Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1077
Revised: July 27, 2011
Accepted: August 5, 2011
Published online: March 14, 2012
AIM: To evaluate cut-off values and performance of acoustic radiation force impulse imaging (ARFI) using transient elastography [FibroScan© (FS)] as a reference.
METHODS: Six hundred and six patients were enrolled in this study. All patients underwent liver stiffness measurement with FS (FS-LS) and ARFI (with shear wave velocity quantification; ARFI-SWV) and the performance of ARFI in comparison to FS was determined. Sixty-eight patients underwent liver biopsy.
RESULTS: Significantly higher success rates for the determination of liver stiffness were found using ARFI as compared to FS [604/606 (99.7%) vs 482/606 (79.5%), P < 0.001]. ARFI-SWV correlated significantly with FS-LS (r = 0.920, P < 0.001). ARFI-SWV increased significantly with the stage of fibrosis (1.09 ± 0.13 m/s for patients with no significant fibrosis (FS-LS < 7.6 kPa); 1.46 ± 0.27 m/s for patients with significant liver fibrosis (7.6 < FS-LS ≤ 13.0 kPa); and 2.55 ± 0.77 m/s for patients with liver cirrhosis (FS-LS > 13.0 kPa)). ARFI-SWV cut-off values were identified for no significant fibrosis (1.29 m/s; sensitivity 91.4% and specificity 92.6%) and for liver cirrhosis (1.60 m/s; sensitivity 92.3% and specificity 96.5%). The optimal cut-off value for predicting liver fibrosis (F ≥ 2) was 1.32 m/s (sensitivity 87.0% and specificity 80.0%) and for liver cirrhosis (F4) 1.62 m/s (sensitivity 100% and specificity 85.7%), for patients who underwent liver biopsy. An excellent inter-and intraobserver reproducibility was observed for ARFI-SWV determinations.
CONCLUSION: An ARFI-SWV cut-off value of 1.29 m/s seems to be optimal for patients with no significant liver fibrosis and 1.60 m/s for patients with liver cirrhosis.
- Citation: Kircheis G, Sagir A, Vogt C, vom Dahl S, Kubitz R, Häussinger D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol 2012; 18(10): 1077-1084
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1077.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1077
Liver biopsy is currently considered the gold standard for assessing hepatic fibrosis or cirrhosis[1]. However, it is an invasive procedure with rare but potentially life threatening complications. In addition, the accuracy of liver biopsy for assessment of fibrosis may suffer from sampling errors and interobserver variability[2-6]. Therefore, research has been focused on the evaluation of non-invasive methods for assessment of liver fibrosis, such as routine biological and hematologic tests, surrogate serum fibrosis markers and measurement of liver elasticity[7-11]. Considerable experience exists for transient elastography[12]. Transient elastography [FibroScan© (FS)] is a rapid, non-invasive, and reproducible method for measuring liver stiffness (FS-LS). A strong association between FS-LS and the degree of liver fibrosis was demonstrated in patients with chronic hepatitis[13-15]. A cut-off value of 13 kPa has been established for the discrimination between liver fibrosis and cirrhosis[13]. For discrimination of fibrosis from no significant fibrosis a cut-off value of 7.6 kPa was suggested[16]. However, FS is limited in patients with ascites or a body mass index above 28 kg/m2[17]. Further limitations of FS have been described in several studies[18-21].
Another noninvasive tool for the detection of liver fibrosis is the acoustic radiation force impulse (ARFI) imaging technology[22-24]. ARFI imaging has been incorporated into a conventional ultrasonographic (US) device (Acuson S2000; Siemens Medical Solutions, Mountain View, CA, United States). ARFI imaging technology involves the mechanical excitation of tissue using short-duration acoustic pulses in a region of interest, producing shear waves that spread away from the region of interest[25-27]. By recording the shear wave-front and correlating these measurements with the elapsed time, the shear wave velocity-SWV (m/s) can be quantified (ARFI-SWV). The SWV increases with stiffness. Thus, the measured SWV is an intrinsic and reproducible property of the tissue[28-30]. A significant correlation between ARFI imaging, serum fibrosis marker tests, and the histologic fibrosis stage was reported in a few pilot studies[31-33].
In this study, we compared FS-LS with ARFI-SWV. Using the known cut-off values of 7.6 kPa and 13 kPa for FS we established cut-off values for ARFI-SWV for discriminating no significant liver fibrosis from significant liver fibrosis and significant liver fibrosis from liver cirrhosis, respectively. Furthermore, inter- and intraobserver reproducibility was studied for ARFI.
This study was approved by the ethics committee of Heinrich Heine University. A total of 606 patients who had consulted the Hepatology Unit of the University Hospital Düsseldorf, Germany were included in this study. Aetiology of the liver disease was determined according to standard diagnostic criteria. Patients´ characteristics and aetiologies of liver diseases are shown in Table 1.
| Patients,n | 606 |
| Male, n (%) | 363 (59.9%) |
| Age (yr) | 53 ± 17 |
| ALT (IU/L) | 59 ± 159 |
| AST (IU/L) | 53 ± 142 |
| GGT (IU/L) | 104 ± 218 |
| AP (IU/L) | 97 ±84 |
| Total bilirubin (mg/dL) | 1.1 ± 0.4 |
| Prothrombin time (% of normal) | 96 ± 22 |
| Chronic liver diseases, n (%) | |
| Non-alcoholic steatohepatitis | 236 (38.9%) |
| Chronic hepatitis B | 48 (7.9%) |
| Chronic hepatitis C | 97 (16.0%) |
| Alcoholic liver disease | 52 (8.6%) |
| Autoimmune hepatitis | 18 (3.0%) |
| PBC/PSC | 12 (2.0%) |
| Others | 14 (2.3%) |
| Healthy controls | 129 (21.3%) |
| Liver biopsy available, n (%) | 68 (11.2%) |
Details of the technical background and examination procedure have been described previously[12]. The tip of the probe transducer was placed on the skin between the ribs over the right liver lobe. The measurement depth was between 25 mm and 65 mm below the skin surface. Ten measurements were obtained in each patient. Determination of liver stiffness was considered valid, when a success rate of at least 60% was obtained. The results were expressed in kPa. The median value was taken as representative.
In all patients, ARFI imaging (Acuson S2000, Virtual Touch Tissue Quantification mode) and transient elastography (TE; FibroScan; Echosens, Paris, France) were performed on the same day. The examination was performed in the right lobe of the liver, through the intercostal space, at the same site as the transient elastography measurement. A measurement depth of 2 cm below the liver capsule was chosen to standardize the examination for ARFI-SWV. The mean value of ten measurements was taken as representative.
A subgroup of 68 patients underwent liver biopsy in the previous six months. Patients with histological proven liver cirrhosis in the previous two years were also included. Liver biopsy specimens were fixed in formalin and embedded in paraffin. Liver fibrosis was evaluated semiquantitatively according to the METAVIR scoring system. Fibrosis was staged on a 0-4 scale as follows: F0: No fibrosis; F1: Portal fibrosis without portal septa; F2: Portal fibrosis with few septa; F3: Numerous septa without cirrhosis; and F4: Cirrhosis.
Data were entered in SPSS (version 19.0, Inc., Munich, Germany). A χ2 or Fisher´s exact test (F-test) was used to compare categorical variables, and a Mann-Whitney test was used for the comparison of continuous variables. The significance level was set at 0.05, and all P values were two-tailed. A Pearson´s test was performed to study the correlation between FS-LS and ARFI-SWV.
For no significant fibrosis (FS ≤ 7.6 kPa) and liver cirrhosis (FS < 13.0 kPa), the diagnostic performance of ARFI was assessed using receiver operating characteristic (ROC) curves. The ROC curve is a plot of sensitivity vs 1-specificity for all possible cut-off values. The most commonly used index of accuracy is area under the ROC curve (AUROC). AUROC-values close to 1.0 indicated high diagnostic accuracy. ROC curves were generated for patients with FS ≤ 7.6 kPa, and patients with FS > 13 kPa. Optimal cut-off values for ARFI were chosen to maximize the sum of sensitivity and specificity, positive and negative predictive values were computed for these cut-off values. Using this analysis SWV cut-off values were identified for patients with no significant fibrosis (FS ≤ 7.6 kPa) and patients with liver cirrhosis (FS > 13.0 kPa).
Intraobserver and interobserver agreement was analysed using the intraclass correlation coefficient (ICC)[34]. ICC values range from +1 (100% agreement; all the variability being due to patient characteristics) to -1 (100% disagreement; all the variability being due to the rater’s performance). Interobserver agreement was calculated as the agreement between the first liver ARFI measurements of the two observers. Intraobserver agreement was calculated as the agreement between the first and the second ARFI evaluation. The agreement of liver stiffness between the right liver lobe and left liver lobe was calculated using the ICC. Agreement was classified as poor (ICC = 0.00 to 0.20), fair to good (ICC = 0.40 to 0.75) or excellent (ICC = 0.75).
A total of 606 patients were enrolled in this study. Their characteristics at the time of the FibroScan/ARFI examination are summarised in Table 1 (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1856085/table/tbl1/). Aetiologies of chronic liver diseases were non-alcoholic steatohepatitis (n = 236), hepatitis C virus (n = 97) or hepatitis B virus infection (n = 48), alcoholic liver disease (n = 52), primary biliary cirrhosis/primary sclerosing cholangitis (n = 12), autoimmune hepatitis (n = 18), and others (n = 14). In addition, another 129 patients without any liver diseases were included in the study.
FS-LS ranged from 2.3 kPa to 75.0 kPa (median 6.0 kPa) and ARFI-SWV ranged from 0.77 m/s to 4.72 m/s (mean 1.5 ± 0.77 m/s). Mean depth of the area where ARFI-SWV measurement was performed was 4.51 ± 0.56 cm. The overall success rate was 77.8% ± 28.5% for FS compared to 93.3% ± 9.87% for ARFI (P < 0.001). A liver stiffness measurement success rate of 100% was observed in 262 (43.2%) patients by FS compared to 373 patients (61.6%, P < 0.001) by ARFI.
A valid liver stiffness determination (success rate of at least 60%) was observed in 482/606 (79.5%) by FS compared to 604/606 (99.7%) by ARFI (P < 0.001). This difference was mostly due to a large distance between the skin surface and the liver capsule, which is associated with overweight. The success rate of FS was significantly dependent upon the distance between the skin surface and liver capsule (success rate 0%: 3.27 ± 0.34 cm; success rate between 1% and 59%: 2.81 ± 0.56 cm; and success rate ≥ 60%: 2.41 ± 0.52 cm; P < 0.001 for all differences).
After exclusion of all patients with an invalid liver stiffness determination (success rates below 60%) in one of the techniques, 482 patients remained for the following analysis.
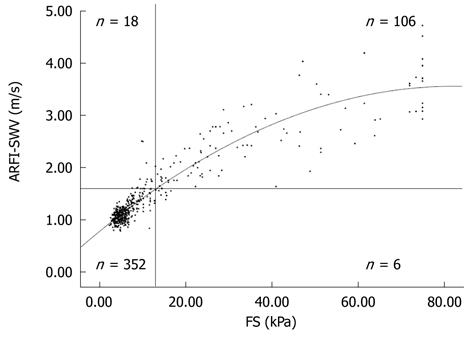
To analyse the correlation between FS-LS and ARFI-SWV, a Pearson test was performed. There was a significant correlation between these two methods (P < 0.001; r = 0.920; Figure 1)
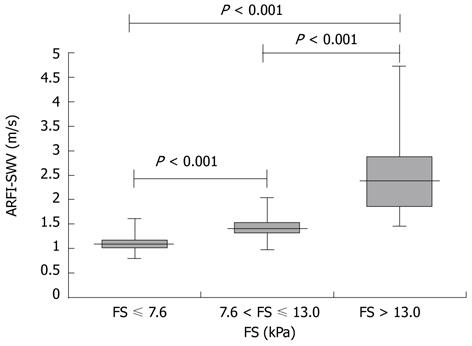
In consideration of the cut-off values for the different stages of liver fibrosis for FS, the following frequencies were observed: 297 (61.6 %) no significant fibrosis (FS-LS ≤ 7.6 kPa), 73 (15.2%) significant fibrosis (7.6 kPa < FS-LS ≤ 13.0 kPa), and 112 (23.2%) cirrhosis (FS-LS > 13.0 kPa). Mean ARFI-SWV was 1.09 ± 0.13 m/s (range 0.80-1.61 m/s) for patients with no significant fibrosis (FS-LS < 7.6 kPa), compared to 1.44 ± 0.26 m/s (range 0.98-2.03 m/s) for patients with significant liver fibrosis, and 2.55 ± 0.77 m/s (range 1.47-4.72 m/s) for patients with liver cirrhosis. ARFI-SWV was significantly different between patients according to their fibrosis stage (P < 0.001). Figure 2 shows box plots of ARFI-SWV for the three groups.
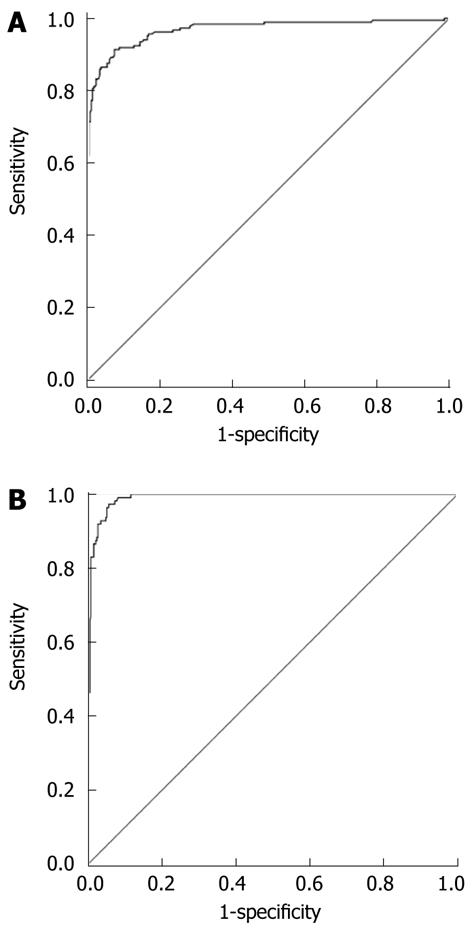
The diagnostic value (ROC curves) of liver stiffness measurement for patients with FS-LS < 7.6 kPa and patients with FS-LS > 13.0 kPa is shown in Figure 3. Corresponding AUROC values and 95% confidence intervals were 0.969 (95% CI: 0.952-0.985) for FS-LS < 7.6 kPa and 0.991 (95% CI: 0.985-0.997) for FS-LS > 13 kPa. Based on the ROC curves the optimal cut-off values for ARFI were chosen to maximize the sum of sensitivity and specificity. These cut-off levels were 1.29 m/s (sensitivity 91.4% and specificity 92.6% for FS-LS < 7.6 kPa) and 1.60 m/s (sensitivity 92.3% and specificity 96.5% for FS-LS > 13 kPa). The corresponding positive predictive value was 0.93 and the negative predictive value was 0.90 for FS-LS ≤ 7.6 kPa. When 1.60 m/s was chosen as the cut-off value for liver cirrhosis, the positive and negative predictive values were 0.85 and 0.98, respectively (Table 2).
| AUROC | Cut-off | PPV | NPV | Sensitivity (%) | Specificity (%) | |
| Comparison to all patients [ARFI-SWV (n = 482)] | ||||||
| FS < 7.6 kPa | 0.969 | 1.29 m/s | 0.93 | 0.90 | 91.4 | 92.6 |
| FS > 13.0 kPa | 0.991 | 1.60 m/s | 0.85 | 0.98 | 92.3 | 96.5 |
| Comparison with liver biopsy [ARFI-SWV (n = 68) and FS-LS (n = 59)] | ||||||
| Non significant liver fibrosis | ||||||
| ARFI-SWV (n = 23) | 0.929 | 1.32 m/s | 0.83 | 0.91 | 87.0 | 80.0 |
| FS-LS (n = 20) | 0.920 | 7.6 kPa | 0.85 | 0.95 | 94.9 | 85.0 |
| Liver cirrhosis | ||||||
| ARFI-SWV (n = 28) | 0.934 | 1.62 m/s | 1.0 | 0.85 | 100 | 85.7 |
| FS-LS (n = 24) | 0.958 | 13.0 kPa | 1.0 | 0.91 | 100 | 91.4 |
Using these cut-off values for ARFI-SWV, 458 of the 482 patients (95.1%) were classified correctly. Six (1.2%) patients were classified false-negative (FS-LS ≥ 13 kPa and ARFI-SWV < 1.60 m/s), and 18 (3.7%) were classified false-positive (FS-LS < 13 kPa and ARFI-SWV ≥ 1.60 m/s; Figure 1).
After dividing the patients according to the aetiologies of chronic liver diseases [non-alcoholic steatohepatitis (n = 157) vs others (n = 325)], the following cut-off values for ARFI were chosen (FS-LS > 13kPa): 1.52 m/s for patients with non-alcoholic steatohepatitis (sensitivity 100% and specificity 96.6%; AUROC 0.990; 95% CI: 0.977-1.000) compared to 1.64 m/s for patients with liver diseases other than non-alcoholic steatohepatitis (sensitivity 94.2% and specificity 96.2%; AUROC 0.988; 95% CI: 0.980-0.996).
68 patients underwent liver biopsy. A valid liver stiffness determination (success rate of at least 60%) was observed in 59/68 (86.8%) by FS compared to 68/68 (100%) by ARFI (P = 0.003).
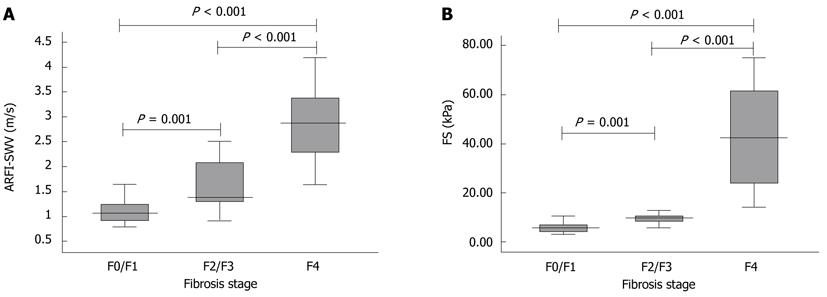
Liver stiffness measurements by ARFI ranged from 0.79 m/s to 4.17m/s. For patients without significant fibrosis (F ≤ F1, n = 23), mean ARFI-SWV was 1.11 ± 0.24 m/s, 1.78 ± 0.88 m/s for patients with moderate fibrosis (F2 and F3; n = 17), and 2.87 ± 0.76 m/s for liver cirrhosis (F4; n = 28). Liver stiffness measured by ARFI was significantly different between patients according to their fibrosis stage (P = 0.001 for F ≤ F1 vs F2/F3; P < 0.001 for F2/F3 vs F4; and P < 0.001 for F ≤ F1 vs F4; Figure 4A).
AUROC values and 95% confidence intervals were 0.934 (95% CI: 0.870-0.998) for liver cirrhosis (F4) and 0.929 (95% CI: 0.870-0.987) for F ≤ F1. Based on the ROC curves, the discriminating cut-off values for ARFI were chosen to maximize the sum of sensitivity and specificity. These cut-off levels were 1.32 m/s for F ≤ F1 (sensitivity 87.0% and specificity 80.0%) and 1.62 m/s for liver cirrhosis (F4) (sensitivity 100% and specificity 85.7%). The corresponding positive predictive value was 0.83 and the negative predictive value was 0.91 for non-significant fibrosis and 1.0 and 0.85 for liver cirrhosis (Table 2).
Liver stiffness measurements by FS ranged from 2.9 kPa to 75 kPa. For patients without significant fibrosis (F ≤F1, n = 20), mean FS-LS was 7.6 ± 8.1 kPa, 13.8 ± 17.1 kPa for patients with moderate fibrosis (F2 and F3; n = 15), and 42.6 ± 20.5 kPa for liver cirrhosis (F4; n = 24). Liver stiffness measured by FS was significantly different between patients according to their fibrosis stage (P = 0.001 for F ≤ F1 vs F2/F3; P < 0.001 for F2/F3 vs F4; and P < 0.001 for F ≤ F1 vs F4; Figure 4B).
AUROC value for non-significant fibrosis (F0/F1) was 0.920 (95% CI: 0.841-0.998) with a sensitivity of 94.9% and specificity of 85.0% when 7.6 kPa was chosen as the cut-off value. AUROC value and 95% confidence intervals were 0.958 (95% CI: 0.900-1.000) for liver cirrhosis (F4) with a sensitivity of 100% and specificity of 91.4% when 13.0 kPa was chosen as the cut-off value. The corresponding positive predictive value was 0.85 and the negative predictive value was 0.95 for non-significant liver fibrosis (F0/F1) and 1.0 and 0.91 for liver cirrhosis (F4; Table 2).
In order to evaluate the reproducibility of ARFI-SWV measurements, 18 patients were examined repeatedly. For interobserver reproducibility, the patients were examined by two observers consecutively. An excellent agreement between the observers was found (ICC = 0.945; 95% CI: 0.844-0.981). For intraobserver reproducibility, one observer examined the patients twice directly in series. The intraobserver reproducibility was also excellent (ICC = 0.975; 95% CI: 0.906-0.993).
In order to study if there was a difference when ARFI was performed in the right liver lobe or left liver lobe, 18 patients underwent measurement of ARFI-SWV in both liver lobes. The mean distance between skin surface and the liver capsule for the right and left liver lobes were 2.24 ± 0.52 cm and 2.54 ± 0.62 cm, respectively (P = 0.143). ARFI-SWV did not differ significantly between the two liver lobes (1.36 ± 0.41 m/s vs 1.51 ± 0.53 m/s, P = 0.143). The agreement between both liver lobes, however, was moderate (ICC = 0.589; 95% CI: 0.135-0.851).
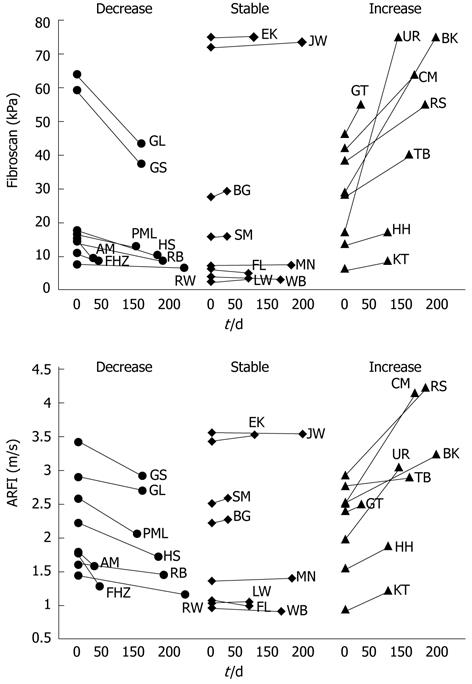
Fifty patients underwent liver stiffness measurement at least twice (mean ± SD, 2.3 ± 1.2). The mean interval between the two measurements was 73.6 ± 56.6 d. FS-LS did not change in 24 patients, increased in 9 and decreased in 17 patients. ARFI-SWV changed in parallel to FS-LS. The behaviour of ARFI-SWV over time was assessed in 24 patients, in which FS-LS remained constant, increased or decreased over time (8 patients in each group) (Figure 5).
Liver biopsy is currently considered to be the gold standard for detection of liver fibrosis/cirrhosis, but is associated with serious complications[1]. On the other hand, cut-off values for FS-LS are available, which were evaluated in a meta-analysis including more than 8000 patients[16]. All these patients underwent liver biopsy, and these cut-off values were compared with the gold standard. Therefore, we used TE as the standard to evaluate ARFI technology. This allowed us to avoid any liver biopsy-associated complications.
The data in this study suggest that non-invasive fibrosis/cirrhosis assessed by measuring ARFI-SWV shows an excellent agreement with the established FS-LS measurements and can be successfully employed in patients, where FS-LS measurements were unsuccessful. Furthermore, as already reported for FS-LS determinations[35], both intra- and interobserver variability were excellent for ARFI-SWV measurements.
A significantly higher success rate was observed for ARFI compared to FS (78.6% vs 99.8%, P < 0.001). A significant inverse relationship between the success rate by FS and the distance between the skin surface and liver capsule was observed. ARFI is less dependent on this factor. Another advantage is that ARFI is performed under the control of conventional B-mode sonography. The observer can select and place the region of interest under visual control.
Mean ARFI-SWV increased significantly with the stage of fibrosis [1.09 ± 0.13 m/s (range 0.80-1.61 m/s)] for patients with no significant fibrosis (FS-LS < 7.6 kPa); 1.44 ± 0.26 m/s (range 0.98-2.03 m/s) for patients with significant liver fibrosis (7.6 < FS-LS ≤ 13.0 kPa); and 2.55 ± 0.77 m/s (range 1.47-4.72 m/s) for patients with liver cirrhosis (13.0 < FS-LS).
We defined cut-off values for patients with no significant fibrosis and patients with liver cirrhosis. They were chosen so that the sum of sensitivity and specificity was maximal. A cut-off value for ARFI-SWV of 1.29 m/s was associated with a sensitivity of 91.4% and specificity of 92.6% for patients with FS-LS < 7.6 kPa and a cut-off value of 1.60 m/s for patients with FS-LS > 13.0 kPa with a sensitivity of 92.3% and specificity of 96.5%. Both cut-off values indicated high diagnostic accuracy for no significant fibrosis or liver cirrhosis, respectively. These cut-off values were confirmed by the subgroup-analyses. The cut-off values identified in patients who underwent liver biopsy did not differ significantly from the cut-off values taken from the correlation between both liver stiffness measurements (1.29 m/s vs 1.32 m/s for patients without significant liver fibrosis; 1.60 m/s vs 1.62 m/s for patients with liver cirrhosis).
Experience with ARFI is limited and there are only a few published studies on small numbers of patients[31-33,36-40].The largest study published by Palmeri et al[36] included 172 patients with non-alcoholic fatty liver disease. The present study included patients with different liver diseases and used FS-LS cut-off values from a meta-analysis of studies which included patients with different liver diseases[16]. In the above-mentioned studies, ARFI-SWV cut-off values between 1.30 m/s and 1.37 m/s were reported for no significant liver fibrosis and 1.75-2.00 m/s for liver cirrhosis[31,32,38,40]. These cut-off values differ from those determined in our study, and may be due to differences in the liver diseases studied, sample size and the sensitivities and specificities chosen. In our substudy, which included patients with non-alcoholic steatohepatitis, the cut-off value was lower than for other patients, which may due to the softening effect of steatosis. This effect has been described previously[41]. While liver stiffness measurement by FS is possible only in the right liver lobe, measurement of liver stiffness by ARFI is practicable in both liver lobes. Agreement of the measured liver stiffness between both liver lobes was moderate, but should be investigated in a larger study population. Thus, at present ARFI should be performed in the right liver lobe. Dynamics in the liver stiffness measured by FS have been described previously[18,19,21]. A congruent behaviour of ARFI-SWV and FS-LS dynamics over time was observed.
In conclusion, ARFI-SWV correlated significantly with FS-LS. ARFI can be performed in a significantly higher proportion of patients compared to FS. The most important advantage of ARFI over FS is the visual control by B-mode sonography and the variable depth of the measurement. A cut-off value of 1.29 m/s seems to be optimal for no significant fibrosis and 1.60 m/s for liver cirrhosis. ARFI-SWV did not depend on the observer. The sensitivity and specificity for the detection of liver cirrhosis seems to be comparable for both methods when liver biopsy is taken as the reference.
Liver biopsy is currently considered the gold standard for assessing hepatic fibrosis or cirrhosis, but is associated with complications. Thus, research has been focused on the evaluation of methods for the assessment of liver fibrosis. Transient elastography [FibroScan© (FS)] and acoustic radiation force impulse imaging (ARFI) are two methods used to detect liver fibrosis/cirrhosis.
A strong association between FS-LS and the degree of liver fibrosis was demonstrated in patients with chronic hepatitis. The experience with ARFI is limited. In this study the authors found a strong correlation between FS and ARFI. Using FS as a reference they evaluated cut-off values for ARFI. To evaluate the reproducibility of ARFI, an intra- and interobserver study was performed, without any significant results. It is known that liver stiffness shows a dynamic development, this point was also observed using ARFI.
Non-invasive methods for the assessment of liver fibrosis are of great interest. FS and ARFI are two methods used to detect liver fibrosis/cirrhosis. The experience with ARFI is very limited. Significantly higher success rates for the determination of liver stiffness were found using ARFI as compared to FS. A strong correlation between liver stiffness measured by FS and ARFI was shown. An ARFI-SWV cut-off value of 1.29 m/s seems to be optimal for patients with no significant liver fibrosis and 1.60 m/s for patients with liver cirrhosis.
ARFI is an additional non-invasive tool to detect liver fibrosis/cirrhosis. An ARFI-SWV cut-off value of 1.29 m/s seems to be optimal for patients with no significant liver fibrosis and 1.60 m/s for patients with liver cirrhosis.
FS and ARFI are two non-invasive methods to detect liver fibrosis/cirrhosis by measuring liver stiffness. Both methods show a strong correlation between liver stiffness and the stage of liver fibrosis. These methods did not show any dependence on the observer. These tools can reduce the number of liver biopsies which is associated with complications.
The authors reported the efficacy of acoustic radiation force impulse for determination of liver stiffness. The report is well written.
Peer reviewer: Yuichiro Eguchi, MD, Department of Internal Medicine, Saga Medical School, 5-1-1 Nabeshima, Saga 849-8501, Japan
S- Editor Gou SX L- Editor Webster JR E- Editor Zhang DN
| 1. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. NEngl J Med. 2001;344:495-500. [PubMed] |
| 2. | Abdi W, Millan JC, Mezey E. Sampling variability on percutaneous liver biopsy. Arch Intern Med. 1979;139:667-669. [PubMed] |
| 3. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1398] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 4. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 731] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 5. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 462] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1567] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 7. | Anastasiou J, Alisa A, Virtue S, Portmann B, Murray-Lyon I, Williams R. Noninvasive markers of fibrosis and inflammation in clinical practice: prospective comparison with liver biopsy. Eur J GastroenterolHepatol. 2010;22:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Angulo P. Noninvasive assessment of fibrosis and steatosis in NASH and ASH. Gastroenterol Clin Biol. 2009;33:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Attallah AM, Toson EA, Shiha GE, Omran MM, Abdel-Aziz MM, El-Dosoky I. Evaluation of serum procollagenaminoterminalpropeptide III, laminin, and hydroxyproline as predictors of severe fibrosis in patients with chronic hepatitis C. J Immunoassay Immunochem. 2007;28:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Oudry J, Chen J, Glaser KJ, Miette V, Sandrin L, Ehman RL. Cross-validation of magnetic resonance elastography and ultrasound-based transient elastography: a preliminary phantom study. J MagnReson Imaging. 2009;30:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Huwart L, van Beers BE. MR elastography. GastroenterolClinBiol. 2008;32:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1933] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 13. | Erhardt A, Lörke J, Vogt C, Poremba C, Willers R, Sagir A, Häussinger D. [Transient elastography for diagnosing liver cirrhosis]. Dtsch Med Wochenschr. 2006;131:2765-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1847] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 15. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 953] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 16. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 17. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J GastroenterolHepatol. 2006;18:411-412. [PubMed] |
| 18. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 573] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 19. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 20. | Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 462] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 22. | Nightingale K, Bentley R, Trahey G. Observations of tissue response to acoustic radiation force: opportunities for imaging. Ultrason Imaging. 2002;24:129-138. [PubMed] |
| 23. | Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 391] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Nightingale K, Palmeri M, Trahey G. Analysis of contrast in images generated with transient acoustic radiation force. UltrasoundMedBiol. 2006;32:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Frizzell LA, Carstensen EL. Shear properties of mammalian tissues at low megahertz frequencies. J AcoustSoc Am. 1976;60:1409-1411. [PubMed] |
| 26. | Palmeri ML, Frinkley KD, Zhai L, Gottfried M, Bentley RC, Ludwig K, Nightingale KR. Acoustic radiation force impulse (ARFI) imaging of the gastrointestinal tract. Ultrason Imaging. 2005;27:75-88. [PubMed] |
| 27. | Dahl JJ, Pinton GF, Palmeri ML, Agrawal V, Nightingale KR, Trahey GE. A parallel tracking method for acoustic radiation force impulse imaging. IEEE Trans UltrasonFerroelectrFreq Control. 2007;54:301-312. [PubMed] |
| 28. | Zhai L, Palmeri ML, Bouchard RR, Nightingale RW, Nightingale KR. An integrated indenter-ARFI imaging system for tissue stiffness quantification. Ultrason Imaging. 2008;30:95-111. [PubMed] |
| 29. | Nightingale K, Nightingale R, Stutz D, Trahey G. Acoustic radiation force impulse imaging of in vivo vastusmedialis muscle under varying isometric load. Ultrason Imaging. 2002;24:100-108. [PubMed] |
| 30. | Mauldin FW, Zhu HT, Behler RH, Nichols TC, Gallippi CM. Robust principal component analysis and clustering methods for automated classification of tissue response to ARFI excitation. Ultrasound Med Biol. 2008;34:309-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 32. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 33. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420-428. [PubMed] |
| 35. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 654] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 36. | Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Sporea I, Sirli R, Popescu A, Danilă M. Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason. 2010;12:26-31. [PubMed] |
| 38. | Grgurevic I, Cikara I, Horvat J, Lukic IK, Heinzl R, Banic M, Kujundzic M, Brkljacic B. Noninvasive assessment of liver fibrosis with acoustic radiation force impulse imaging: increased liver and splenic stiffness in patients with liver fibrosis and cirrhosis. Ultraschall Med. 2011;32:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Haque M, Robinson C, Owen D, Yoshida EM, Harris A. Comparison of acoustic radiation force impulse imaging (ARFI) to liver biopsy histologic scores in the evaluation of chronic liver disease: A pilot study. Ann Hepatol. 2010;9:289-293. [PubMed] |
| 40. | Piscaglia F, Salvatore V, Di Donato R, D'Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G. Accuracy of Virtual Touch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |