlights that continued gluten intake and other diagnoses can be concomitant, such that they should be considered in the diagnostic work up.
This is a good descriptive study in which authors investigate all patients referred to our centre with non-responsive celiac disease to establish a cause for their continued symptoms.The results are interesting and suggest that an algorithm for managing patients with non-responsive celiac disease.
Peer reviewer: Salvatore Auricchio, MD, PhD, Professor, Scientific Director of European Laboratory for the Investigation of Food-Induced Diseases, University Federico II, Via S. Pansini 5, I-80131 Naples, Italy
S- Editor Lv S L- Editor Kerr C E- Editor Zheng XM
| 1. | Pink IJ, Creamer B. Response to a gluten-free diet of patients with the coeliac syndrome. Lancet. 1967;1:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Cooke WT, Holmes GK. "Non-responsive" coeliac disease. Br Med J. 1977;1:1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | O'Mahony S, Howdle PD, Losowsky MS. Review article: management of patients with non-responsive coeliac disease. Aliment Pharmacol Ther. 1996;10:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Schuppan D, Kelly CP, Krauss N. Monitoring non-responsive patients with celiac disease. Gastrointest Endosc Clin N Am. 2006;16:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59:547-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 6. | Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 248] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 460] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Cellier C, Brousse N. Classification and outcome of refractory coeliac sprue. Coeliac Dsease: Proceedings of the Xth International Symposium on Coeliac disease. Montrouge: John Libbey eurotext 2003; 215-223. |
| 10. | Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | United European Gastroenterology. When is a coeliac a coeliac? Report of a working group of the United European Gastroenterology Week in Amsterdam, 2001. Eur J Gastroenterol Hepatol. 2001;13:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Ciclitira PJ, King AL, Fraser JS. AGA technical review on Celiac Sprue. American Gastroenterological Association. Gastroenterology. 2001;120:1526-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Baert F, Wouters K, D'Haens G, Hoang P, Naegels S, D'Heygere F, Holvoet J, Louis E, Devos M, Geboes K. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795-1803. [PubMed] |
| 16. | Stevens FM, Kearns MC, McCarthy CF. Abnormal pancreolauryl tests in coeliac disease: lack of correlation with the degree of intestinal mucosal damage. J Clin Pathol. 1997;50:1001-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, García-Sanz R. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2284] [Cited by in RCA: 2375] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 18. | Brüggemann M, White H, Gaulard P, Garcia-Sanz R, Gameiro P, Oeschger S, Jasani B, Ott M, Delsol G, Orfao A. Powerful strategy for polymerase chain reaction-based clonality assessment in T-cell malignancies Report of the BIOMED-2 Concerted Action BHM4 CT98-3936. Leukemia. 2007;21:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Hadithi M, von Blomberg BM, Crusius JB, Bloemena E, Kostense PJ, Meijer JW, Mulder CJ, Stehouwer CD, Peña AS. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med. 2007;147:294-302. [PubMed] |
| 20. | Stern M. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. J Pediatr Gastroenterol Nutr. 2000;31:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Rostami K, Kerckhaert J, Tiemessen R, von Blomberg BM, Meijer JW, Mulder CJ. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am J Gastroenterol. 1999;94:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 324] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Codex standard for foods for special dietary use for persons intolerant to gluten. Codex Stan 118-1979. Revised. Montrouge: John Libbey eurotext 2008; Available from: http://www.codexalimentarius.net/web/standard_list.jsp. |
| 23. | Attar A, Flourié B, Rambaud JC, Franchisseur C, Ruszniewski P, Bouhnik Y. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: a crossover, randomized trial. Gastroenterology. 1999;117:794-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Tursi A, Giorgetti GM, Brandimarte G, Elisei W. High prevalence of celiac disease among patients affected by Crohn's disease. Inflamm Bowel Dis. 2005;11:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Yang A, Chen Y, Scherl E, Neugut AI, Bhagat G, Green PH. Inflammatory bowel disease in patients with celiac disease. Inflamm Bowel Dis. 2005;11:528-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Regan PT, DiMagno EP. Exocrine pancreatic insufficiency in celiac sprue: a cause of treatment failure. Gastroenterology. 1980;78:484-487. [PubMed] |
| 27. | Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, Lobo AJ. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Mauriño E, Niveloni S, Cherñavsky AC, Sugai E, Vázquez H, Pedreira S, Periolo N, Mazure R, Smecuol E, Moreno ML. Clinical characteristics and long-term outcome of patients with refractory sprue diagnosed at a single institution. Acta Gastroenterol Latinoam. 2006;36:10-22. [PubMed] |
| 29. | Sategna-Guidetti C, Pulitanó R, Grosso S, Ferfoglia G. Serum IgA antiendomysium antibody titers as a marker of intestinal involvement and diet compliance in adult celiac sprue. J Clin Gastroenterol. 1993;17:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Dickey W, Hughes DF, McMillan SA. Disappearance of endomysial antibodies in treated celiac disease does not indicate histological recovery. Am J Gastroenterol. 2000;95:712-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Wahab PJ, Crusius JB, Meijer JW, Uil JJ, Mulder CJ. Cyclosporin in the treatment of adults with refractory coeliac disease--an open pilot study. Aliment Pharmacol Ther. 2000;14:767-774. [PubMed] |
| 32. | Al-Toma A, Goerres MS, Meijer JW, von Blomberg BM, Wahab PJ, Kerckhaert JA, Mulder CJ. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin Gastroenterol Hepatol. 2006;4:1322-137; quiz 1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Al-toma A, Visser OJ, van Roessel HM, von Blomberg BM, Verbeek WH, Scholten PE, Ossenkoppele GJ, Huijgens PC, Mulder CJ. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood. 2007;109:2243-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |