Published online Feb 14, 2011. doi: 10.3748/wjg.v17.i6.789
Revised: November 14, 2010
Accepted: November 21, 2010
Published online: February 14, 2011
AIM: To establish the end-to-end anastomosis (EEA) model of guinea pig bile duct and evaluate the healing process of bile duct.
METHODS: Thirty-two male guinea pigs were randomly divided into control group, 2-, 3-, and 6-mo groups after establishment of EEA model. Histological, immunohistochemical and serologic tests as well as measurement of bile contents were performed. The bile duct diameter and the diameter ratio (DR) were measured to assess the formation of relative stricture.
RESULTS: Acute and chronic inflammatory reactions occurred throughout the healing process of bile duct. Serology test and bile content measurement showed no formation of persistent stricture in 6-mo group. The DR revealed a transient formation of relative stricture in 2-mo group in comparation to control group (2.94 ± 0.17 vs 1.89 ± 0.27, P = 0.004). However, this relative stricture was released in 6-mo group (2.14 ± 0.18, P = 0.440).
CONCLUSION: A simple and reliable EEA model of guinea pig bile duct can be established with a good reproducibility and a satisfactory survival rate.
- Citation: Zhang XQ, Tian YH, Xu Z, Wang LX, Hou CS, Ling XF, Zhou XS. An end-to-end anastomosis model of guinea pig bile duct: A 6-mo observation. World J Gastroenterol 2011; 17(6): 789-795
- URL: https://www.wjgnet.com/1007-9327/full/v17/i6/789.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i6.789
Bile duct injury (BDI) is a severe consequence of gastrointestinal surgery. Unrecognized or improperly treated biliary injuries can lead to severe complications such as biliary cirrhosis, hepatic failure, and death[1,2]. Treatment of BDI remains a challenge for gastrointestinal surgeons. Reconstruction of bile ducts following iatrogenic injuries is associated with a high risk of stricture and stricture recurrence in the anastomosis[3,4]. Therefore, effective and safe bile duct reconstruction is very important.
Although the method of biliary tract reconstruction has been extensively studied, no consensus is reached concerning the ideal model of biliary tract reconstruction. The most frequently recommended procedure is Roux-Y hepaticojejunostomy (HJ) for its reconstruction[3-5].
However, Roux-Y HJ has its obvious drawbacks, including a large number of postoperative complications, such as a high occurrence of biliary tract stenosis leading to secondary biliary cirrhosis. The diagnostic and therapeutic endoscopic access to the biliary tract becomes impaired or hindered since the reconstruction of biliary tract with Roux-en-Y HJ is not anatomical[6]. The changed bile flow pathway is also a cause of disturbance in fat metabolism[7]. In case of poor drainage of the excluded loop, especially when applied in thin biliary tract with an intense inflammatory process, ascending cholangitis may also ensue. As the reconstruction of biliary tract with Roux-en-Y HJ is not physiological, the bile bypass induces gastric hypersecretion leading to a pH change secondary to altered bile synthesis and release of gastrin, therefore peptic ulcer occurs frequently in the long term[8,9].
End-to-end anastomosis (EEA) of bile duct is seldom used in surgical treatment of BDI. However, this procedure is routinely performed during hepatic transplantation with good results[10,11] and can achieve a better long-term outcome than Roux-en-Y HJ. Establishing a physiological bile pathway allows proper digestion and absorption. Also, control endoscopic examination in these patients is possible. Therefore, some authors recommend EEA as the first choice of bile duct reconstruction[6,12].
However, no large-scale clinical trial and a suitable animal model of EEA are available to evaluate the bile duct healing process. Therefore, to gain a better understanding of the healing process after EEA, and provide some valuable information for the etiology, development and prophylaxis of BDI, an animal model of bile duct reconstruction with EEA was established after total resection of common bile duct (CBD) in guinea pigs in this study. Guinea pigs were raised for 2, 3, and 6 mo after operation to observe the short- and long-term healing and possible complications. General conditions, survival rate and histological characteristics of the animals were detected and serology test was performed, as well as content and size of bile duct were measured before and after operation.
Thirty-two male guinea pigs weighing 350-400g, purchased from Laboratory of Experimental Animals, Peking University Health Science Center, Beijing, were housed under controlled conditions at a temperature of 21 ± 2°C and a relative humidity of 30%-70% in a 12-h dark and light cycle. The animals were fasted with free access to water 8 h before and after operation. This study was performed in accordance with the rules for the protections of animals and approved by the Animal Ethical and Welfare Committee of Peking University Health Science Center (LA2008-021).
The animals were randomly divided into control group (group 1), and 2-, 3-, 6-mo groups (groups 2-4) after EEA model was established, 8 in each group.
Surgical microscope (XTS-4A Jiangsu Surgical Instruments Company, China), microsurgical instruments (SSW-4, Shanghai Surgical Instruments Company, China), and 10-0 monofilament sutures (Double Arrow, China) were used.
The animals were anesthetized with pentobarbital (30 mg/kg) by intraperitoneal injection under sterile conditions. Peritoneal cavity was accessed via a midline incision (approximately 3 cm). After the liver lobes were pressed to the upper region and the duodenum was tracked toward left, the CBD was identified. Gallbladder was drained through a cystic duct joined with hepatic duct into the CBD. The CBD in control group was exposed and freed with forceps. A complete transection between the portal hilus and duodenum was performed with sharp dissection in the other 3 groups. The bile duct was reconstructed with the microsurgical instruments.
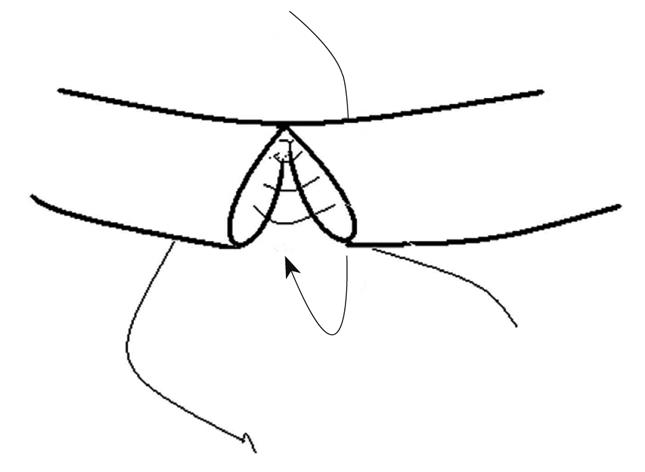
EEA was performed with interrupted sutures passing through the layers of the duct wall. The first stitch was placed at the side wall to join the two cut ends. Then, posterior walls of the proximal and distal cut ends were clockwise sutured. After the posterior wall was sutured, anterior wall was sutured in the same way (Figure 1). The proximal and distal cut ends were connected precisely and all stitches were distributed evenly with no tension on the approximate distal and proximal ends. Leaked bile was constantly wiped out to keep the operation area clean. The peritoneal cavity was flushed with normal saline to wash away the remaining bile after suturing was completed. The whole process took about 40 min with 8-10 sutures. No closer was used to disturb the blood supply and bile flow during operation. No T-tube or drainage was placed.
The general conditions and body weights of guinea pigs were carefully observed. The causes of death of guinea pigs were examined by autopsy. Overall survival rates of the animals were calculated and recorded.
By the end of two, three and six months after the EEA model was established, guinea pigs in the 4 groups were sacrificed by exsanguination. The maximum diameter of the proximal end (MDP) of CBD and the maximum diameter of anastomosis (MDA) were measured using a sliding caliper. The diameter ratio (DR, DR = MDP/MDA) in every group was calculated to assess the formation of relative stricture. Tissue samples were harvested from the anastomosed bile duct for histological examination.
Tissue samples, taken from the anastomosed bile duct, were fixed in 10% formalin, embedded in paraffin, and cut into 5-μm thick cross-sections which were stained with hematoxylin and eosin (HE). Immunohistochemical staining was performed using proliferating cell nuclear antigen (PCNA monoclonal antibody, Medical and Biological Laboratories, Japan). Histological characteristics were reported by the professional pathologist.
Cells were considered positive when their nuclei were stained distinctly brown. Negative control sections were fixed with a safe buffer and positive control sections were used.
The sections of PCNA stained with immunohistochemistry were examined under a light microscope and graded using a modified 0-4 numerical scale provided by Hunt et al[13] (Table 1).
| 0 | No evidence |
| 1 | Occasional evidence |
| 2 | Light scattering |
| 3 | Abundant evidence |
| 4 | Prominent distribution |
Serum alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) levels were measured and analyzed with an automatic biochemical analyzer (Type 5421-04, MISHIMA OLYMPUS CO., Shizuoka-ken, Japan).
The changes of bile contents in 3- and 6-mo groups were analyzed, and the presence of biliary sludge and gallstones was detected. The bile was drained in sterile conditions to examine its pH value. The levels of total bilirubin (TBIL), total bile acid (TBA), and calcium ions were measured with an automatic biochemical analyzer (Type 5421-04, Mishima Olympus Co., Shizuoka-ken, Japan).
Levene’s test for equality of variances was performed to assess the equality between groups. Independent sample t test and Mann-Whiney U test were used to compare the differences in the 4 groups. Data were expressed as mean ± SE. Statistical analysis was conducted using the SPSS for Windows (Chicago, Illinois, USA). P < 0.01 was considered statistically significant.
The animals in 4 groups survived throughout the experiment with a survival rate of 97%. One guinea pig in the control group died of anaesthetic intolerance before surgery. The total survival rate was 91% and two guinea pigs (one in 3-mo group and 1 in 6-mo group) died of bile leak age within seven days after operation. No fever, bile leakage, jaundice, infection, cholangitis, peritonitis, or other postoperative complications were noted in the surviving animals during the follow-up. All animals fed with usual diet gained their weight gradually (Table 2) and remained in a good condition till euthanized.
The mean value of MDA in groups 2-4 was 3.51 ± 0.12 mm, 3.15 ± 0.23 mm, and 3.47 ± 0.16 mm, respectively, which was significantly higher than that (1.71 ± 0.17 mm) in control group (P < 0.01). The mean value of MDP in groups 2-4 was 10.24 ± 0.48 mm, 8.66 ± 0.47 mm, and 8.05 ± 0.52 mm, respectively, which was also significantly higher than that (2.95 ± 0.15 mm) of control group (P < 0.01). The MDP value in group 2 was the highest. The MDP values were comparable between groups 3 and 4.
The DR indicates the formation of relative stricture and the postoperative DR suggests the remolding status and restoration of physiological function. A significant difference was observed in DR between the control and 2-mo groups (1.89 ± 0.27 vs 2.94 ± 0.17, P = 0.004). The DR in groups 3 and 4 was 3.24 ± 0.65 and 2.14 ± 0.18, respectively (Table 3).
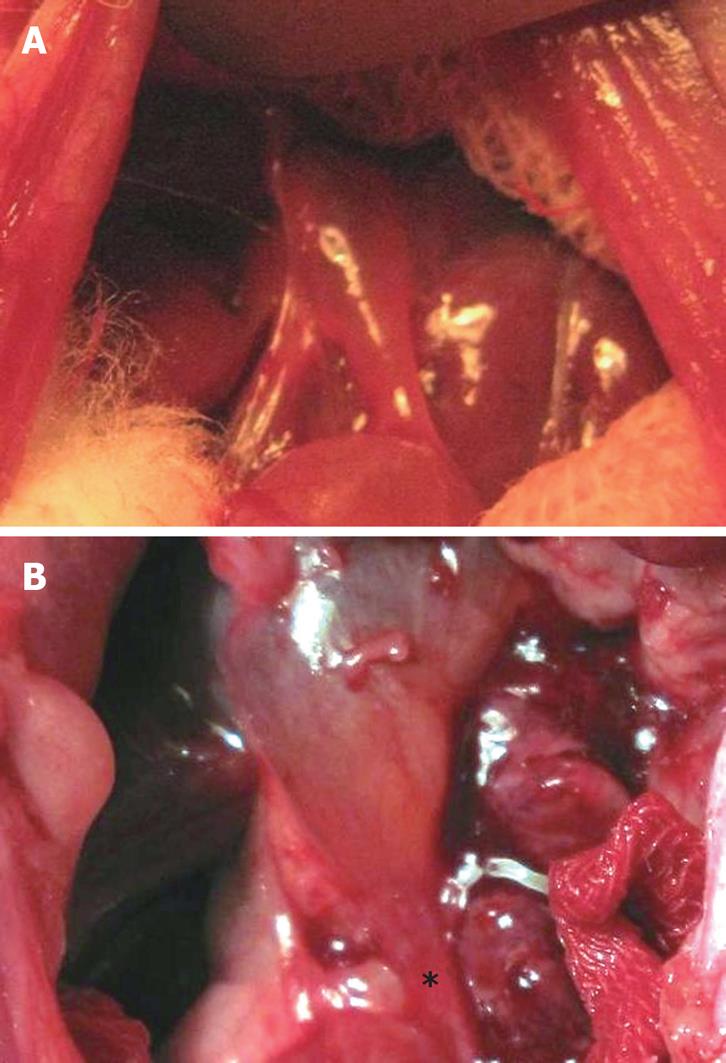
Gross inspection of the bile duct after operation revealed that the anastomosed bile duct was narrowed due to local inflammation and edema. The thickened bile duct wall looked like a “stricture ring” (Figure 2).
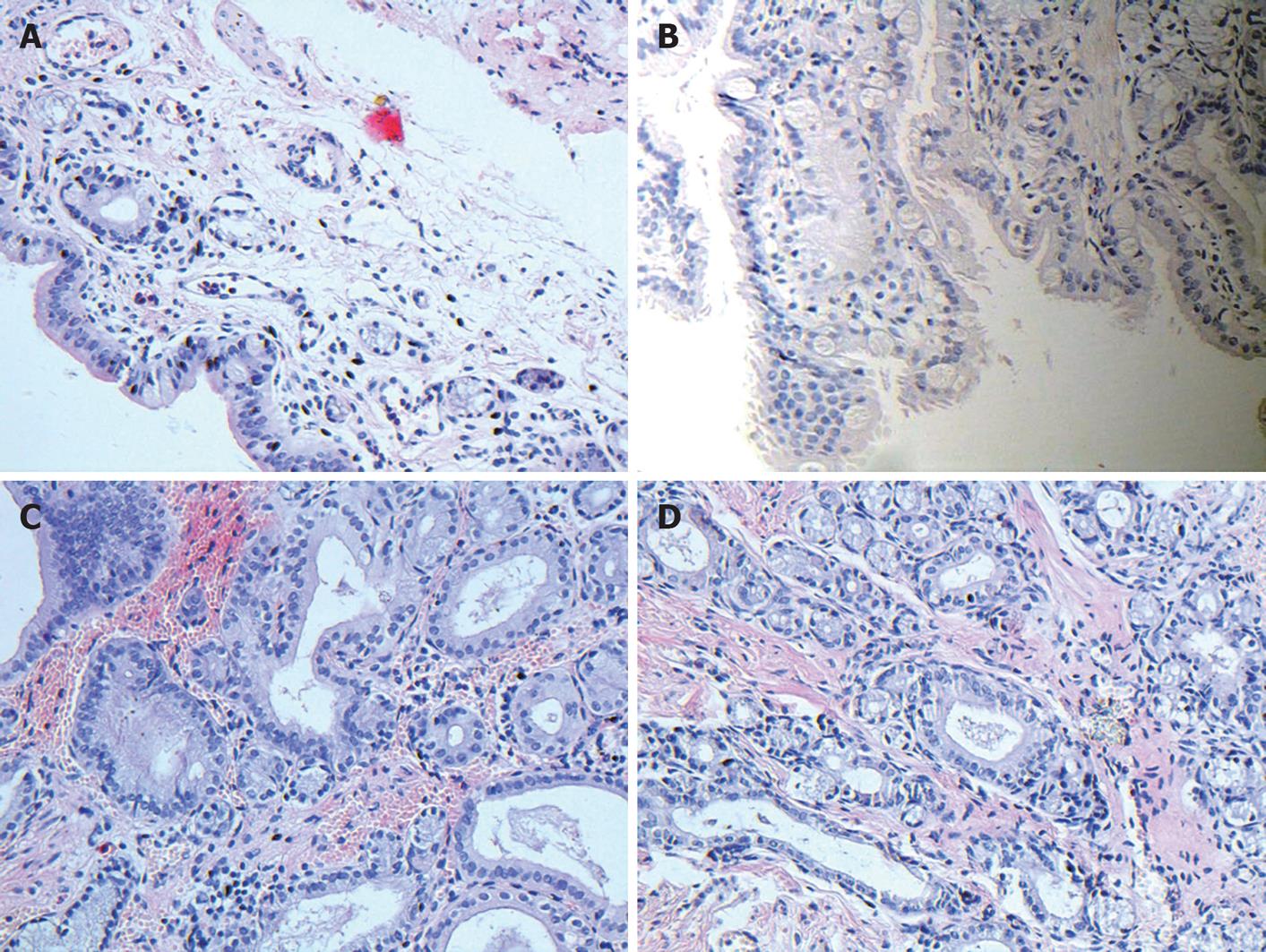
In this study, normal bile duct contained abundant elastic fibers with a good contract property. Its intima was lined with a single glandular epithelium. Lamina propria contained a small amount of gland elements. Scattered smooth muscle cells were distributed unevenly in the media wall. Adventitia contained fibroblasts and other connective tissues (Figure 3A).
Two months after operation, significant epithelial proliferation, intra- and extramural glandular hyperplasia, fibrous thickening of the duct and dense infiltration of inflammatory cells were noted in the anastomosis (Figure 3B). Inflammatory reactions were gradually subsidized in 3- and 6-mo groups. Hyperplasia of gland elements was observable in 6-mo group (Figure 3C and D).
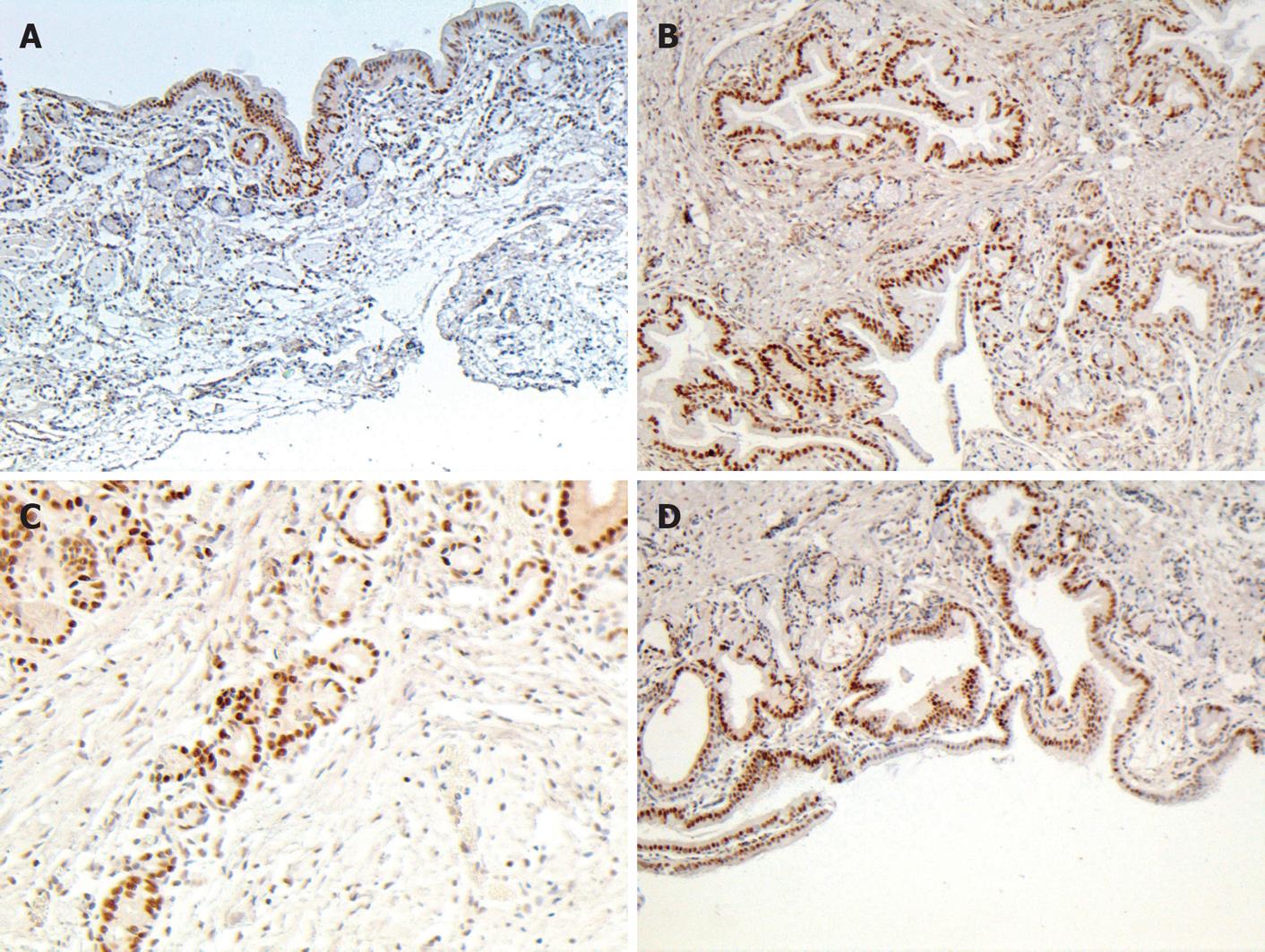
PCNA, a 36-kDa nuclear protein, is an auxiliary protein for DNA polymerase -delta. PCNA expression and distribution are correlated with cell proliferation, DNA synthesis, and cell proliferative activity[14,15]. In this study, positive PCNA cells were concentrated on the glandular-epithelia and peribiliary glands of control group. After bile duct reconstruction in 2- and 3-mo groups, an increased number of positive cells were distributed in all layers of the duct wall. By the end of six months, positive PCNA cells were located mainly in glandular elements and epithelial cells (Figure 4). The number of proliferated PCNA cells was significantly greater in groups 2-3 than in control group (P < 0.01) (Table 4).
One serum sample taken from control group was abandoned due to contamination. The mean serum ALP and GGT level was 55.33 ± 8.44 U/L and 14.67 ± 2.76 U/L, respectively, in control group and groups 2-4 (P < 0.01, Table 5).
The bile contents were measured to show whether there is a tendency to form gallstones. No biliary sludge or gallstones were found in the biliary system of all groups, and no significant difference was observed in levels of TBIL, TBA, calcium ions and pH value among the 4 groups (Table 6).
| Groups | n | TBIL (μmol/L) | TBA (μmol/L) | Ca2+ (mmol/L) | pH | ||||
| mean ± SE | P | mean ± SE | P | mean ± SE | P | mean ± SE | P | ||
| Group 1 | 7 | 32.44 ± 3.43 | 11 668.38 ± 1549.42 | 0.78 ± 0.06 | 8.87 ± 0.07 | ||||
| Group 3 | 7 | 28.5 ± 7.92 | 0.6221 | 15 981.67 ± 2295.02 | 0.1361 | 1.17 ± 0.13 | 0.0141 | 8.86 ± 0.08 | 0.9081 |
| Group 4 | 7 | 39.19 ± 9.97 | 0.5652 | 18 260.25 ± 4164.40 | 0.1641 | 1.24 ± 0.17 | 0.0642 | 8.82 ± 0.07 | 0.6421 |
In this study, EEA was performed instantly after total transection of CBD in guinea pigs. A few weeks after EEA, the anastomosed bile duct was narrowed due to local inflammation, edema and proliferation of glandular elements. The thickened bile duct wall looked and functioned as a “stricture ring”, leading to a significantly higher hydrodynamic pressure on the proximal bile duct end and noticeable dilation of the proximal bile duct end above the stenosis zone. The increased pressure of the proximal bile duct end would pass on the pressure to the anastomosed area of bile duct and influence its remolding. The “stricture ring” took on adaptive changes with inflammation gradually subsidized after a few months. The MDA and MDP values were significantly higher in groups 2-4 than in control group. Therefore, a single MDA parameter could not reliably reflect the remolding process of the anastomosed bile duct. Moreover, the body weight of guinea pigs increased throughout the experiment in 6-mo group. The DR parameter of (DR = MDP/MDA) was used to assess the remolding status and evaluate whether there is a relative stricture formation. If the DR was significantly higher in groups 2-4 than in control group, a relative stricture would form.
The DR was significantly higher in 2-mo group than in control group, which might be the indication for relative stricture formation. The animals remained in a good condition with their weight gradually increased. The MDP, MDA and DR values were lower in 6-mo group than in 2-mo group, while comparable to those in 3-mo group. No significant difference was noted in levels of ALP, GGT, and bile contents among 4 groups, indicating that normal bile duct anatomy and physiological function can be gradually restored after a transient formation of “relative stricture”.
Although the body weight of guinea pigs was notably increased in six months, the bile duct parameters did not increase in proportion, suggesting that the growth of animals is not the only cause of bile duct enlargement. We hypothesize that besides hydrodynamic pressure of bile flow to the side walls and normal growth of the animals, neurological factors may also account for the changes during bile duct remolding.
Consequently, the postoperative remolding process in 6-mo group was a synergetic and balanced result of tissue injury and repair, as well as hydrodynamic and neurological changes in injured CBD. Further study is needed to explain the neurological mechanism underlying the postoperative remolding process of bile duct.
It has been shown that biliary stasis can induce pigment stone formation in animals[16,17] and in patients with biliary stricture[18]. It was reported that the increased bile pH and changes in bile contents when ligation results in bile duct stricture are the early events, leading to the formation of gallstone[17,19]. In our experiment, the levels of TBIL, TBA, calcium ions and the pH value were measured. No biliary sludge or gallstones were found in the biliary system of guinea pigs.
Guinea pig is an ideal animal for the reconstruction of bile duct with EEA. The EEA model is frequently used in studies of biliary system, especially in investigation of gallstone formation[17,19]. Rats have no gallbladder and the diameter of their CBD is only 1 mm[20]. The anatomy of bile duct in guinea pigs is very similar to that of human beings. Since the diameter of bile duct in guinea pigs is approximately 1.7 mm, it is easy to reconstruct the bile duct with a good reproducibility. The bile contents differ in species. The bile in guinea pigs contains the same bilirubinic acid as in human beings, while the bile in rabbits contains biliverdinic acid which renders it unusable in this respect[21]. Moreover, although dogs might be the better candidates for the reconstruction of bile duct, guinea pigs were chosen in this preliminary experiment from the animal welfare and ethical point of view.
In the present study, the histological characteristic of normal CBD tissue taken from guinea pigs were similar to those taken from human beings, which are consistent with the reported findings[22-24], indicating that guinea pigs are ideal for the establishment of EEA models.
The tissue repair of injured biliary tract is a scar healing process. Two important changes in tissue repair can restore the morphological consistency and physiological function, namely the formation of granulation tissue with contractile properties and the epithelial cell proliferation, migration and the closure of the wound[25]. MFB is the major constituent of inflammatory and reparative granulation tissues. By forming a net work of contracting system, MFB may last scar contraction and result in stricture formation. MFB disappear due to apoptosis when the epithelialization is completed and the remolding process becomes stable[26,27]. The presence of MFB can lead to excessive scarring and fibrotic conditions. Geng and his colleagues established the bile duct anastomosis model of dogs by making an incision on the anterior wall of CBD with one third of its circumference, and found that the number of myofibroblasts can reach its peak 3 mo after operation, and decrease due to apoptosis 6 mo after operation[28], suggesting that six months is enough for the examination of the healing process of bile duct anastomosis. However, the long-term outcome is critical in surgical treatment of BDI in clinical practice. No biliary anastomosis stricture formation is a proof of successful surgical management. Therefore, further study is needed to observe the longer postoperative outcome.
In this preliminary study, we presented a simple and reliable animal EEA model for bile duct reconstruction with a good reproducibility and satisfactory survival rate. No permanent biliary anastomosis stricture was noticed in 6-mo group and no serology or bile content revealed stricture formation. The overall animal survival rate was 91%. The animals gained their weight with no postoperative biliary obstruction found in all groups.
In conclusion, the EEA animal model of bile duct established in this study can be used in studies of BDI etiology, development, and possible prophylaxis, and provide some valuable information for the postoperative healing process of bile duct.
Treatment of bile duct injury (BDI) remains a great challenge for gastrointestinal surgeons. No consensus has been reached concerning the ideal method for bile duct reconstruction. No large scale clinical study is available on bile duct reconstruction.
Some surgeons prefer end-to-end anastomosis (EEA) as a more physiological method in bile duct reconstruction. However, no large-scale clinical study or suitable animal model is available or analyzed. In this study, by establishing a reliable animal EEA model of common bile duct (CBD) and observing the postoperative results of histological, immunohistochemical exanimation, serologic and bile content analysis, as well as bile duct parameters, the authors demonstrated that EEA can be utilized in treatment of BDI.
The authors provided a simple and reliable animal EEA model of CBD with a good outcome in this study, which may shed light on studies of BDI etiology, development, and possible prophylaxis.
The animal EEA model of CBD we established in the present study can be utilized in studies on BDI etiology, development, and possible prophylaxis as well as provide some valuable information for the post-operative healing process of EEA.
EEA, an end-to-end anastomosis procedure, is a more preferable choice of treatment than Roux-en-Y maneuver. MFB are the myofibroblasts with α-SMA expression in stress fibers. In wound healing, inflammation mediators and mechanical tension lead to generation of actin-containing microfilaments or stress fibers which confer contractile property to fibroblasts, and convert them into terminally differentiated MFB. MFB are the major constituent of inflammatory and reparative granulation tissue and can last scar contraction and stricture formation.
In this study, the animals gained their weight and no postoperative biliary obstruction was observed in any group after bile duct reconstruction with EEA in this study, showing that EEA is a simple and reliable procedure for bile duct reconstruction with a satisfactory survival rate, which provides some valuable information for the postoperative healing process of bile duct.
Peer reviewer: Emiko Mizoguchi, MD, PhD, Department of Medicine, Gastrointestinal Unit, GRJ 702, Massachusetts General Hospital, Boston, MA 02114, United States
S- Editor Tian L L- Editor Wang XL E- Editor Lin YP
| 1. | Al-Kubati WR. Bile duct injuries following laparoscopic cholecystectomy: A clinical study. Saudi J Gastroenterol. 2010;16:100-104. |
| 2. | Lau WY, Lai EC, Lau SH. Management of bile duct injury after laparoscopic cholecystectomy: a review. ANZ J Surg. 2010;80:75-81. |
| 3. | Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, Sita A. The long-term outcome of hepaticojejunostomy in the treatment of benign bile duct strictures. Ann Surg. 1996;224:162-167. |
| 4. | Ahrendt SA, Pitt HA. Surgical therapy of iatrogenic lesions of biliary tract. World J Surg. 2001;25:1360-1365. |
| 5. | Lillemoe KD, Melton GB, Cameron JL, Pitt HA, Campbell KA, Talamini MA, Sauter PA, Coleman J, Yeo CJ. Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg. 2000;232:430-441. |
| 6. | Imamura M, Takahashi M, Sasaki I, Yamauchi H, Sato T. Effects of the pathway of bile flow on the digestion of fat and the release of gastrointestinal hormones. Am J Gastroenterol. 1988;83:386-392. |
| 7. | Jabłońska B, Lampe P. Iatrogenic bile duct injuries: etiology, diagnosis and management. World J Gastroenterol. 2009;15:4097-4104. |
| 8. | Nielsen ML, Jensen SL, Malmstrøm J, Nielsen OV. Gastrin and gastric acid secretion in hepaticojejunostomy Roux-en-Y. Surg Gynecol Obstet. 1980;150:61-64. |
| 9. | Sato T, Imamura M, Sasaki I, Kameyama J. Effect of biliary reconstruction procedures on gastric acid secretion. Am J Surg. 1982;144:549-553. |
| 10. | Yamamoto S, Sato Y, Oya H, Nakatsuka H, Kobayashi T, Hara Y, Watanabe T, Kurosaki I, Hatakeyama K. Risk factors and prevention of biliary anastomotic complications in adult living donor liver transplantation. World J Gastroenterol. 2007;13:4236-4241. |
| 11. | Ishiko T, Egawa H, Kasahara M, Nakamura T, Oike F, Kaihara S, Kiuchi T, Uemoto S, Inomata Y, Tanaka K. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235-240. |
| 12. | de Reuver PR, Busch OR, Rauws EA, Lameris JS, van Gulik TM, Gouma DJ. Long-term results of a primary end-to-end anastomosis in peroperative detected bile duct injury. J Gastrointest Surg. 2007;11:296-302. |
| 13. | Hunt TK, Mueller RV. Wound healing. Current surgical diagnosis and treatment. 10th edition. New Jersey: Appleton and Lange 1994; 80-93. |
| 14. | Bravo R, Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985;4:655-661. |
| 15. | Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515-517. |
| 16. | Soloway RD, Trotman BW, Ostrow JD. Pigment gallstones. Gastroenterology. 1977;72:167-182. |
| 17. | Xu Z, Ling XF, Zhang WH, Zhou XS. Can pigment gallstones be induced by biliary stricture and prevented by medicine in Guinea pigs? World J Gastroenterol. 2007;13:2703-2706. |
| 18. | Jabłońska B, Lampe P, Olakowski M, Górka Z, Lekstan A, Gruszka T. Hepaticojejunostomy vs. end-to-end biliary reconstructions in the treatment of iatrogenic bile duct injuries. J Gastrointest Surg. 2009;13:1084-1093. |
| 19. | Chen CY, Shiesh SC, Lin XZ. Biliary sludge and pigment stone formation in bile duct-ligated guinea pigs. Dig Dis Sci. 1999;44:203-209. |
| 20. | Tez M, Keskek M, Ozkan O, Karamursel S. External metallic circle in microsurgical anastomosis of common bile duct. Am J Surg. 2001;182:130-133. |
| 21. | Jimenez R, Gullon J, Monte MJ, Esteller A. Biliary bilirubin and biliverdin excretion in rabbits during fasting and feeding. Cornell Vet. 1988;78:99-104. |
| 22. | Ludwick JR. Observations on the smooth muscle and contractile activity of the common bile duct. Ann Surg. 1966;164:1041-1050. |
| 23. | Duch BU, Andersen HL, Smith J, Kassab GS, Gregersen H. Structural and mechanical remodelling of the common bile duct after obstruction. Neurogastroenterol Motil. 2002;14:111-122. |
| 24. | Nakayama F, Koga A. Hepatolithiasis: present status. World J Surg. 1984;8:9-14. |
| 25. | Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500-503. |
| 26. | Xu J, Geng ZM, Ma QY. Microstructural and ultrastructural changes in the healing process of bile duct trauma. Hepatobiliary Pancreat Dis Int. 2003;2:295-299. |
| 27. | Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56-66. |
| 28. | Geng ZM, Yao YM, Liu QG, Niu XJ, Liu XG. Mechanism of benign biliary stricture: a morphological and immunohistochemical study. World J Gastroenterol. 2005;11:293-295. |