Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1028
Revised: January 16, 2012
Accepted: February 8, 2012
Published online: March 14, 2012
AIM: To investigate the expression of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in human gastric cancer and it’s mechanism in apoptosis and cell cycle arrest.
METHODS: Expression of 15-PGDH mRNA and protein was examined by immunohistochemistry, immunocytochemistry, reverse transcriptase polymerase chain reaction (RT-PCR) and Western blotting in tissue from human gastric cancer, gastric precancerous state (gastric polyps and atrophic gastritis), normal stomach, and gastric cancer cell lines. The relationship between gastric cancer, gastric precancerous state and 15-PGDH expression was determined. The association between expression of 15-PGDH and various clinicopathological parameters in gastric cancer was evaluated. Human gastric cancer cell line SGC-7901 was transfected with 15-PGDH expression plasmids. The effect of 15-PGDH on the cell cycle was examined by flow cytometry. The effect of 15-PGDH on apoptosis was examined by transmission electron microscopy, flow cytometry and transferase mediated nick end labeling (TUNEL) assay. Expression of cell cycle (p21, p27, p16 and p53) and apoptosis (Survivin, BCL-2, BCL-XL, BAK and BAX) genes was analyzed by RT-PCR.
RESULTS: Expression of 15-PGDH mRNA and protein in human gastric cancer tissues was significantly lower than in normal gastric tissues (P < 0.01). Expression in human gastric cancer cell lines MKN-28 and MKN-45 was reduced, and absent in SGC-7901 cells (P < 0.05). Reduction of 15-PGDH expression was also found in precancerous tissues, such as gastric polyps and atrophic gastritis (P < 0.01). There was a significant difference in expression of 15-PGDH among various gastric cancer pathological types (P < 0.05), with or without distant metastasis (P < 0.05) and different TNM stage (P < 0.01). Flow cytometry demonstrated a significant increase in apoptotic cells in SGC-7901 cells transfected with pcDNA3/15-PGDH plasmid for 24 h and 48 h (P < 0.01), and an increased fraction of sub-G1 phase after transfection (P < 0.05). TUNEL assay showed an increased apoptotic index in cells overexpressing 15-PGDH (P < 0.01). After transfection, expression of proapoptotic genes, such as BAK (P < 0.05), BAX and p53 (P < 0.01), was increased. Expression of antiapoptotic genes was decreased, such as Survivin, BCL-2 and BCL-XL (P < 0.01). Expression of cyclin-dependent kinase inhibitors p21 and p16 (P < 0.01) was significantly upregulated in cells overexpressing 15-PGDH.
CONCLUSION: Reduction of 15-PGDH is associated with carcinogenesis and development of gastric carcinoma. 15-PGDH induces apoptosis and cell cycle arrest in SGC-7901 cells.
- Citation: Lou LH, Jing DD, Lai YX, Lu YY, Li JK, Wu K. 15-PGDH is reduced and induces apoptosis and cell cycle arrest in gastric carcinoma. World J Gastroenterol 2012; 18(10): 1028-1037
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1028.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1028
Gastric carcinoma is one of the most common malignant tumors in humans and continues to be a major unresolved health problem. New approaches for the management of gastric cancer are needed. NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH) is the key enzyme responsible for the biological inactivation of prostaglandins (PGs) and related eicosanoids. It catalyzes the oxidation of the 15(S)-hydroxyl group of PGs and lipoxins. The products, 15-keto-metabolites, exhibit greatly reduced biological activities[1]. 15-PGDH is widely distributed in various mammalian tissues such as lung, breast, prostate, placenta and gut. The stomach is one of the most active tissues expressing 15-PGDH[2]. Recent studies[3-6] have shown a reduction of 15-PGDH in some cancers, such as colorectal, breast, prostate and lung. Some studies[3,4,6,7-9] have revealed that 15-PGDH may have tumor-suppressive properties. Recently, some studies[10-15] have indicated that 15-PGDH is downregulated in gastric cancer and is a suppressor of human gastric cancer. It provides a new target for the chemoprevention and treatment of cancer, especially in gastric carcinoma. However, to the best of our knowledge, there has been no study on 15-PGDH expression in gastric precancerous tissue. The association between 15-PGDH expression and various clinicopathological parameters of gastric cancer needs to be validated. Only a few studies have been published on its mechanism of tumor inhibition. Therefore, more studies on the mechanism of 15-PGDH suppression of gastric cancer are necessary.
In this study, we investigated the expression of 15-PGDH in human gastric cancer and gastric precancerous tissues, and determined the relationship between occurrence, development, infiltration, metastasis, cell differentiation of gastric carcinoma and 15-PGDH expression. We also examined the association of 15-PGDH with gastric cancer cell proliferation, apoptosis and the cell cycle. We studied the role of 15-PGDH reduction in carcinogenesis and development of gastric cancer and the possible mechanism of gastric cancer inhibition of 15-PGDH. Our results suggest the use of 15-PGDH in chemoprevention and treatment of gastric cancer.
Human gastric carcinoma specimens (n = 30) were obtained from surgical resections, with the approval of the Shanghai First People’s Hospital Ethics Committee. The specimens were frozen and stored in liquid nitrogen and 10% formaldehyde solution. Each tumor sample was matched with adjacent tissues (3 cm and 6 cm from the border of tumor) collected during the process. Other gastric tissues, including normal gastric tissues (n = 10), gastric polyps (n = 10) and chronic atrophic gastritis (n = 10), were obtained from gastroscopic biopsy and stored in liquid nitrogen and 10% formaldehyde solution. Specimens were dissected macroscopically by trained pathologists.
Human gastric carcinoma cell lines MKN-45, MKN-28 and SGC-7901 (obtained from Shanghai Institute of Biochemistry and Cell Biology) were maintained in RPMI-1640 (Gibco, United States) medium supplemented with 10% fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin in a 5% CO2 atmosphere at 37 °C. These cells were plated in six-well plates at about 2 × 105 cells/well in duplicate, and grown for 24 h before transfection.
The mammalian expression vector pcDNA3 containing the cDNA of the wild-type 15-PGDH and pcDNA3 expression vector were donated by Dr. Tai HH (Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, United States). Both pcDNA3/15-PGDH and pcDNA3 (200 ng) plasmids were transfected into SGC-7901 cells by Lipofectamine 2000 reagent for 24 h and 48 h, according to the manufacturer’s directions. Expression of the wild-type 15-PGDH mRNA and protein was monitored by reverse transcriptase polymerase chain reaction (RT-PCR), cellular immunohistochemistry and Western blotting.
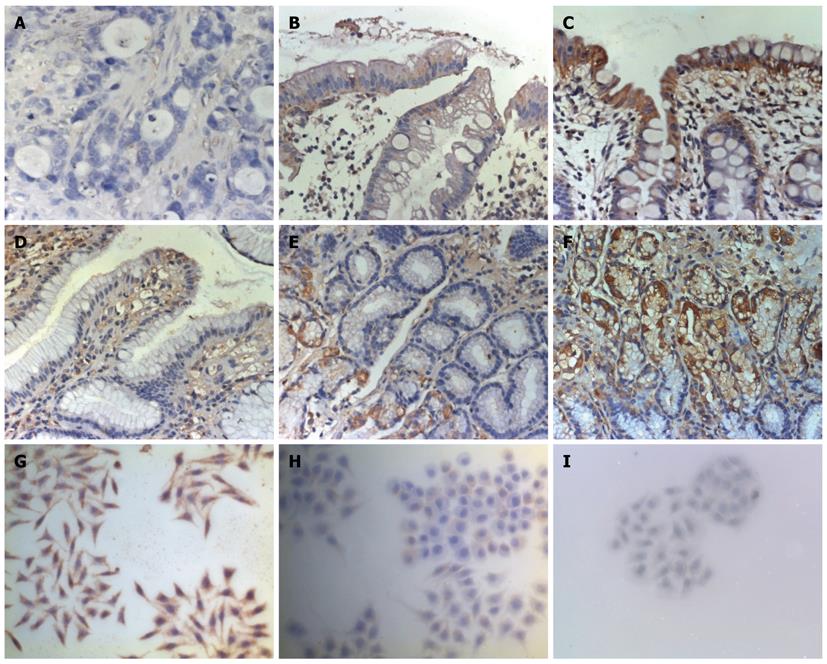
Paraffin-embedded tissue sections (3 μm) were dried, deparaffinized, and rehydrated. Endogenous peroxidase was blocked with 3% hydrogen peroxide in ion-free water for 30 min. After nonspecific binding sites, tissue slides were blocked with 10% goat serum. Cellular slides were treated by 4% paraformaldehyde for 30 min. Both kinds of slides were incubated at 4 °C overnight with a 1:50 dilution of rabbit polyclonal 15-PGDH antibody (Cayman, United States), followed by a 30-min incubation in horseradish peroxidase (HRP)-conjugated sheep anti-rabbit IgG (Changdao, China), rinsed with PBS, developed with the DAB kit (DakoCytomation, United States), and then counterstained with haematoxylin. Each slide was scanned at 100 and 400 × magnification. Immunohistochemistry score = intensity score (absent, 0; weak, 1; moderate, 2; strong, 3) × percentage score (< 5%, 0; 5%-25%, 1; 25%-50%, 2; 50%-75%, 3; > 75% of total tumor area, 4).
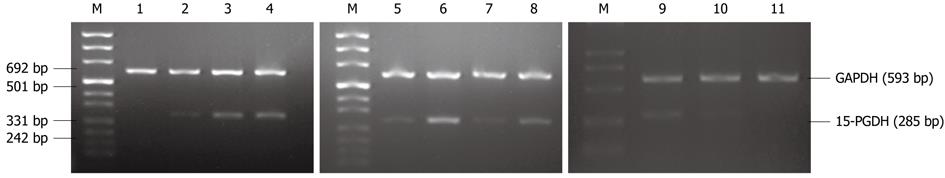
Total RNA of tissues and gastric cancer cells was extracted with TRIzol (Invitrogen, United States) following the manufacturer’s instructions. cDNA was synthesized from 2 μg total RNA using the M-MLV RT-PCR kit (Promega, United States) in a 20 μL volume, according to the manufacturer’s instructions. Two μL of cDNA, 2 μL each primer (50 pmol/L), 1 μL dNTP mix (10 mmol/L) and 1 μL Taq DNA polymerase (Sangon, China) were used for PCR analysis. The PCR amplification cycles consisted of denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 60 s, annealing for 60 s, extension at 72 °C for 60 s, and final elongation at 72 °C for 10 min. The PCR products were separated on a 1.5% agarose gel, stained with 0.5 mg/mL ethidium bromide, and visualized by UV light. Gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase and shown as the ratio of absorbance values. The primer sequences and annealing temperature are listed in Table 1.
| Target genes | Primer sequence | Size (bp) | Annealing temperature (°C) | |
| GAPDH | Sense | 5'-CCACCCATGGCAAATTCCATGGCA-3' | 593 | 62 |
| Antisense | 5'-AACAAAGCCTGGACAAAT-3' | |||
| 15-PGDH | Sense | 5'-GCTGGAGTGAATAATGAGA-3' | 285 | 55 |
| Antisense | 5'-GCTGAGCGTGTGAATCCAACT-3' | |||
| Survivin | Sense | 5'-GGCATGGGTGCCCCGAGGTT-3' | 320 | 58 |
| Antisense | 5'-AGAGGCCTCAATCCATGGCA-3' | |||
| BCL-2 | Sense | 5'-GGTGCCACCTGTGGTCCACCT-3' | 458 | 54 |
| Antisense | 5'-CCTCACTTGTGGCCCAGATAGG-3' | |||
| BAX | Sense | 5'-CTGACATGTTTTCTGACGGC-3' | 289 | 54 |
| Antisense | 5'-TCAGCCCATCTTCTTCCAGA-3' | |||
| BCL-XL | Sense | 5'-TTGGACAATGGACTGGTTG-3' | 765 | 54 |
| Antisense | 5'-GTAGAGTGGATGGTCAGTG-3' | |||
| BAK | Sense | 5'-TGAAAAATGGCTTCGGGGCAAGGC-3' | 642 | 54 |
| Antisense | 5'-TCATGATTTGAAGAATCTTCGTACC-3' | |||
| p53 | Sense | 5'-CCTTCCCAGAAAACCTACCA-3' | 371 | 59 |
| Antisense | 5'-TCATAGGGCACCACCACACT-3' | |||
| p21 | Sense | 5'-CAGGGGACAGCAGAGGAAGA-3' | 335 | 63 |
| Antisense | 5'-GGGCGGCCAGGGTATGTAC-3' | |||
| p27 | Sense | 5'-ATGTCAAACGTGCGAGTGTC-3' | 395 | 58 |
| Antisense | 5'-TCTGTAGTAGAACTCGGGCAA-3' | |||
| p16 | Sense | 5'-GGGCTCTCACAACTAGGAA-3' | 371 | 59 |
| Antisense | 5'-CGGAGGAGGTGCTATTAACTC-3' |
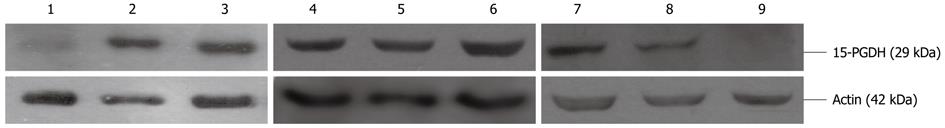
Tissues and gastric cancer cells were lysed with lysis buffer containing 0.5% NP-40, 40 mmol/L Tris-HCl (pH 8.0), 120 mmol/L NaCl, and a protease cocktail inhibitor (Complete Mini; Pierce, Rockford, IL, United States). Samples (40 μg protein per lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then blotted onto polyvinylidene difluoride membranes. Membranes were blocked for 2 h at room temperature with 5% skimmed milk and then probed with 1:200 dilution of rabbit polyclonal 15-PGDH antibody overnight at 4 °C. Membranes were washed and incubated for 1 h at room temperature with anti-rabbit IgG-HRP. Results were visualized by ECL chemiluminescence detection kit (Kangcheng, China). Protein expression was normalized to ACTIN.
The effect of 15-PGDH on the cell cycle and apoptosis in SGC-7901 cells was analyzed by flow cytometry. Cells floating in medium combined with the adherent layer were trypsinized and fixed with 2 mL citrate buffer for 1 h. Cells were then incubated with RNase A (1500 μL) and stained with propidium iodide (1500 μL). Samples were immediately analyzed by flow cytometry for cell cycle and apoptosis assays. Cells were observed under transmission electron microscopy (TEM) at Shanghai Medical College of Fudan University. The number of apoptotic cells was counted per 100 cells. Terminal deoxynucleotidyl transferase mediated nick end labeling (TUNEL) assay, in which residue of digoxigenin-labeled dUTP was catalytically incorporated into the DNA by terminal deoxynucleotidyl transferase II, was performed according to the manufacturer’s instructions (Boster, Wuhan, China). The positive particles of DAB staining were viewed under an optical microscope. The number of apoptotic cells was counted under a microscope (400 ×) and expressed as the apoptotic index (AI = the number of apoptotic bodies/1000 cells).
Quantitative results were expressed as mean ± SD. Statistical analysis was assessed by Student’s t test (between two groups) or Student-Newman-Keuls test (among three or more groups), with SAS version 8.02 software. P < 0.05 was considered statistically significant.
Immunohistochemistry analysis confirmed that 15-PGDH protein was expressed mainly in the cytoplasm of epithelial, inflammatory and gastric cancer cells in the lamina propria. Of the 30 gastric cancer cases, 15-PGDH expression was undetectable in 10 tumors (33.3%). Immunohistochemistry score of 15-PGDH was decreased in gastric cancer, paracancerous tissues 3 cm and 6 cm from the tumor, gastric polyps and atrophic gastritis compared with normal gastric tissues. Immunocytochemical analysis showed that expression of 15-PGDH in various differentiated gastric cell lines was dissimilar. Poorly differentiated gastric cell line SGC-7901 displayed no 15-PGDH, whereas MKN-28 and MKN-45 displayed little 15-PGDH (Figure 1).
RT-PCR analysis showed that expression of 15-PGDH mRNA in gastric cancer, paracancerous tissues, gastric polyps and atrophic gastritis was significantly lower than in norma1 gastric tissues. We also found loss of 15-PGDH in nine tumors (30%). Expression of 15-PGDH was absent in SGC-7901 cells, and significantly decreased in MKN-45 and MKN-28 cells (Figure 2).
Western blotting demonstrated that 15-PGDH protein expression was absent in nine of 30 gastric cancer cases (30%), and an average 5.7- and 8.3-fold less 15-PGDH expression was found in cancer tissues compared with paracancerous tissues at 3 cm and 6 cm from the tumor. There was a twofold reduction in gastric polyps and 2.1-fold reduction in atrophic gastritis tissues compared with normal gastric mucosa. Expression of 15-PGDH protein in gastric cancer cells was the same as shown by RT-PCR (Figure 3).
Expression of 15-PGDH protein was significantly different among the various gastric cancer pathological types (P < 0.05). Reduction of 15-PGDH was more distinct in gastric cancer with distant metastasis than in tumor without distant metastasis (P < 0.05 at protein level, P < 0.01 at mRNA level). There was also a significant difference in expression of 15-PGDH among tumors of different TNM stage (P < 0.01 at both protein and mRNA level). More reduced 15-PGDH expression was associated with worse TNM stage (Table 2).
| Clinicopathological parameters | Cases(n = 30) | IHC score | P values | Ratio (15-PGDH/GAPDH mRNA) | P values |
| Age (yr) | 0.847 | 0.970 | |||
| < 60 | 12 | 1.25 ± 1.22 | 0.21 ± 0.25 | ||
| ≥ 60 | 18 | 1.67 ± 1.10 | 0.21 ± 0.22 | ||
| Sex | 0.092 | 0.814 | |||
| Male | 23 | 1.39 ± 1.16 | 0.20 ± 0.21 | ||
| Female | 7 | 0.57 ± 0.79 | 0.23 ± 0.31 | ||
| Location | 0.891 | 0.407 | |||
| Antrum | 16 | 1.28 ± 1.27 | 0.20 ± 0.23 | ||
| Fundus and corpus | 6 | 1.00 ± 0.82 | 0.35 ± 0.36 | ||
| Cardia | 8 | 1.13 ± 0.99 | 0.17 ± 0.15 | ||
| Size (diameter) | 0.578 | 0.388 | |||
| < 5 cm | 14 | 1.07 ± 1.21 | 0.17 ± 0.15 | ||
| ≥ 5 cm | 16 | 1.31 ± 1.08 | 0.24 ± 0.28 | ||
| Pathological type | 0.019 | 0.138 | |||
| Well differentiated adenocarcinoma | 4 | 2.50 ± 1.00 | 0.24 ± 0.10 | ||
| Moderately differentiated adenocarcinoma | 9 | 1.44 ± 1.24 | 0.31 ± 0.25 | ||
| Poorly differentiated adenocarcinoma | 9 | 1.11 ± 0.78 | 0.24 ± 0.27 | ||
| Mucinous adenocarcinoma | 5 | 0.60 ± 0.89a | 0.06 ± 0.11a | ||
| Signet ring cell carcinoma | 3 | 0.00 ± 0.00a | 0.00 ± 0.00a | ||
| Distant metastasis | 0.038 | 0.002 | |||
| Negative | 21 | 1.48 ± 1.17c | 0.27 ± 0.24d | ||
| Positive | 9 | 0.56 ± 0.73 | 0.06 ± 0.09 | ||
| TNM stage | 0.007 | 0.001 | |||
| I | 3 | 2.67 ± 1.15e | 0.43 ± 0.25e | ||
| II | 5 | 1.60 ± 1.52 | 0.47 ± 0.27e | ||
| III | 7 | 1.57 ± 0.79 | 0.17 ± 0.15 | ||
| IV | 15 | 0.60 ± 0.74 | 0.09 ± 0.14 |
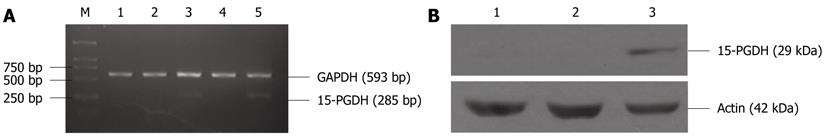
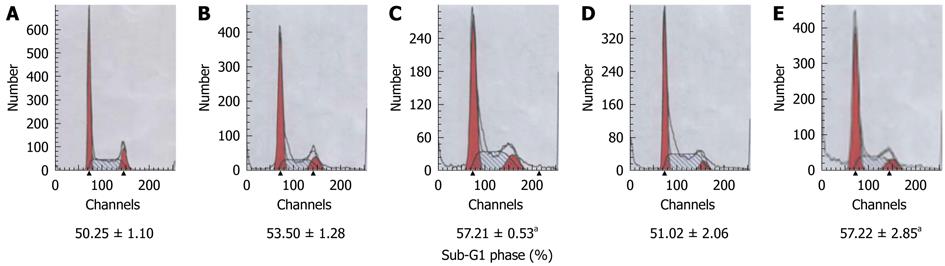
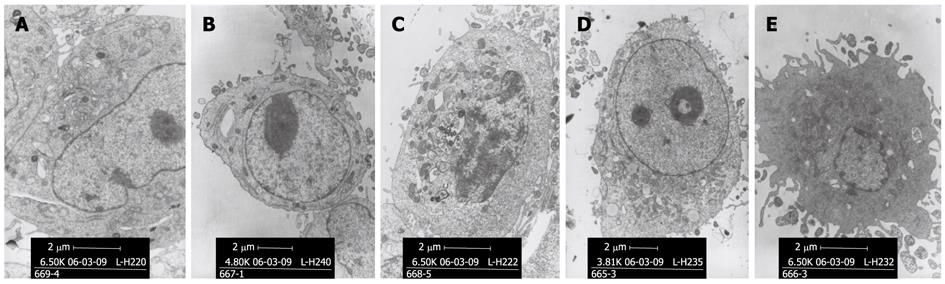
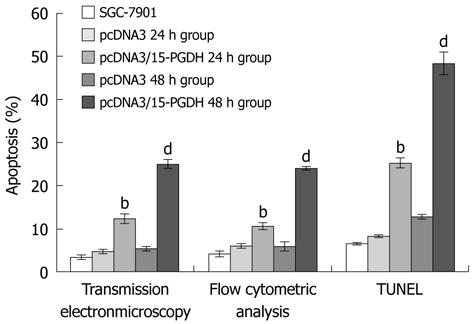
After transfection by pcDNA3/15-PGDH plasmid, SGC-7901 cells were induced to overexpress 15-PGDH (Figure 4). At the same time, an increased fraction of sub-G1 phase (57.21% ± 0.53% for 24 h transfection and 57.22% ± 2.85% for 48 h transfection, P < 0.05) was found by flow cytometry (Figure 5). It showed that 15-PGDH promoted cell cycle arrest in the sub-G1 phase. To assess the effect of 15-PGDH on induction of cell apoptosis in gastric cancer, we observed SGC-7901 cells under TEM and by flow cytometry, and then performed a TUNEL assay. Under TEM, nuclear and cytoplasmic shrinkage, condensation and margination of chromatin against the nuclear membrane, and formation of apoptotic bodies were observed in SGC-7901 cells that overexpressed 15-PGDH (Figure 6). The proportion of apoptotic cells was significantly increased after transfection for 24 h and 48 h (12.33% ± 1.15% and 25.00% ± 1.00% vs 3.33% ± 0.58%, P < 0.01). Flow cytometric analysis showed induction of apoptosis (10.49% ± 0.81% and 24.02% ± 0.37% vs 4.17% ± 0.68%, P < 0.01), which was further confirmed by TUNEL assay (AI: 25.27% ± 1.19% and 48.37% ± 2.67% vs 6.50% ± 0.30%, P < 0.01) (Figure 7). It indicated that cell cycle arrest and increased apoptosis was one mechanism of cancer suppression of 15-PGDH in SGC-7901 cells.
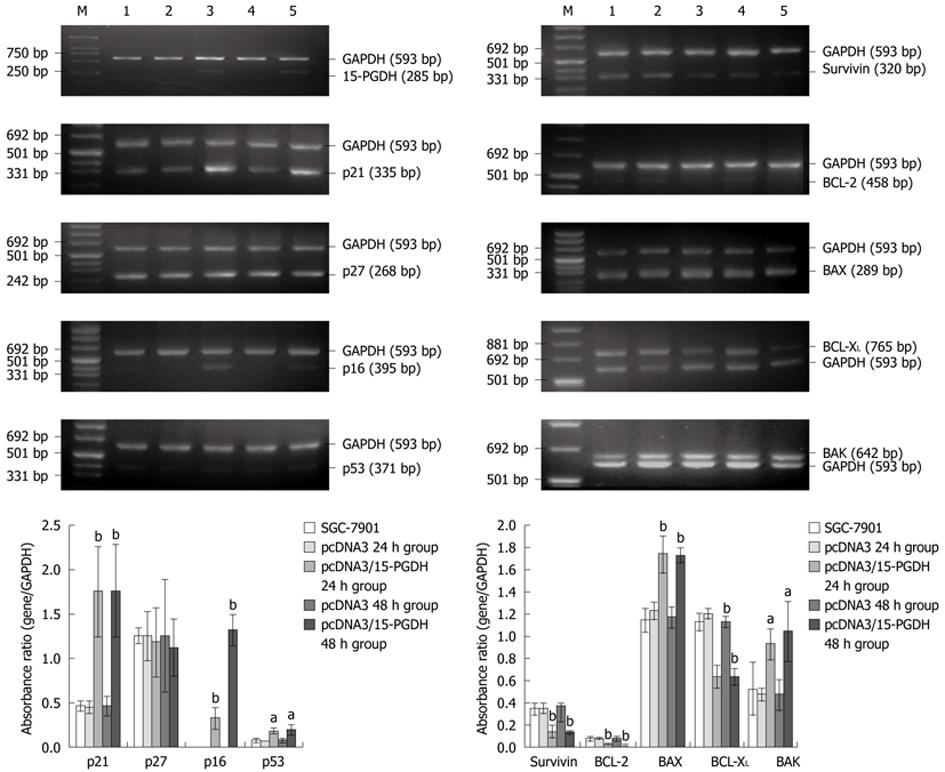
Expression of genes associated with the cell cycle (p21, p27, p16 and p53) and apoptosis (Survivin, BCL-2, BCL-XL, BAK and BAX) was determined by RT-PCR in SGC-7901 cells transfected with pcDNA3/15-PGDH plasmids. p21 (1.75 ± 0.51 for 24 h transfection and 1.76 ± 0.52 for 48 h transfection vs 0.46 ± 0.06 SGC-7901, P < 0.01), p16 (0.33 ± 0.12 and 0.32 ± 0.17 vs absence, P < 0.01) and p53 genes (0.19 ± 0.04 and 0.19 ± 0.06 vs 0.08 ± 0.02, P < 0.01) were significantly upregulated in cells treated with 15-PGDH for 24 h group and 48 h, whereas the level of p27 mRNA did not change (P > 0.05). Expression of the proapoptotic genes, such as BAK (0.92 ± 0.14 and 1.04 ± 0.27 vs 0.52 ± 0.24, P < 0.05) and BAX (1.73 ± 0.17 and 1.72 ± 0.07 vs 1.14 ± 0.11, P < 0.01) was significantly increased. The antiapoptotic genes, such as Survivin (0.14 ± 0.06 and 0.13 ± 0.02 vs 0.34 ± 0.06, P < 0.01), BCL-2 (0.02 ± 0.01 and 0.02 ± 0.01 vs 0.08 ± 0.03, P < 0.01) and BCL-XL (0.63 ± 0.11 and 0.63 ± 0.08 vs 1.12 ± 0.08, P < 0.01), were significantly downregulated (Figure 8).
These findings demonstrate that the reduction of 15-PGDH is related to occurrence and development of gastric cancer in humans. 15-PGDH also induces cell cycle arrest and apoptosis in gastric cancer cells. It may be a suppressor of gastric cancer through these two pathways.
15-PGDH catalyzes the oxidation of the 15(S)-hydroxyl group of PGs and lipoxins. 15-PGDH is one of the target genes. Some cytokines, factors and cell signaling pathways affect carcinogenesis and tumor progression through 15-PGDH. It shows that epidermal growth factor (EGF) and EGF receptor tyrosine kinase inhibitor[16,17], histone deacetylase inhibitors, transforming growth factor-β (TGF-β)[18], hepatocyte nuclear factor 3β[7], interleukin (IL)-4[19], tumor necrosis factor α[20], IL-1β[21], peroxisome proliferator-activated receptor γ ligands[22], hepatocyte growth factor receptor, Met[23], bile acids[24] adjust tumor growth through 15-PGDH. Recent studies have shown an obvious reduction of 15-PGDH in some cancers, for example, colorectal, breast, prostate and lung[3-6]. It also has been reported that 10%-80% of gastric cancer exhibits downregulation of 15-PGDH expression[10,11-14]. Our finding that 15-PGDH expression is decreased in gastric cancer is consistent with these reports. 15-PGDH protein and mRNA expression is absent in 33.3% of gastric cancer tissues, and reduced in almost all gastric cancer tissues and cell lines examined. There was also a significant reduction of 15-PGDH expression in paracancerous and precancerous tissues, for example, gastric polyps and atrophic gastritis. Downregulation of 15-PGDH expression was positively correlated with differentiation in gastric cancer tissues, distant metastasis and different TNM stages of gastric cancer. This result is similar to that in previous studies. It has also been reported that expression of 15-PGDH is reduced and associated with tumor differentiation, lymph node metastasis, clinical stage[11,13] and prognosis[10] in gastric cancer. We verified the relationship between differentiation of gastric cancer cells and 15-PGDH expression in vitro. We showed that poorer differentiation in carcinoma was associated with lower 15-PGDH expression. Taken together, reduction of 15-PGDH is related to the carcinogenesis and development of gastric cancer. Evaluation of 15-PGDH expression in tumor and precancerous tissues is a useful diagnostic or prognostic marker for gastric carcinoma.
After determining the relationship between 15-PGDH expression and gastric cancer, we suggest that reduction of 15-PGDH promotes occurrence and development of gastric cancer and that it is an inhibitor of human gastric cancer. Some studies have already demonstrated that 15-PGDH suppresses some tumors. Overexpression of 15-PGDH by transfection with plasmid or adenovirus vectors encoding 15-PGDH reduces occurrence and growth of tumor[3-5,8-10], whereas silencing of 15-PGDH using siRNA enhances cell proliferation and growth of cancer[3,10]. 15-PGDH gene knockout increases the colon tumor incidence in the APC+/Min mouse model[25]. The antitumor effect in human gastric cancer has only been shown in one study[15]. However the mechanism is still not clear.
The mechanism of the antitumor effect of 15-PGDH can be explained by the following hypothesis. 15-PGDH substantially inhibits production of PGE2 and changes the microenvironment to suppress tumor formation by Ras gene[8]; controls growth of tumor by regulation of cyclooxygenase-2[21]; suppresses synthesis, secretion and activation of matrix metalloproteinase-2, inhibits cell adhesion to extracellular matrix and reduces CD44 expression, which contributes to the inhibition of the growth, invasion and metastasis of cancer cells[4,9]; reduces expression of antiapoptotic protein Bcl-2, which indicates a role for Bcl-2 in mediating or triggering the event of apoptosis[4]; inhibits endothelial cell proliferation by 15-oxo-5,8,11,13-(Z,Z,Z,E)-eicosatetraenoic acid, a metabolite of 15-PGDH, suppressing DNA synthesis and implicating a potential antiangiogenic role[26]; and attenuates tumor-induced immune suppression and substantially reduces the secretion of immunosuppressive mediators and cytokines such as PGE2, IL-10, IL-13 and IL-6 to regulate the local antitumor immune response[27].
Our present research showed that apoptosis occurred in gastric cancer cells overexpressing 15-PGDH. In SGC-7901 cells transfected with pcDNA3/15-PGDH plasmid, we found by TEM nuclear and cytoplasmic shrinkage, condensation and margination of chromatin against the nuclear membrane, and formation of apoptotic bodies. Flow cytometric analysis and TUNEL assay showed that the proportion of apoptotic cells was increased by 15-PGDH. Previous research has found the same phenomenon in lung cancer[4]. Overexpression of this enzyme induces apoptosis in lung cancer cell line A459. When A549 cells overexpress 15-PGDH by transfection with Ad-15-PGDH, they become apoptotic, as shown by DNA fragmentation, activation of pro-caspase-3 and cleavage of poly ADP ribose polymerase[4]. Furthermore, we analyzed genes associated with apoptosis. There was a reduction in expression of antiapoptotic genes (Survivin, BCL-2 and BCL-XL) and increased expression of proapoptotic genes (BAK, BAX and p53). As we know, Survivin, BCL-2 members and p53 genes are crucial regulators of apoptotic cell death[28-32]. BCL-2 prevents the release of apoptosis-inducing factor and cytochrome c from the mitochondria, which is assumed to be a key event during apoptosis[30,31]. Overexpression of 15-PGDH regulates expression of Survivin, BCL-2 members and p53, indicating a role in mediating or triggering apoptosis. The results are inconsistent with previous findings, in which apoptosis induced by 15-PGDH iswas independent of the p53 pathway. 15-PGDH only decreases expression of antiapoptotic protein BCL-2 in lung cancer[4]. The mechanism of apoptosis varies in different tumors. In gastric cancer, 15-PGDH induces apoptosis by Survivin, BCL-2 and the p53 pathway.
In our study, we also observed cell cycle arrest in SGC-
7901 cells that overexpressed 15-PGDH. We showed an increased accumulation of cells in the sub-G1 phase compared with the control group, and upregulated expression of p21, p16 and p53 without altering p27 expression. p16, p21 and p27 all belong to the cyclin-dependent kinase (CDK) inhibitors[33-37]. The product of p16 gene is an inhibitor of CDK4. Its function is to cyclin E and CDK2 complexes. p27 functions as a negative regulator of G1 progression and is a possible mediator of TGF-β-induced G1 phase arrest[35]. p53 is known as a suppressor gene that can adjust the cell cycle[29].
In conclusion, our study provides evidence that loss or reduction of 15-PGDH is related to human gastric cancer. 15-PGDH induces cell cycle arrest and apoptosis of gastric cancer cells in vitro, and it may be the mechanism by which it suppresses human gastric cancer and other tumors. Further research is needed to establish the role of 15-PGDH as a target for treatment and chemoprevention of gastric cancer.
Gastric carcinoma is one of the most common malignant tumors in humans and continues to be a major unresolved health problem. New approaches for the management of gastric cancer are needed. Recent studies have shown a reduction of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in some cancers, such as colorectal, breast, prostate and lung. Some studies have revealed that 15-PGDH may have tumor-suppressive properties. It provides a new target for the chemoprevention and treatment of cancer, especially in gastric carcinoma. However, to the best of our knowledge, there has been no study on 15-PGDH expression in gastric precancerous tissue. The association between 15-PGDH expression and various clinicopathological parameters of gastric cancer needs to be validated. Only a few studies have been published on its mechanism of tumor inhibition.
NAD+-dependent 15-PGDH is the key enzyme responsible for the biological inactivation of prostaglandins (PGs) and related eicosanoids. It may have tumor-suppressive properties. The research hotspot is first study on 15-PGDH expression in gastric precancerous tissue, The association between 15-PGDH expression and various clinicopathological parameters of gastric cancer and its mechanism of tumor inhibition.
We found that 15-PGDH expression in human gastric cancer was reduced and even absent. Reduction of 15-PGDH expression was also found in precancerous state tissues, e.g., gastric polyps and atrophic gastritis. There was significant difference in expression of 15-PGDH among various pathologic type, with or without distant metastasis and different TNM stage. Apoptosis and cell cycle arrest was found after tranfected with pcDNA3/15-PGDH plasmid. In 15-PGDH over-expression cells, expression of the pro-apoptotic genes, such as BAK and p53, was increased. Expression of anti-apoptotic genes was decreased, e.g., Survivin, BCL-2 and BCL-XL. Cyclin-dependent kinase inhibitors p21 and p16 expression was significantly up-regulated. The researchers drew a conclusion that reduction of 15-PGDH is associated with carcinogenesis and development of gastric carcinoma. 15-PGDH induces apoptosis and cell cycle arrest in gastric cancer. It will be a novel chemotherapy strategy in gastric cancer.
The study results suggest the use of 15-PGDH in chemoprevention and treatment of gastric cancer.
NAD+-dependent 15-PGDH is the key enzyme responsible for the biological inactivation of PGs and related eicosanoids. It catalyzes the oxidation of the 15(S)-hydroxyl group of PGs and lipoxins. The products, 15-keto-metabolites, exhibit greatly reduced biological activities.
This is an interesting and well presented study, supported by primary data. It presents the studies on the expression of 15-PGDH in normal and cancerous gastric mucosal tissue, and its effect on apoptosis and cell cycle progression. Further, the authors examined the effect of 15-PGDH overexpression in gastric cell line, SGC-7901, on cell cycle progression and apoptosis. Based on the presented results, it is concluded that the loss or reduction in the mucosal cell 15-PGDH expression is associated with carcinogenesis and the development of gastric carcinoma.
We thank Dr. Tai HH (Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, United States) for donating 15-PGDH expression vector.
Peer reviewers: Bronislaw L Slomiany, Professor, Research Center, C875, UMDNJ-NJ Dental School, 110 Bergen Street, PO Box 1709, Room C875, Newark, NJ 07103-2400, United States; Hikaru Nagahara, MD, PhD, Professor, Aoyama Hospital, Tokyo Women's Medical University, 2-7-13 Kita-Aoyama, Minatoku, Tokyo 107-0061, Japan
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Jarabak J, Fried J. Comparison of substrate specificities of the human placental NAD- and NADP-linked 15-hydroxyprostaglandin dehydrogenases. Prostaglandins. 1979;18:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ensor CM, Tai HH. 15-Hydroxyprostaglandin dehydrogenase. J Lipid Mediat Cell Signal. 1995;12:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, Tai HH, Karlan BY, Koeffler HP. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006;66:7818-7823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, Milne GL, Katkuri S, DuBois RN. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Tong M, Tai HH. Synergistic induction of the nicotinamide adenine dinucleotide-linked 15-hydroxyprostaglandin dehydrogenase by an androgen and interleukin-6 or forskolin in human prostate cancer cells. Endocrinology. 2004;145:2141-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Huang G, Eisenberg R, Yan M, Monti S, Lawrence E, Fu P, Walbroehl J, Löwenberg E, Golub T, Merchan J. 15-Hydroxyprostaglandin dehydrogenase is a target of hepatocyte nuclear factor 3beta and a tumor suppressor in lung cancer. Cancer Res. 2008;68:5040-5048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Pham H, Eibl G, Vincenti R, Chong B, Tai HH, Slice LW. 15-Hydroxyprostaglandin dehydrogenase suppresses K-RasV12-dependent tumor formation in Nu/Nu mice. Mol Carcinog. 2008;47:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Li M, Xie J, Cheng L, Chang B, Wang Y, Lan X, Zhang D, Yin Y, Cheng N. Suppression of invasive properties of colorectal carcinoma SW480 cells by 15-hydroxyprostaglandin dehydrogenase gene. Cancer Invest. 2008;26:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Tatsuwaki H, Tanigawa T, Watanabe T, Machida H, Okazaki H, Yamagami H, Shiba M, Watanabe K, Tominaga K, Fujiwara Y. Reduction of 15-hydroxyprostaglandin dehydrogenase expression is an independent predictor of poor survival associated with enhanced cell proliferation in gastric adenocarcinoma. Cancer Sci. 2010;101:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Liu Z, Wang X, Lu Y, Han S, Zhang F, Zhai H, Lei T, Liang J, Wang J, Wu K. Expression of 15-PGDH is downregulated by COX-2 in gastric cancer. Carcinogenesis. 2008;29:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Yoo NJ, Jeong EG, Lee SH, Lee SH. Expression of 15-hydroxyprostaglandin dehydrogenase, a COX-2 antagonist and tumour suppressor, is not altered in gastric carcinomas. Pathology. 2007;39:174-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Jang TJ, Ji YS, Jung KH. Decreased expression of 15-hydroxyprostaglandin dehydrogenase in gastric carcinomas. Yonsei Med J. 2008;49:917-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Thiel A, Ganesan A, Mrena J, Junnila S, Nykänen A, Hemmes A, Tai HH, Monni O, Kokkola A, Haglund C. 15-hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clin Cancer Res. 2009;15:4572-4580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Liu Z, Wang X, Lu Y, Du R, Luo G, Wang J, Zhai H, Zhang F, Wen Q, Wu K. 15-Hydroxyprostaglandin dehydrogenase is a tumor suppressor of human gastric cancer. Cancer Biol Ther. 2010;10:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Mann JR, Backlund MG, Buchanan FG, Daikoku T, Holla VR, Rosenberg DW, Dey SK, DuBois RN. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006;66:6649-6656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Yang L, Amann JM, Kikuchi T, Porta R, Guix M, Gonzalez A, Park KH, Billheimer D, Arteaga CL, Tai HH. Inhibition of epidermal growth factor receptor signaling elevates 15-hydroxyprostaglandin dehydrogenase in non-small-cell lung cancer. Cancer Res. 2007;67:5587-5593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Tong M, Ding Y, Tai HH. Histone deacetylase inhibitors and transforming growth factor-beta induce 15-hydroxyprostaglandin dehydrogenase expression in human lung adenocarcinoma cells. Biochem Pharmacol. 2006;72:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Chi X, Tai HH. Interleukin-4 up-regulates 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in human lung cancer cells. Exp Cell Res. 2010;316:2251-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Otani T, Yamaguchi K, Scherl E, Du B, Tai HH, Greifer M, Petrovic L, Daikoku T, Dey SK, Subbaramaiah K. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G361-G368. [PubMed] |
| 21. | Tong M, Ding Y, Tai HH. Reciprocal regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase expression in A549 human lung adenocarcinoma cells. Carcinogenesis. 2006;27:2170-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Hazra S, Batra RK, Tai HH, Sharma S, Cui X, Dubinett SM. Pioglitazone and rosiglitazone decrease prostaglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Mol Pharmacol. 2007;71:1715-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Moore AE, Greenhough A, Roberts HR, Hicks DJ, Patsos HA, Williams AC, Paraskeva C. HGF/Met signalling promotes PGE(2) biogenesis via regulation of COX-2 and 15-PGDH expression in colorectal cancer cells. Carcinogenesis. 2009;30:1796-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Miyaki A, Yang P, Tai HH, Subbaramaiah K, Dannenberg AJ. Bile acids inhibit NAD+-dependent 15-hydroxyprostaglandin dehydrogenase transcription in colonocytes. Am J Physiol Gastrointest Liver Physiol. 2009;297:G559-G566. [PubMed] |
| 25. | Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA. 2006;103:12098-12102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Wei C, Zhu P, Shah SJ, Blair IA. 15-oxo-Eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Mol Pharmacol. 2009;76:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Eruslanov E, Kaliberov S, Daurkin I, Kaliberova L, Buchsbaum D, Vieweg J, Kusmartsev S. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor-infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J Immunol. 2009;182:7548-7557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Wall NR, O'Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230-235. [PubMed] |
| 29. | Aranda M, Náquira N, Karque R, Mendoza H, Sepúlveda C, Silva C. [Mutations of the p53 suppressor gene in gastric adenocarcinoma. Rev Med Chil. 1998;126:525-532. [PubMed] |
| 30. | Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3067] [Cited by in RCA: 2968] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 31. | Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899-1911. [PubMed] |
| 32. | Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403-406. [PubMed] |
| 33. | Zhang H, Hannon GJ, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 491] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 34. | Li R, Waga S, Hannon GJ, Beach D, Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994;371:534-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 478] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 35. | Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67-74. [PubMed] |
| 36. | He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515-521. [PubMed] |
| 37. | Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |