Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4532
Revised: June 15, 2011
Accepted: June 22, 2011
Published online: October 28, 2011
AIM: To evaluate the difference between the performance of the (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) equations in cirrhotic patients.
METHODS: From Jan 2004 to Oct 2008, 4127 cirrhotic patients were reviewed. Patients with incomplete data with respect to renal function were excluded; thus, a total of 3791 patients were included in the study. The glomerular filtration rate (GFR) was estimated by the 4-variable MDRD (MDRD-4), 6-variable MDRD (MDRD-6), and CKD-EPI equations.
RESULTS: When serum creatinine was 0.7-6.8 mg/dL and 0.6-5.3 mg/dL in men and women, respectively, a significantly lower GFR was estimated by the MDRD-6 than by the CKD-EPI. Similar GFRs were calculated by both equations when creatinine was > 6.9 mg/dL and > 5.4 mg/dL in men and women, respectively. In predicting in-hospital mortality, estimated GFR obtained by the MDRD-6 showed better accuracy [81.72%; 95% confidence interval (CI), 0.94-0.95] than that obtained by the MDRD-4 (80.22%; 95%CI, 0.96-0.97), CKD-EPI (79.93%; 95%CI, 0.96-0.96), and creatinine (77.50%; 95%CI, 2.27-2.63).
CONCLUSION: GFR calculated by the 6-variable MDRD equation may be closer to the true GFR than that calculated by the CKD-EPI equation.
- Citation: Chen YW, Chen HH, Wang TE, Chang CW, Chang CW, Wu CJ. Difference between CKD-EPI and MDRD equations in calculating glomerular filtration rate in patients with cirrhosis. World J Gastroenterol 2011; 17(40): 4532-4538
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4532
Routine tests for serum creatinine (Scr) have been found to significantly improve the prognostic accuracy of Child-Pugh score and to be an independent predictor of survival in patients with end-stage liver disease[1]. In the early 2000s, the Model for End-stage Liver Disease (MELD) score emerged as a simple and more objective score than Child-Pugh score, with Scr as one of the 3 variables included [the other 2 being international normalized ratio (INR) and serum bilirubin][2-4]. Unlike those of the Child-Pugh score, the 3 variables of the MELD score are selected on the basis of statistical analysis and not empirical analysis. Different from INR and serum bilirubin, which are the basic markers of liver function, Scr is essentially a marker of renal function; and highlights the prognostic significance of the interactions between liver and renal functions in patients with cirrhosis[5].
Kidney injury is an ominous and common event in cirrhotic patients[6]. Although Scr shows a strong prognostic value in patients with cirrhosis, it is considered an insensitive predictor in such patients because of reduced muscle mass, protein-deficient diet, severe hyperbilirubinemia, and diminished hepatic biosynthesis of serum creatinine, all of which lead to an overestimation of creatinine clearance as compared with inulin clearance[7]. Therefore, Scr level and creatinine-based equations also tend to overestimate glomerular filtration rate (GFR) in patients with cirrhosis.
Recently, a new creatinine-based equation known as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation has been proposed as a more accurate formula than the Cockcroft and Modification of Diet in Renal Disease (MDRD) equations[8]. However, the CKD-EPI equation has not been tested in patients with cirrhosis. The aim of the present study was to evaluate the difference between the performance of the MDRD and CKD-EPI equations when evaluating renal function in a broader population of patients with cirrhosis than liver transplant registries.
A retrospective, cross-sectional, single-center study design was used, and the study protocol was approved by the local ethics committee. Patients diagnosed with cirrhosis were selected from those admitted to Mackay Memorial Hospital between January 2004 and October 2008.
Of a total of 228 345 admitted patients, the records of 4127 patients with cirrhosis were reviewed. Patients who survived and were followed up in the outpatient department were defined as survivors, and the most recent laboratory data available for them were collected. Patients whose records indicated death any time during the hospital stay were defined as non-survivors (cases of in-hospital mortality), and laboratory data for these patients were those collected during the admission in which death occurred. In the case of patients with multiple admissions, the records before those of the last admission were excluded. Demographic data, Child-Pugh scores, and information regarding underlying comorbidities were obtained from the most recent laboratory examinations. Patients with incomplete data with respect to Child-Pugh score and renal function or with cirrhosis due to congenital abnormality were excluded; thus, a total of 3791 patients were included in the study. None of the included patients had received liver transplants. The data on renal function in the common populace were based on the results of health examinations conducted among the residents of Taipei city, Taiwan, which were recently published as part of an epidemiologic study conducted at our institution[9].
We calibrated serum creatinine values using the modified Jaffe method (Beckman Coulter, Inc. UniCel® DxC 800 Synchron® Clinical System) which were further standardized using the isotope dilution mass spectrometry (IDMS) reference method at Mackay Memorial Hospital Laboratory
The GFR was calculated according to the listed formulae: MDRD-4 = 175 × (Scr)-1.154× (Age)-0.203× (0.742 if female) × (1.178 if black)[10], MDRD-6 = 170 × (Scr)-0.999× (Age)-0.176× (0.762 if patient is female) × (1.180 if black) × (SUN)-0.170× (Albumin)0.318[10], CKD-EPI = 141 × min (Scr/κ, 1)α× max (Scr/κ, 1)-1.209× 0.993Age× 1.018 (if female) × 1.159 (if black)[8],where MDRD-4 is the 4-variable MDRD, MDRD-6 is the 6-variable MDRD, age is given in years, albumin in g/dL, Scr is serum creatinine (mg/dL), SUN is serum urea nitrogen concentration (mg/dL), κ is 0.7 for females and 0.9 for males, α is -0.329 for females and -0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of serum creatinine/κ or 1.
Continuous variables are summarized as mean ± standard deviation unless otherwise stated. We initially compared the demographic data and laboratory variables of survivors, non-survivors, and the common populace using the analysis of variance (ANOVA) test and chi-square test. Student’s t test was used to assess differences in estimated GFR (eGFR) by CKD-EPI between cirrhotic patients and the common populace, and the difference in eGFR in cirrhotic patients calculated by MDRD6 or CKD-EPI, respectively. Logistic regression analyses were conducted to investigate the accuracy of predicting in-hospital mortality by the different creatinine-based equations. The results of these analyses were used to construct a receiver-operating characteristic (ROC) curve from which we sought the optimum cutoff point for predicting successful sites. The optimum cutoff point was defined as the point on the ROC curve closest to the point (0.1), where the false-positive rate was zero and the sensitivity was 100%. The area under the curve (AUC) and 95% confidence interval (CI) were calculated. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL, United States).
Table 1 shows the demographic data, clinical characteristics, and laboratory data of patients with cirrhosis and the common populace. Older age, poorer renal function, and worse nutritional status were noted in the patients with cirrhosis than in the common populace. Thus, the average eGFR in patients with cirrhosis was significantly lower than that in the common populace, irrespective of the equation used for calculation (MDRD-4, MDRD-6, or CKD-EPI equation).
| Variables (n, %) | Survived cirrhotic patients(n = 2337) | Expired cirrhotic patients(n = 1454) | Common populace(n = 4292) | P value |
| Age (yr) | 59.03 ± 14.03 | 63.61 ± 13.62 | 52.11 ± 12.13 | < 0.001 |
| Gender (male/female) | 1620/717 | 990/464 | 2270/2022 | < 0.001 |
| Albumin (3.5-5 g/dL) | 3.24 ± 0.68 | 2.48 ± 0.55 | 4.50 ± 0.29 | < 0.001 |
| BUN (8-12 mg/dL) | 17.31 ± 14.77 | 60.81 ± 40.77 | 13.39 ± 3.78 | < 0.001 |
| Creatinine (0.4-1.2 mg/dL) | 1.20 ± 1.06 | 2.93 ± 1.99 | 0.89 ± 0.20 | < 0.001 |
| eGFR (MDRD4) | 79.27 ± 35.43 | 36.75 ± 33.55 | 81.72 ± 16.38 | < 0.001 |
| eGFR (MDRD6) | 65.70 ± 30.28 | 26.76 ± 24.63 | 69.65 ± 13.16 | < 0.001 |
| eGFR (CKD-EPI) | 78.50 ± 29.82 | 36.03 ± 30.23 | 88.31 ± 15.78 | < 0.001 |
| Total bilirubin (0.3-1.2 mg/dL) | 2.24 ± 3.58 | 9.76 ± 10.68 | < 0.001 | |
| INR | 1.36 ± 0.43 | 2.70 ± 2.53 | < 0.001 | |
| Hepatoma | 647 (27.69) | 717 (49.31) | < 0.001 | |
| Ascites | 817 (34.96) | 1018 (70.01) | < 0.001 | |
| Hepatic encephalopathy | 431 (18.44) | 649 (44.64) | < 0.001 | |
| Child-Pugh points | 7.12 ± 1.97 | 10.37 ± 2.09 | < 0.001 |
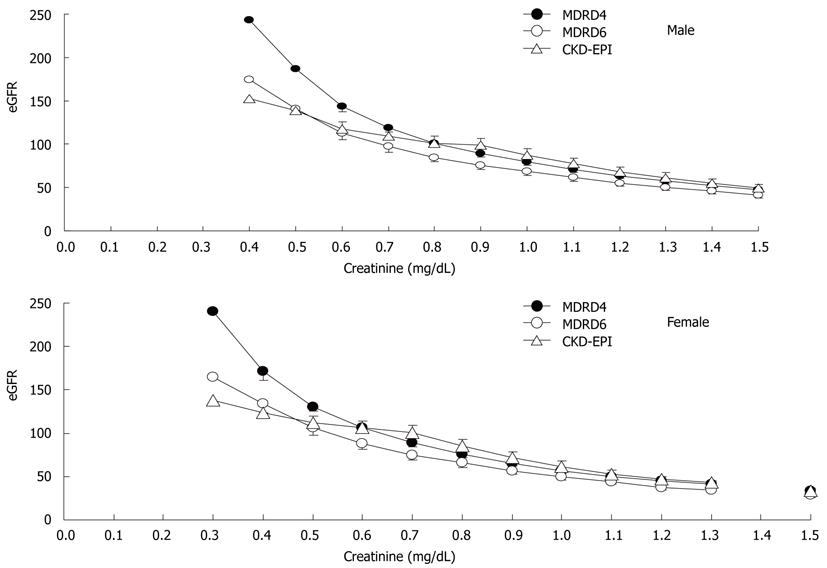
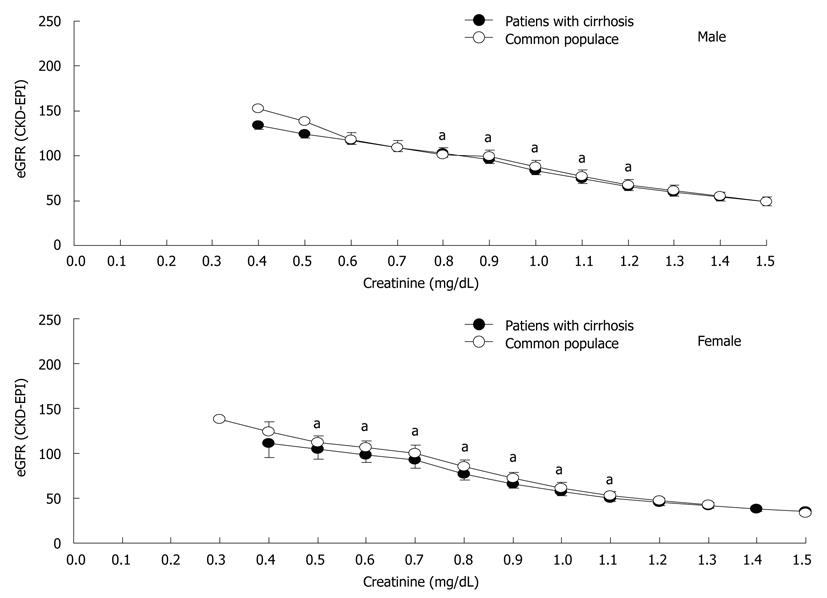
Figure 1 shows the application of the 3 creatinine-based equations for calculating GFR in the common populace. The slope of the CKD-EPI equation was similar to that of the MDRD-4 equation when the Scr level was > 0.8 mg/dL and > 0.6 mg/dL in men and women, respectively, but less steep below the knots, which leads to less overestimation of GFR by the CKD-EPI equation at a lower Scr level[8]. Figure 2 shows the application of the CKD-EPI equation in calculating GFR in both the patients with cirrhosis and the common populace. At the same Scr level, the CKD-EPI equation tended to estimate a significantly lower value of GFR in patients with cirrhosis when the Scr level was 0.8-1.2 mg/dL and 0.5-1.1 mg/dL in men and women, respectively. Figure 3
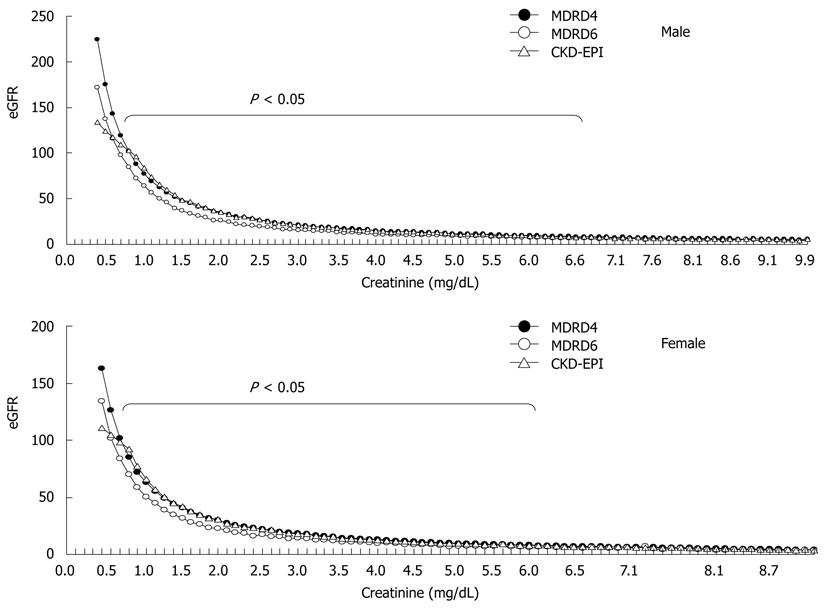
shows the eGFR obtained by the 3 creatinine-based equations in patients with cirrhosis. The eGFRs obtained by the 3 equations were similar when the Scr level was > 6.9 mg/dL and > 5.4 mg/dL in men and women, respectively. Interestingly, significantly lower eGFR was obtained by the MDRD-6 equation than by the CKD-EPI equation when the Scr level was 0.7-6.8 mg/dL and 0.6-5.3 mg/dL in men and women, respectively. When the Scr level was < 0.5 mg/dL in men (1.8% of men with cirrhosis) and < 0.4 mg/dL in women (1.4% of women with cirrhosis), a lower eGFR was obtained by the CKD-EPI equation than by the MDRD-6 equation.
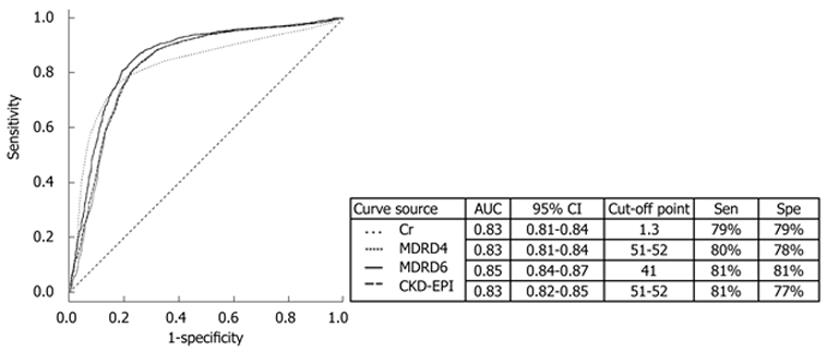
The eGFR obtained by the MDRD-6 equation showed better accuracy (81.72%; 95% CI, 0.94-0.95) in predicting in-hospital mortality than that obtained by the MDRD-4 equation (80.22%; 95% CI, 0.96-0.97) and CKD-EPI equation (79.93%; 95% CI, 0.96-0.96). In general, eGFR showed a better prognostic value as a surrogate of renal function than Scr level (accuracy, 77.50%; 95% CI, 2.27-2.63). In the ROC curve (Figure 4),
the cutoff point for eGFR obtained by the MDRD-6 equation was 41 (AUC, 0.85; 95% CI, 0.84-0.87). Interestingly, the cutoff point for Scr level was 1.3 mg/dL (AUC, 0.83; 95% CI, 0.81-0.84), which was lower than 1.5 mg/dL, a value suggested to indicate renal failure in patients with cirrhosis and the threshold value for the diagnosis of hepatorenal syndrome.
This retrospective, cross-sectional, single-center study involved a broader population of patients with cirrhosis than liver transplant registries to obtain eGFR using different creatinine-based equations. A significantly lower eGFR was obtained by the MDRD-6 equation than by the CKD-EPI equation when the Scr level was 0.7-6.8 mg/dL and 0.6-5.3 mg/dL in men and women, respectively. In view of the overall overestimation of GFR by the creatinine-based equations in patients with cirrhosis, eGFR obtained by the MDRD-6 equation may be closer to the true GFR than that obtained by the CKD-EPI equation. The use of eGFR obtained by the MDRD-6 equation as a surrogate of renal function offered better accuracy in predicting in-hospital mortality than that of eGFR obtained by the MDRD-4 equation, CKD-EPI equation, or Scr level.
The prognostic significance of renal function in patients with cirrhosis is reflected by the inclusion of Scr in the MELD score, which predicts short-term mortality (3 mo) and is used for the prioritization of transplant recipients in the United States[2-5,11]. However, it has recently been suggested that Scr weighs too heavily on the MELD score[12]: the assumption that mortality is constant at the Scr level of < 1 mg/dL is likely to be false. On the other hand, Scr level and creatinine-based equations tend to overestimate GFR, and creatinine clearance from the time of urine collection also leads to overestimation of GFR. As a result, a modified MELD score with a lower weighting for Scr than that in the current MELD score has been proposed and has been shown to be slightly superior[12]. However, even after these adjustments, Scr is still a determinant of prognosis.
The creatinine-based Cockcroft and MDRD equations are widely used to estimate GFR in the general population, and MDRD is considered the gold standard in nephrology[5,13]. However, both the Cockcroft and MDRD equations tend to overestimate GFR: a series has shown that only 66% of estimates were within 30% of the measured GFR[14,15]. Unfortunately, most of the cited studies evaluated GFR in patients in liver transplant registries, who tend to have more advanced cirrhosis and decreased GFR, in part, due to the liver disease and malnourishment. The present study included a broader population that may have been better nourished or not as ill as that in previous studies.
The CKD-EPI equation, a newly developed equation for estimating GFR, has been proposed to be more accurate than the MDRD equation, especially when GFR is high. Moreover, it shows less bias, improved precision, and greater accuracy[8]. Our study results agreed with this fact since the slope of the CKD-EPI equation was less steep when the Scr level was < 0.8 mg/dL and < 0.6 mg/dL in men and women, respectively. When the CKD-EPI equation was applied at the same Scr level in patients with cirrhosis and the common populace, a lower GFR was calculated in the former than in the latter. This result was probably related to the older age of the patients with cirrhosis, with the same Scr level. When the CKD-EPI, MDRD-4, and MDRD-6 equations were applied in the case of patients with cirrhosis, the performance of the CKD-EPI and MDRD-4 equations was similar to that in the common populace. However, a significantly lower GFR was estimated by the MDRD-6 equation than by the CKD-EPI equation when the Scr level was 0.7-6.8 mg/dL and 0.6-5.3 mg/dL in men and women, respectively. This result was probably related to the higher blood urea nitrogen (BUN) and lower albumin level-the additional 2 variables used in the MDRD-6 equation-in patients with cirrhosis. Although the CKD-EPI equation also yielded a lower eGFR than the MDRD-6 equation when the Scr level was < 0.5 mg/dL and < 0.4 mg/dL in men and women, respectively, the value was only found in 1.8% men and 1.4% women in all the study subjects. In view of the overall overestimation of GFR by the creatinine-based equations in patients with cirrhosis, eGFR obtained by the MDRD-6 equation seemed to be closer to the true GFR than that obtained by the CKD-EPI equation.
Creatinine shows a significant prognostic value in patients with cirrhosis[2,5,11]. Theoretically, the creatinine-based equations show a similar prognostic value. However, the Cockcroft equation is less accurate than the MDRD equation since it incorporates body weight, which is markedly biased in patients with edema and/or ascites[16]. The MDRD-4 (simplified MDRD) equation is usually and most often used to calculate GFR, since it is considered as accurate as the original MDRD-6 equation[17]. However, its usefulness has not been proved in healthy individuals, and its accuracy may be low in specific clinical settings[15,18]. Therefore, the MDRD-6 equation is considered the best, possibly because it incorporates BUN and albumin level, the 2 variables which are abnormal in patients with cirrhosis[18]. Our data also showed that eGFR obtained by the MDRD-6 equation was more accurate than that obtained by the MDRD-4 equation, CKD-EPI equation, or even Scr level in predicting in-hospital mortality. It is most likely that the improved predication due to BUN and albumin, in particular serum albumin is an excellent predictor of mortality. Thus, the use of eGFR obtained by the MDRD-6 equation as a surrogate of renal function offers a better prognostic value than that of eGFR obtained by the other equations. However, the accuracy of the MDRD equation has only been estimated on a large scale, in patients with chronic kidney disease. This suggests that a specific formula should be derived for patients with cirrhosis.
The present study has several limitations. First, there was no comparison between the CKD-EPI equation and the gold standard for GFR estimation such as that using 125I-iothalamate or inulin. Thus, the true performance of the CKD-EPI equation in patients with cirrhosis could not be evaluated. Second, due to the variation in assay of BUN and albumin across labs which is not standardized, these results may not be useful in other populations. Third, the study was retrospective and cross-sectional in nature, and therefore, a prospective, cohort study is needed to test and verify our conclusions.
In conclusion, in view of the overall overestimation of GFR in patients with cirrhosis by creatinine-based equations, GFR calculated by the MDRD-6 equation may be closer to the true GFR than that calculated by the CKD-EPI equation and, hence, more suitable as a surrogate of renal function. However, a formula specifically derived for calculating GFR in patients with cirrhosis is warranted.
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation has been proposed to be more accurate than the Cockcroft and Modification of Diet in Renal Disease (MDRD) equations. However, the CKD-EPI equation has not been tested in patients with cirrhosis.
This is a retrospective study. Glomerular filtration rate (GFR) calculated by the 6-variable MDRD equation is closer to the true GFR than that calculated by the CKD-EPI equation. The 6-variable MDRD equation is a better way of calculating GFR in cirrhotic patients.
To our knowledge, this is the first study to evaluate the difference between the performance of the CKD-EPI and MDRD equations in cirrhotic patients. Although the CKD-EPI equation been proposed to be more accurate than the MDRD equation in the general population, the 6-variable MDRD equation remains the best way to calculate GFR in cirrhotic patients.
GFR should be calculated by the 6-variable MDRD equation in cirrhotic patients.
It is a good study. However the authors have adequately mentioned the limitation of the same.
Peer reviewer: Yogesh K Chawla, Dr., Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN
| 1. | Abad-Lacruz A, Cabré E, González-Huix F, Fernández-Bañares F, Esteve M, Planas R, Llovet JM, Quer JC, Gassull MA. Routine tests of renal function, alcoholism, and nutrition improve the prognostic accuracy of Child-Pugh score in nonbleeding advanced cirrhotics. Am J Gastroenterol. 1993;88:382-387. [PubMed] |
| 2. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2061] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 3. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3668] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 4. | Chen YW, Wu CJ, Chang CW, Lee SY, Sun FJ, Chen HH. Renal function in patients with liver cirrhosis. Nephron Clin Pract. 2011;118:c195-c203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Francoz C, Glotz D, Moreau R, Durand F. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol. 2010;52:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Chen YW, Wu CJ, Wang TE, Chang CW, Chang CW, Chen HH. The mortality survey of older patients with cirrhosis in Taiwan--a single-center experience. J Am Geriatr Soc. 2010;58:2230-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Demirtas S, Bozbas A, Akbay A, Yavuz Y, Karaca L. Diagnostic value of serum cystatin C for evaluation of hepatorenal syndrome. Clin Chim Acta. 2001;311:81-89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [PubMed] |
| 9. | Chen HH, Chen YW, Wu CJ. Primary hyperparathyroidism in Taiwan: clinical features and prevalence in a single-center experience. Endocrine. 2010;37:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [PubMed] |
| 11. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1863] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 12. | Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2001] [Cited by in RCA: 2069] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 14. | Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Cholongitas E, Shusang V, Marelli L, Nair D, Thomas M, Patch D, Burns A, Sweny P, Burroughs AK. Review article: renal function assessment in cirrhosis - difficulties and alternative measurements. Aliment Pharmacol Ther. 2007;26:969-978. [RCA] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-254. [PubMed] |
| 18. | Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 845] [Article Influence: 42.3] [Reference Citation Analysis (0)] |