INTRODUCTION
The esophagogastric junction is located between the esophagus and the stomach. The high-pressure zone at the junction between the esophagus and the stomach is composed of the lower esophageal sphincter (LES) and the crural diaphragm[1,2]. Circular smooth muscle from the esophageal body generates little if any tone at rest, whereas the circular smooth muscle of LES is characterized by a spontaneously generated basal tone that prevents the reflux of gastric contents into the esophagus[3,4]. The basal tone of the LES is primarily myogenic in origin, but can be modulated by both neural and hormonal factors[5]. In response to esophageal distension and swallowing, the LES relaxes[6]. The abnormal dynamics of LES function are considered to be the most important factors in the pathogenesis of gastroesophageal reflux disease (GERD)[7-10]. GERD is described as the reflux of gastric contents into the esophagus leading to reflux symptoms and esophagitis sufficient to affect patient wellbeing and/or induce complications. These complications range from esophagitis to adenocarcinoma of the distal esophagus. Furthermore it may cause extra esophageal symptoms, such as cough, laryngitis and asthma[11,12]. GERD is a highly prevalent in the general population, affecting up to 10%-30% of the adult population in western countries[13].
Pharmacological therapy is necessary in the majority of patients. GERD is currently treated with acid suppressing drugs, such as proton pump inhibitors (PPIs); however, for those refractory to pharmacological treatment, surgery is often recommended[13,14]. PPIs are the mainstay of medical management for GERD[11]. They have been widely used since the 1980s and have been considered as ideal drugs because of their highly specific pharmacologic actions[15,16]. Although PPIs have been used as a common treatment modality in GERD, there is a lack of experimental studies of their effects on isolated LES preparations.
The aim of this study was to investigate the effect of a PPI, pantoprazole, on the tone of the isolated rat LES preparations contracted by carbachol. This study provides a significant contribution to this somewhat ignored area of research.
MATERIALS AND METHODS
The experimental protocol was approved by the Ethical Committee of Yeditepe University Experimental Medicine Research Institute and the use of animals was in compliance with US National Institutes of Health Guide for Care and Use of Laboratory Animals.
Sixteen rats weighing 250-300 g, provided by the Yeditepe University Experimental Research Center (YÜDETAM), were used throughout the study. They were kept in plexiglass cages in a room whose temperature and humidity were controlled with 12-h light/dark cycle, and had free excess to food and water.
Rats were anesthetized with a combination of 10 mg/kg xylazine HCl (Rompun® 2%, Bayer HealthCare AG, Leverkusen-Germany) and 100 mg/kg ketamine HCl (Ketasol® 10%, Richter Pharma AG, Weis-Austria) before decapitation.
A midline incision was performed to open up the abdominal cavity and the LES was carefully dissected out and placed in a petri dish containing Krebs solution at room temperature. Thereafter, the mucosal lining was removed and the sphincteric muscle was set up, as a ring segment 2 mm in width, in Krebs solution contained in a standard 30-mL organ bath. The modified Krebs solution comprised NaCl, 118.07 mmol/L; KCl, 4.69 mmol/L; CaCl2, 2.52 mmol/L; MgSO4, 1.16 mmol/L; KH2PO4, 1.2 mmol/L; NaHCO3, 25 mmol/L, and glucose, 11.10 mmol/L. Krebs solution was continuously aerated with 95% oxygen-5% carbon dioxide gas mixture and kept at 37 ± 0.5 °C throughout the experimental period. The tissues were tied to stainless steel hooks at one end of the organ bath; the other end was connected to a force transducer (FDT 05, May, COMMAT Iletisim Co, Ankara-Turkey) under a resting tension of around 1 g. LES ring activities were recorded on an online computer via a 4-channel transducer data acquisition system (MP35, BIOPAC Systems Inc. Goleta, CA, United States) using the software BSL PRO v 3.7 (BIOPAC Systems Inc. Goleta, CA, United States), which also analyzed the data.
The following compounds were used: carbachol chloride (Carbamylcholine chloride, Sigma-Aldrich Chemical Co. St. Louis, MO, United States) and pantoprazole (Pantoprazole sodium, Dr. Reddy’s Laboratories Ltd. Hyderabad-India). Solutions were prepared daily in distilled water and kept at 4 °C during the experiments. Pantoprazole was treated with 1 mol/L HCl and its pH was adjusted to 4.0 before application to the organ bath. Following a 60-min equilibration period for stabilization, the contractile response to carbachol was obtained by application of a single dose of charbachol to a final concentration of 10-6 mol/L in the organ bath. After the contractions reached a plateau, concentration-response relationships for pantoprazole (final organ bath concentrations of 5 × 10-6 mol/L, 5 × 10-5 mol/L and 1.5 × 10-4 mol/L, with 15 min allotted between each dose) were obtained in a cumulative manner. (These doses were calculated to be the equivalent of Human doses for the rats). Control experiments were also run with only acidified distilled water added to the organ bath. The relaxations were quantified by integrating the area under the curve for each concentration and control group. At the end of the each experiment, tissues were weighed and the final pH of the Krebs solution was measured.
Statistical analysis
For statistical evaluation, analysis of variance (One way ANOVA) was performed with the program SPSS for windows version 18 (SPSS Inc. Chicago, Illinois). Values of P < 0.05 were considered as statistically significant.
RESULTS
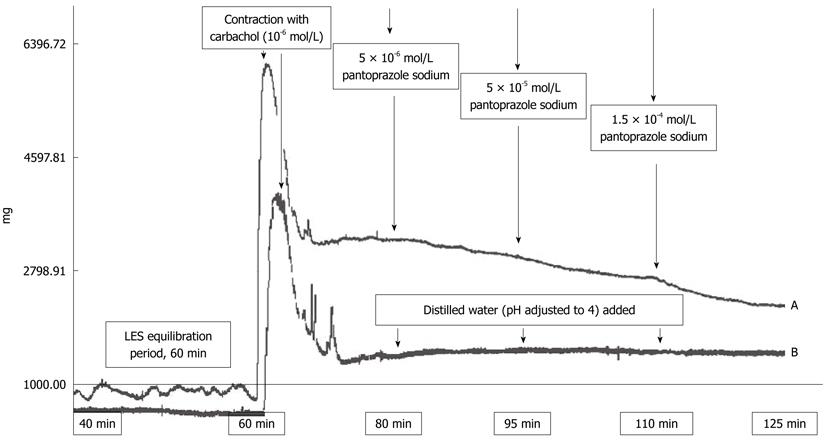
The experiment design is outlined in Figure 1. Pantoprazole caused dose dependent relaxation of the carbachol-contracted LES preparations. No such effect was observed in the control group (Figure 1B). The relaxations were quantified by integrating the area under the curve for each concentration.
Figure 1 Outline of the experimental procedure.
A: The tissues were allowed to stabilize for 60 min in Krebs-containing organ baths. Following that period, their contractile response to 10-6 mol/L carbachol was obtained. Pantoprazole was treated with 0.1 mol/L HCl and the pH of the drug solution was adjusted to 4.0. Different concentrations of pantoprazole were added directly to the tissue bath to generate cumulative concentrations of 5 × 10-6 mol/L, 5 × 10-5 mol/L and 1.5 × 10-4 mol/L. The relaxations were quantified by integrating area under the curve for each concentration; B: For the control experiments, acidified distilled water was added at the same time points. LES: Lower esophageal sphincter.
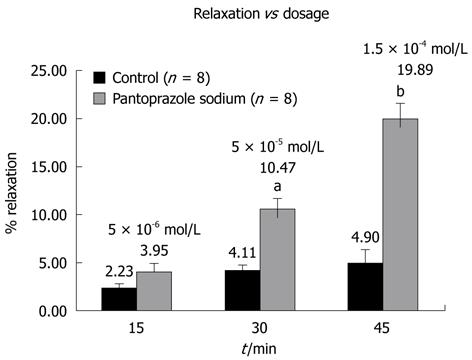
The mean of integral values and percent relaxations of eight preparations were compared for statistical evaluation. As shown in Figure 2, application of pantoprazole sodium in a cumulative manner resulted in significant relaxations of LES preparations at 5 × 10-5 mol/L and 1.5 × 10-4 mol/L concentrations.
Figure 2 Relaxation vs Dosage.
5 × 10-5 mol/L and 1.5 × 10-4 mol/L pantoprazole sodium induced significant relaxation in lower esophageal sphincter preparations in vitro (aP < 0.05 and bP < 0.001). Each bar represents percent relaxation ± SEM for both control and experiment groups. Numbers in parentheses indicate the number of preparations used from different animals.
In the carbachol-contracted LES preparations 5 × 10-6 mol/L pantoprazole caused a 4% relaxation, while higher doses caused significant relaxations. Mean integral relaxation values were 4.11% ± 0.58% (SE) and 10.47% ± 1.2% (SE) for control and 5 × 10-5 mol/L pantoprazole, respectively (P < 0.05). Moreover, these values were 4.90% ± 1.4% (SE) and 19.89% ± 1.7% (SE) for control and 1.5 × 10-4 mol/L concentrations, respectively (P < 0.001) (Figure 2).
DISCUSSION
The aim of the present work was to assess the in vitro effects of pantoprazole on LES tone in rats. The reason why pantoprazole was chosen was the drug’s frequent use in our Clinic. The major finding of our study was that pantoprazole caused a dose-dependent decrease in LES tone. This is the first study to demonstrate that pantoprazole has such an effect on isolated LES.
LES is an important specialized smooth muscle in the gastrointestinal tract and has been the subject of investigation by many authors[17-20]. GERD is a highly prevalent condition and is a major burden to society as well as the afflicted individual. Although numerous clinical studies have been conducted to clarify the mechanism of GERD, a clear consensus has not been reached. Regarding the pathophysiologies of GERD, decrease of LES basal tone and transient relaxations of the LES (TLOSRs) as a response to gastric distension[21], and excessive exposure of the esophagus to gastric acid, have been reported to be important[21-24].
GERD is, in most cases, successfully treated with PPIs, which have largely replaced Histamine H2 receptor blockers because of their well documented efficacy and because they are well tolerated, with relatively few serious adverse effects. However, a significant number of patients do not receive full symptomatic relief[25,26]. Thus, a significant question that has to be addressed is why some GERD patients are resistant to the effects of PPIs? In addition to neonates and infants who respond poorly to PPIs[27], some adults do not benefit from them either. In a study conducted by Hemmink et al[28] in 2008, there were fewer acid reflux episodes in patients on PPI therapy; however, weak acidic reflux episodes increased under the influence of PPIs. The total number of reflux episodes, on the other hand, was not affected. In addition to these, there have been recent papers regarding the adverse effects of PPIs[29,30]. Corley et al[31] showed that PPIs are associated with hip fractures among at-risk patients. They can also cause neutropenia in some patients[32]. Acid suppression also causes nosocomial Clostridium difficile infections in a dose-dependent manner[33].
These results point out the necessity of developing novel approaches for GERD. Coman et al[34] demonstrated the significance of adding prokinetic drugs to the treatment of GERD, in a study conducted on 1118 patients. The effects of specific GABA B receptor agonists have also been studied[35]. Drugs that reduce TLOSRs have also been suggested as pharmacological agents for GERD[36].
At present, the mechanism of the pantoprazole-induced relaxation of LESs can only be speculated. However, there are 2 types of muscles in the LES, circular muscle and sling muscle. Circular smooth muscle is tonically contracted with cholinergic stimulation. In response to swallowing, a peristaltic contraction travels down the length of the esophagus and the LES relaxes.
Nitric oxide (NO)[37,38] and vasoactive intestinal polypeptide (VIP)[39,40] are proposed as neurotransmitters that control relaxation. Both VIP and NO can be released from esophageal nerves with an appropriate stimulus, and NO synthase and VIP are found in myenteric neurons that innervate the circular smooth muscle of the esophagus. Sarioglu et al[41], showed the relaxant effect of omeprazole in rabbit corpus cavernosum in vitro. They concluded that the relaxant effect is probably due to the L-type Ca2+ channel blockage by omeprazole. We can speculate that a similar mechanism is responsible for the effect of pantoprazole on LESs.
The present study is the first to demonstrate a dose-dependent decrease in the carbachol-induced contraction of the LES by pantoprazole. Although this finding has been observed in an isolated tissue, it might have some clinical correlates and might help to understand why the treatment of GERD requires additional pharmacological interventions.
ACKNOWLEDGMENTS
The present study was supported by Yeditepe University. The authors are indebted to Ahmet Ayar Dr. Professor for his valuable methodological advice.
COMMENTS
Background
Gastroesophageal reflux disease (GERD) is a highly prevalent condition in the general population, affecting up to 10%-30% of the adult population in Western countries[13]. The incidence of GERD is rising very rapidly due to the stressful lives. New approaches are necessary for its treatment.
Research frontiers
Not all patients benefit from the proton pump inhibitors (PPIs) that are frequently used for the treatment of GERD. The authors conducted an experiment to investigate the effects of these drugs on isolated rat lower esophageal sphincters (LESs). There was a dose dependent decrease in LES tone.
Innovations and breakthroughs
The study conducted is the first to demonstrate the effects of pantoprazole on the isolated LESs of rat, including the dose dependent decrease in the tone of LESs under the effect of the drug.
Applications
The study suggests that doctors should be cautious about long-term use of PPIs for the treatment of GERD.
Peer review
This paper should be of interest to a broad readership including gastroenterologists, pharmacologists, and physicians of internal medicine. It is also of interest to gastrointestinal surgeons. This paper is very interesting and is an important study to publish.
Peer reviewer: Lygia Stewart, MD, Professor of Clinical Surgery, University of California San Francisco, 4150 Clement Street, San Francisco, CA 94121, United States
S- Editor Tian L L- Editor Stewart GJ E- Editor Zheng XM