Published online Oct 7, 2011. doi: 10.3748/wjg.v17.i37.4184
Revised: April 7, 2011
Accepted: April 14, 2011
Published online: October 7, 2011
AIM: To characterize the inductive effects of isoflurane (ISO) on hepatic heme oxygenase-1 (HO-1) in an animal model of hepatic steatosis.
METHODS: Lean (LEAN) and obese (FAT) Zucker rats were randomized into 4 groups: 1: LEAN + pentobarbital sodium (PEN); 2: LEAN + ISO; 3: FAT + PEN; 4: FAT + ISO. The animals were mechanically ventilated for 6 h. In vitro analyses of liver tissue included determination of HO-1 mRNA and protein expression as well as measurement of HO enzyme activity and immunohistochemical analyses.
RESULTS: Compared to PEN treatment, ISO administration profoundly induced hepatic HO-1 mRNA and protein expression and significantly increased HO enzyme activity in lean Zucker rats. In contrast, no difference in HO-1 gene expression was observed after ISO or PEN anesthesia in obese Zucker rats.
CONCLUSION: The present study demonstrates that ISO is an inducer of hepatic HO-1 gene expression in non-steatotic organs but failed to upregulate HO-1 in steatotic livers.
- Citation: Stoll P, Schwer CI, Goebel U, Buerkle H, Hoetzel A, Schmidt R. Hepatic steatosis prevents heme oxygenase-1 induction by isoflurane in the rat liver. World J Gastroenterol 2011; 17(37): 4184-4190
- URL: https://www.wjgnet.com/1007-9327/full/v17/i37/4184.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i37.4184
The development of hepatic ischemia/reperfusion (I/R) injury is a fundamental problem in major hepatic surgery including liver transplantation, causing a higher rate of morbidity and mortality[1,2]. Surgical interventions such as warm hepatic inflow occlusion (Pringle maneuver) or cold ischemia in the transplant setting followed by reperfusion are important and often unavoidable techniques used to reduce blood loss or preserve organs for subsequent transplantation. I/R injury frequently results in apoptosis and necrosis of hepatocytes, which could consequently lead to organ failure or graft dysfunction[3,4]. In recent years liver surgery has become safer due to improvements in surgical techniques, anesthetic procedures and postoperative care. However, due to the epidemic increase in obesity, the prevalence of hepatic steatosis has significantly increased during the last few decades. The Dallas Heart Study reported a prevalence of hepatic steatosis in their population of about 38% indicating the high relevance for today’s health care system[5]. It has been repeatedly shown that steatotic livers are especially vulnerable to I/R injury. Liver surgery in patients with severe hepatic steatosis is associated with higher morbidity and mortality rates due to the underlying pathogenic features affecting important mechanisms during I/R, liver regeneration and recovery[6-8]. Various strategies have been proposed to improve the postoperative outcome of these patients including pharmacological approaches aiming at upregulation of cytoprotective genes.
Heme oxygenase-1 (HO-1) and its catalytic products have been identified as major players in cell protection in different organs[9-12]. Whereas HO-1 represents the inducible form of the HO family, HO-2 is expressed constitutively. HO catabolizes the first and rate-limiting step in heme degradation producing carbon monoxide (CO), free iron and biliverdin, which is converted into bilirubin by biliverdin reductase[13]. A multitude of HO-1 inducers are well known, but most of them are toxic which limit their therapeutic application in humans[14,15]. We have previously shown that volatile anesthetics are potent non-toxic inducers of HO-1 gene expression in the rat liver[16]. Isoflurane (ISO) pretreatment induces hepatic HO-1 mRNA and protein followed by an increase in HO activity, thereby reducing portal resistance[17]. Experimental and clinical evidence support the hypothesis that administration of volatile anesthetics could be a promising approach to limit I/R injury and to improve the outcome of patients undergoing liver surgery[18,19]. To date, no data are available regarding the effects of anesthetics on hepatic HO-1 induction in steatotic livers. Therefore, the present study was designed to characterize the effects of ISO administration on hepatic HO-1 gene expression in an established animal model of hepatic steatosis using genetically modified Zucker rats.
Isoflurane was obtained from Abbott (Wiesbaden, Germany) and pentobarbital sodium from Alvetra (Neumuenster, Germany). Pancuronium was purchased from Organon (BH Oss, Netherlands). All other reagents used were purchased from Sigma Aldrich (Deisenhofen, Germany), if not specified otherwise.
All animal experiments were approved by the local animal care and use committee and were in accordance with the Guide for the Care and Use of Laboratory Animals. Homozygous obese (FAT) male Zucker rats and heterozygous lean (LEAN) male Zucker rats aged 12 wk were obtained from Charles River (Sulzfeld, Germany). Animals were fasted for 6 h before the beginning of the experiments but were allowed free access to water.
The animals were assigned to 4 groups: group 1, LEAN + PEN (pentobarbital sodium, 40 mg/kg per hour i.v.); group 2, LEAN + ISO; group 3, FAT + PEN; group 4, FAT + ISO. Animals treated with PEN received one initial intraperitoneal injection of pentobarbital sodium (40 mg/kg) followed by an intravenous infusion of 40 mg/kg per hour. For compensation of evaporative losses 10 mL/kg per hour of saline solution 0.9% were continuously infused. Rats in the ISO groups were anesthetized by inhalation of ISO (2.8-3.1 Vol%). After induction of anesthesia, a tail vein was cannulated and a tracheostomy was performed. Relaxation was achieved by injection of pancuronium (1 mg/kg i.v.) and all animals were mechanically ventilated (Rodent Ventilator UB 7025-10, Harvard Apparatus, March-Hugstetten, Germany). Doses of 0.5 mg/kg pancuronium were repeated every 3 h to maintain muscle paralysis. Cannulation of the left carotid artery with polyethylene (PE-50, Smith Medical, Ashford, United Kingdom) tubing was performed for arterial blood pressure monitoring and blood gas analysis. The right jugular vein was cannulated for fluid administration. Blood gas analyses were performed using an autoanalyzer ABL 800 Flex (Radiometer, Willich, Germany). At the end of the experiment (6 h after onset), the animals were killed and blood and liver tissue were removed for subsequent analyses.
Blood samples were collected at the end of each experiment and immediately centrifuged at 4 °C. Rat α-glutathione s-transferase (α-GST) serum concentration was evaluated using an anti-rat α-GST enzyme immunoassay (Argutus Medical, Dublin, Ireland). The procedures were performed according to the manufacturer’s instructions.
Total RNA was extracted from liver tissues using the TRIzol method (Invitrogen, Carlsbad, CA, Untied States) according to the manufacturer’s recommendation. RNA amounts were normalized to a concentration of 50 ng/μL diluted with RNAse-free Water (Qiagen, Hilden, Germany).
Total RNA was reverse transcribed to single-stranded cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc, Foster City, CA 94404 Untied States) according to the manufacturer’s protocol. Briefly 250 ng of purified total RNA were subsequently used in 50 µL reverse transcription reactions employing random hexamers. 50 ng of the resulting cDNA were used in semi-quantitative real-time polymerase chain reaction (PCR) analysis in a 50 µL final volume. Reactions were performed on an ABI Prism 7000 (Applied Biosystems, Foster City, CA, United States) in duplicate for each animal using TaqMan Mastermix reagents (part number 4309169, Applied Biosystems, Foster City, CA, United States) with a specific TaqMan® Probe against HO-1 cDNA (Assay ID: Rn00561387_mL) as described in the manufacturer’s protocol. Parameters for quantitative PCR were as follows: 10 min at 95 °C, followed by 40 cycles of amplification for 15 s at 95 °C, and 1 min at 60 °C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression was used as endogenous control in all real time analyses using a VIC®/MGB labeled Probe (part number: 4352338E, Applied Biosystems, Foster City, CA, United States). The obtained data were analyzed by the ΔΔCT method.
The heme oxygenase (HO) enzyme activity assay was performed as previously described[20]. Briefly, frozen liver tissue was homogenized and added to a reaction mixture containing NADPH, liver cytosol, glucose-6-phosphate, glucose-6-phosphate dehydrogenase and hemin. The reaction was performed at 37 °C for 1 h in the dark and stopped by the addition of chloroform. The extracted bilirubin was calculated by the difference in absorbance between 464 and 530 nm.
For histological analysis, liver sections were fixed with 4% buffered formalin (pH 6.9) and embedded in paraffin. Livers were sliced (5 μm) and stained with hematoxylin-eosin according to a standardized protocol.
Liver tissue samples were formalin-fixed, paraffin embedded and cut in a microtome to 4 microns. The slides were deparaffinized with xylene, rehydrated and then rinsed with tap water. Antigen retrieval was performed by microwave irradiation in a sodium citrate buffer (pH 6) and slides were blocked with a ready-to-use peroxidase blocking reagent (Dako North America, Inc., CA 93013 United States) for 10 min at room temperature. After subsequent treatment with normal goat serum, slides were incubated with the primary antibody (dilution 1:50) as used in Western blotting for 1 h at room temperature. Following three washing steps with phosphate buffered saline (PBS) the slides were incubated with the HRP-conjugated secondary antibody (Goat A-rabbit-HRP, Dako, Denmark) diluted 1:200. The slides were again washed 3 times with PBS and then incubated with liquid diaminobenzidine and substrate (Dako North America, Inc., CA, United States) as the chromogen for 6 min and rinsed with deionized water. Finally the sections were counterstained with Mayer´s hematoxylin (Merck KG,, Darmstadt, Germany), dehydrated and mounted in an organic mounting media. For assessment of the severity of hepatic steatosis, hematoxylin and eosin-stained sections were evaluated without immunohistochemical treatment.
Western blotting analysis was performed with total cell lysates as described previously[19]. Briefly, frozen liver tissue was homogenized on ice in activated RIPA buffer (Santa Cruz, CA, United States). Total protein concentration was determined in the supernatant using the Bradford assay (Bio-Rad Laboratories, Munich, Germany). Each lane of a 10% sodium dodecyl sulfate gel contained 100 μg of total protein. After separation and electroblotting, HO-1 was detected by a rabbit polyclonal anti-HO-1 antibody (1:1000 dilution, SPA 895; Stress Gen Biotechnologies, Victoria, British Colombia, Canada) using the enhanced chemiluminescence detection kit (Amersham Pharmacia) according to the manufacturer’s instructions.
Data are presented as mean ± SE of the mean with n = 5 animals per group as indicated. Statistical differences within each group were determined using a one-way analysis of variance (ANOVA) for repeated measurements and between the different groups by one-way ANOVA followed by the post hoc Student-Newman-Keuls test for pairwise comparisons. When criteria for parametric tests were not met, Kruskal-Wallis ANOVA on ranks followed by Dunn´s test was used. These data are presented as median (box: 25th and 75th percentiles; error bars: 5th and 95th percentiles) for n = 5 animals per group. Data were considered significant when P < 0.05. Statistical analysis was performed using the Sigma Stat and Sigma Plot 11 software package (Jandel Scientific, San Rafael, CA, United States).
The animals in the LEAN + PEN group had a significantly higher mean arterial pressure at the respective time points during the experiments (Table 1). There were no differences in heart rate between the different groups. Body temperature dropped during induction of anesthesia but returned to normal values after at least 2 h in all groups (Table 1). Homozygous Zucker rats (FAT) had a higher body weight compared to the age-matched heterozygous controls (LEAN) (Table 2). There were no significant differences in blood gas parameters.
| Time (h) | LEAN+PEN | LEAN+ISO | FAT+PEN | FAT+ISO | |
| MAP | 0 | 127a± 5 | 82 ± 11 | 106 ± 5 | 99 ± 5 |
| (mmHg) | 2 | 86a± 3 | 72 ± 2 | 77 ± 4 | 73 ± 2 |
| 4 | 85a± 5 | 69 ± 2 | 74 ± 4 | 69 ± 2 | |
| 6 | 84a± 7 | 69 ± 2 | 72 ± 3 | 70 ± 2 | |
| HR | 0 | 305 ± 3 | 302 ± 4 | 314 ± 2 | 310 ± 2 |
| (bpm) | 2 | 302 ± 2 | 300 ± 0 | 319 ± 3 | 312 ± 0 |
| 4 | 310 ± 2 | 305 ± 3 | 310 ± 2 | 310 ± 4 | |
| 6 | 307 ± 3 | 307 ± 3 | 310 ± 2 | 310 ± 2 | |
| Temp. | 0 | 34.4c± 0.3 | 34.7c± 0.6 | 34.5c± 0.2 | 34.7c± 0.3 |
| (°C) | 2 | 36.6 ± 0.3 | 37.2 ± 0.3 | 36.6 ± 0.3 | 36.9 ± 0.1 |
| 4 | 37.1 ± 0.2 | 37.5 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.2 | |
| 6 | 37.1 ± 0.2 | 37.5 ± 0.0 | 37.2 ± 0.1 | 37.0 ± 0.1 |
| LEAN+PEN | LEAN+ISO | FAT+PEN | FAT+ISO | |
| Weight (g) | 321 ± 19 | 294 ± 16 | 462b± 14 | 426b± 18 |
| pH | 7.46 ± 0.03 | 7.48 ± 0.03 | 7.48 ± 0.04 | 7.42 ± 0.01 |
| pCO2 (mmHg) | 40 ± 3 | 37 ± 3 | 32 ± 4 | 33 ± 4 |
| pO2 (mmHg) | 211 ± 52 | 279 ± 69 | 258 ± 33 | 308 ± 74 |
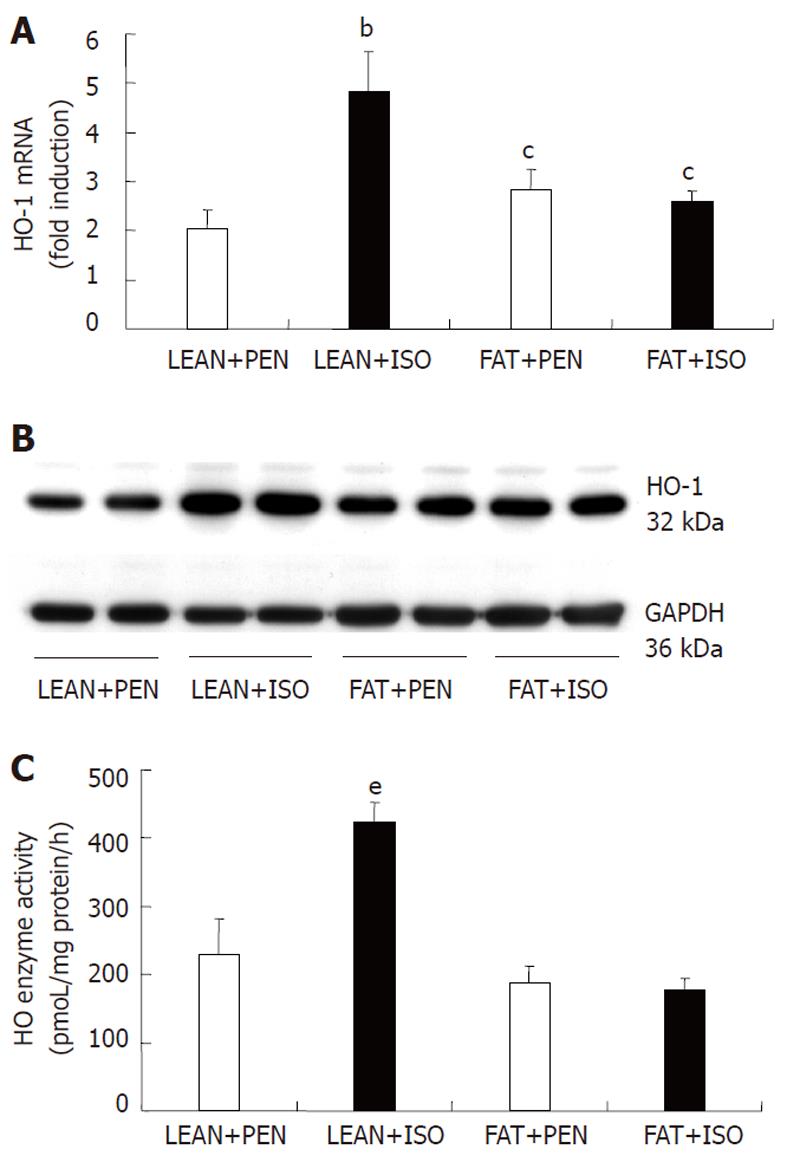
Semi-quantitative HO-1 mRNA real-time analysis of isolated liver extracts is shown in Figure 1A. ISO treatment over 6 h led to a significant increase in HO-1 mRNA in lean rats (4.81 ± 0.82) compared to all other groups. ISO inhalation in FAT Zucker rats (2.57 ± 0.22) did not lead to a significant induction of HO-1 compared to the respective PEN group (2.83 ± 0.39). A significant difference in HO-1 mRNA levels was detected between liver extracts of animals from the FAT+PEN and FAT + ISO group compared to rats assigned to the LEAN + PEN group (2.03 ± 0.38).
In line with RT-analyses, representative Western blotting showed higher HO-1 protein levels in liver extracts from animals in the LEAN + ISO group compared to the other groups (Figure 1B). Equal loading was verified by reprobing the membrane with a GAPDH antibody. In addition, HO enzyme activity was significantly higher in ISO treated lean Zucker rats (Figure 1C). Interestingly, we found no differences in HO activity between the FAT + PEN and FAT + ISO group.
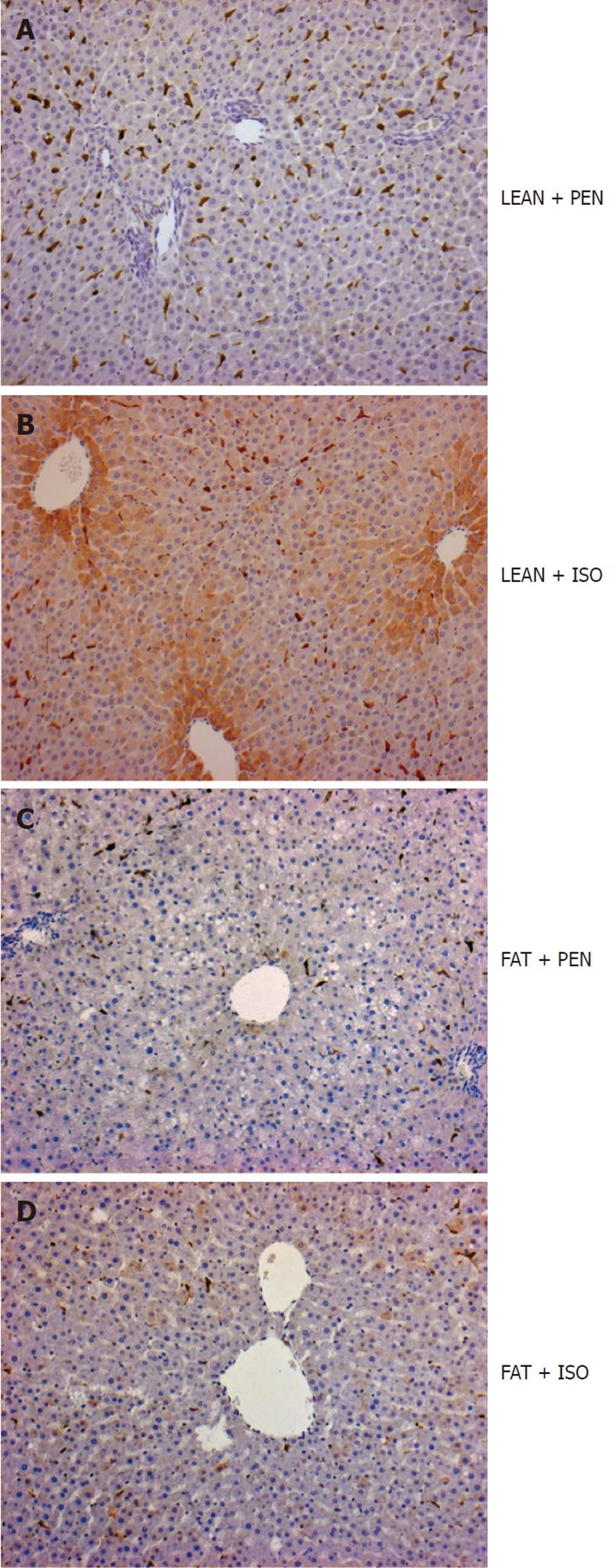
HO-1 immunoreactive protein was restricted to spindle-shaped sinusoidal lining cells in PEN anesthetized control animals (Figure 2A). HO-1 protein was markedly upregulated in hepatocytes predominantly located in the perivenular area after ISO treatment in lean Zucker rats (Figure 2B). In sharp contrast, we did not detect any upregulation of HO-1 protein in hepatocytes of the perivenular area in obese Zucker rats after treatment with ISO (Figure 2C and D).
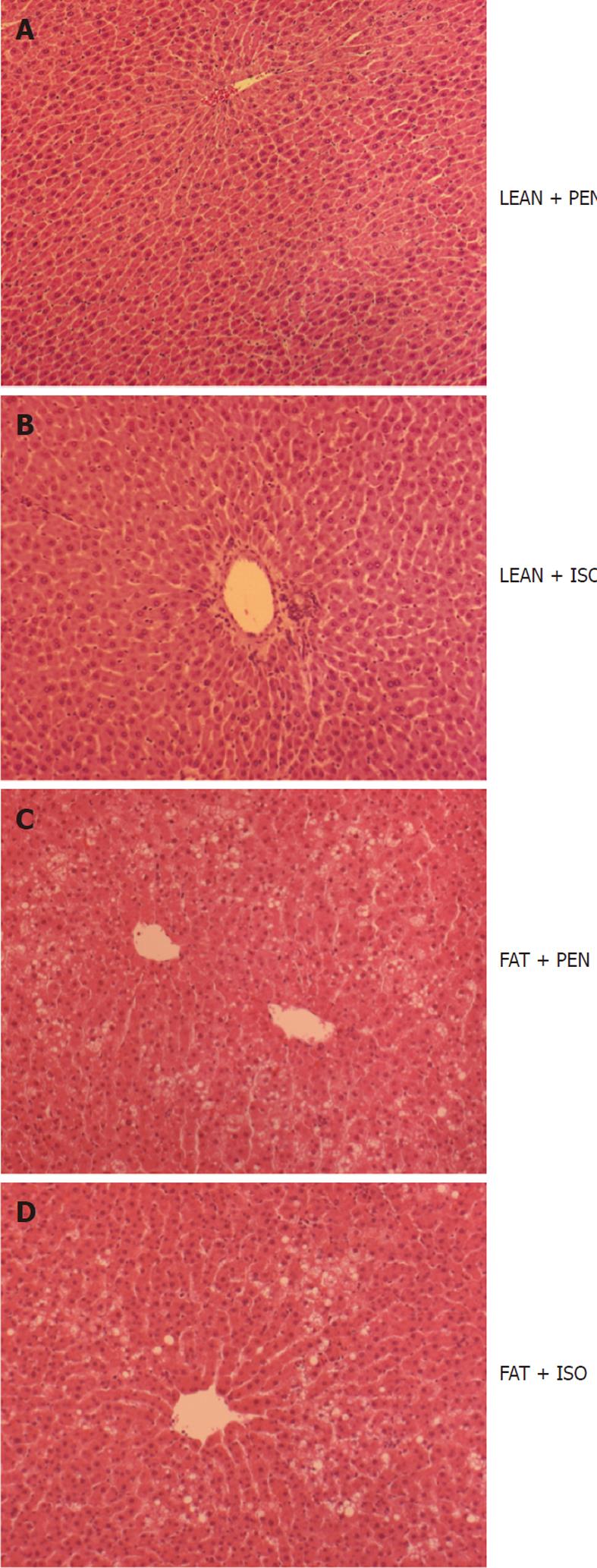
For confirmation of steatosis hepatis, hematoxylin-eosin staining was performed. Obese Zucker rats (FAT) showed severe macrovesicular and microvesicular fatty infiltration in hepatocytes (Figure 3C and D). In contrast, we did not detect any accumulation of lipid droplets in LEAN animals (Figure 3A and B).
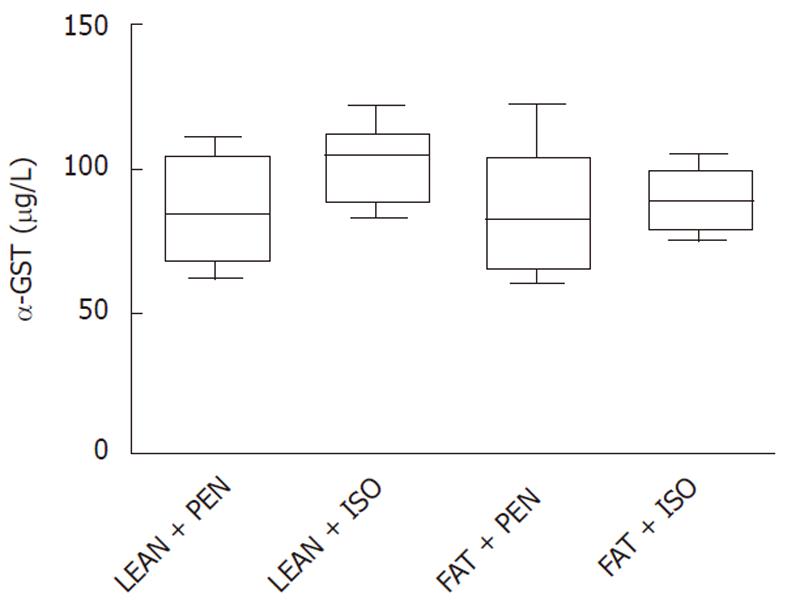
As shown in Figure 4, α-GST, one of the most specific serum enzymes identifying hepatocyte injury did not differ between the groups.
In the present report we demonstrate that ISO induced upregulation of HO-1 gene expression, which was reproducibly shown in normal livers and serves as a major protective mechanism against hepatic ischemia and reperfusion injury, is abrogated in the presence of hepatic steatosis. ISO treatment of heterozygous lean Zucker rats representing a normal phenotype led to a profound induction of HO-1 mRNA and protein with a subsequent increase in HO enzyme activity in the liver. In contrast to these findings, which confirm earlier reports from our laboratory in Sprague Dawley rats, ISO administration to homozygous obese Zucker rats, an established animal model for steatosis hepatis, had no effect on HO-1 gene expression in the liver of these animals.
Volatile anesthetics are non-toxic inducers of hepatic HO-1 gene expression[16,17,19,21]. We previously showed that pretreatment with ISO leads to an improvement in hepatic macro- and microvascular blood flow and reduces portal vascular resistance in the normal liver[17]. Furthermore, ISO induced upregulation of hepatic HO-1 is an important hepatoprotective mechanism against I/R injury. It specifically upregulates HO-1 protein in hepatocytes of the perivenular area, the primary localization of cellular injury in low flow states like I/R. HO-1 induction improves microcirculation in the early reperfusion period, decreases the oxidative burst and significantly reduces serum levels of liver enzymes and morphological signs of hepatic injury after I/R[19]. In addition to these experimental data, Beck-Schimmer and colleagues recently published the first randomized clinical study demonstrating the hepatoprotective effect of the volatile anesthetic, sevoflurane, in patients undergoing liver resection[18]. Interestingly, they found an even more pronounced beneficial effect of sevoflurane in patients with steatotic livers. No information is available regarding sevoflurane treatment on HO-1 gene expression in their study. However, we hypothesize that the protective effect of sevoflurane in this trial is independent of HO-1 since the duration of pretreatment (30 min) and the concentration applied (1.5 of minimal alveolar concentration) is most likely not sufficient to upregulate hepatic HO-1 gene expression. Therefore, volatile anesthetics might mediate liver protection by at least two different mechanisms, one dependent and one independent of HO-1. To exclude the possibility that HO-1 upregulation in homozygous Zucker rats is generally prevented by genetic modifications in these animals, we screened the literature in this regard. It has been repeatedly shown by different authors that the administration of a variety of compounds can profoundly induce HO-1 in obese Zucker rats[22,23]. Therefore, the inability to upregulate HO-1 by ISO seems to be substance specific rather than based on a general lack of inducibility in these animals. As indicated in Figure 1A and B, hepatic HO-1 induction in obese animals was slightly but significantly higher than in the livers of lean controls. However, this did not affect HO enzyme activity in our experiments (Figure 1C).
To exclude hepatotoxic effects of the anesthetics in our experiments, we performed serum α-GST measurements. α-GST levels, which serve as a very specific marker of hepatocyte injury, did not differ between the respective groups indicating the non-toxic action of ISO.
Obese Zucker rats develop hypertensive blood pressure values accompanied by improper autoregulation caused by an impairment of sympathetic baroreceptor reflexes[24-26]. Therefore, anesthetics (e.g., barbiturates) may have an even more pronounced effect on blood pressure in obese rather than lean Zucker rats. This could be an explanation for the higher blood pressure in the LEAN + PEN group compared to the other animals observed in the present study.
Patients with hepatic steatosis are at higher risk for postoperative complications after major hepatic surgery including liver transplantation, and adverse outcomes have been repeatedly documented[8,27-30]. Due to the increasing gap between the number of available organs and the number of patients awaiting an organ, the amount of so-called “marginal livers“ considered for transplantation is increasing. Based on this dilemma, it is important to develop protective strategies particularly for the abovementioned type of organs comprising severely steatotic livers to expand the pool of available liver grafts.
The present study demonstrates that ISO is a potent inducer of HO-1 gene expression in non-steatotic livers but failed to upregulate HO-1 in steatotic organs. If validated in humans, this observation may have an impact on the anesthetic regimen in patients undergoing liver surgery.
The authors are grateful to Martina de Groot and Heide Marniga for their excellent technical assistance.
Experimental and clinical evidence support the hypothesis that administration of volatile anesthetics could be a promising approach to limit ischemia/reperfusion (I/R) injury and to improve the outcome of patients undergoing liver surgery. The volatile anesthetic isoflurane is a potent non-toxic inducer of heme oxygenase-1 (HO-1) gene expression in the normal liver. The authors previously showed that these livers are protected from I/R injury.
There are no studies currently available characterizing the inductive effects of volatile anesthetics on steatotic livers which are especially vulnerable to I/R injury. Therefore, we examined the effects of isoflurane (ISO) on hepatic HO-1 induction in lean (non-steatotic livers) and obese (steatotic livers) Zucker rats.
The findings of the present study demonstrate that isoflurane is a potent inducer of HO-1 gene expression in non-steatotic livers but failed to upregulate HO-1 in steatotic livers.
If verified in humans, this observation may have a crucial impact on the anesthetic regimen in patients undergoing liver surgery.
HO-1 also called heat shock protein-32 and its catalytic products were recently identified as major players in cell protection in different organs. Hepatic I/R injury is a fundamental problem in major hepatic surgery including liver transplantation. The Zucker rat is a genetic research model for obesity and hypertension named after Lois M Zucker. Homozygous Zucker rats have high levels of lipids in their blood and an increased size and number of fat cells. Therefore, these animals serve as a model for steatosis hepatis.
In this manuscript, the authors compare the effects of the volatile anesthetic isoflurane in the induction of HO-1 among lean and obese rats. They measure HO-1 expression at the mRNA and protein level, and also assess the activity of this enzyme. The overall data suggest that isoflurane efficiently induces HO-1 in lean, but not in obese animals. The study is well designed, appropriate controls are included and the experiments are of high technical quality. The conclusion is fully supported by the presented data.
Peer reviewers: Kostas Pantopoulos, Associate Professor, Department of Medicine, McGill University, Lady Davis Institute for Medical Research, 3755 Cote Ste-Catherine Road, Montreal, Quebec, H3T 1E2, Canada; Valentina Medici, MD, Assistant Professor, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of California Davis, 4150 V Street, Suite 3500, Sacramento, CA 95817, United States
S- Editor Tian L L- Editor Stewart GJ E- Editor Xiong L
| 1. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 633] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 2. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [PubMed] |
| 3. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 497] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Nieuwenhuijs VB, De Bruijn MT, Padbury RT, Barritt GJ. Hepatic ischemia-reperfusion injury: roles of Ca2+ and other intracellular mediators of impaired bile flow and hepatocyte damage. Dig Dis Sci. 2006;51:1087-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44:466-471. [PubMed] |
| 6. | Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97-106. [PubMed] |
| 7. | Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, Stewart G, McCaughan G. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Dorman RB, Bajt ML, Farhood A, Mayes J, Jaeschke H. Heme oxygenase-1 induction in hepatocytes and non-parenchymal cells protects against liver injury during endotoxemia. Comp Hepatol. 2004;3 Suppl 1:S42. [PubMed] |
| 10. | Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239-247. [PubMed] |
| 11. | Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688-L694. [PubMed] |
| 12. | Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Barañano DE, Doré S. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152-157. [PubMed] |
| 13. | Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748-755. [PubMed] |
| 14. | Ferrándiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14:473-486. [PubMed] |
| 15. | Schmidt R. Cobalt protoporphyrin as a potential therapeutic agent? FASEB J. 2007;21:2639; author reply 2640. [PubMed] |
| 16. | Hoetzel A, Geiger S, Loop T, Welle A, Schmidt R, Humar M, Pahl HL, Geiger KK, Pannen BH. Differential effects of volatile anesthetics on hepatic heme oxygenase-1 expression in the rat. Anesthesiology. 2002;97:1318-1321. [PubMed] |
| 17. | Schmidt R, Hoetzel A, Baechle T, Loop T, Humar M, Bauer M, Pahl HL, Geiger KK, Pannen BH. Isoflurane pretreatment lowers portal venous resistance by increasing hepatic heme oxygenase activity in the rat liver in vivo. J Hepatol. 2004;41:706-713. [PubMed] |
| 18. | Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, Jochum W, Spahn DR, Graf R, Clavien PA. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg. 2008;248:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, Geiger KK, Pannen BH. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann Surg. 2007;245:931-942. [PubMed] |
| 20. | Hoetzel A, Vagts DA, Loop T, Humar M, Bauer M, Pahl HL, Geiger KK, Pannen BH. Effect of nitric oxide on shock-induced hepatic heme oxygenase-1 expression in the rat. Hepatology. 2001;33:925-937. [PubMed] |
| 21. | Hoetzel A, Leitz D, Schmidt R, Tritschler E, Bauer I, Loop T, Humar M, Geiger KK, Pannen BH. Mechanism of hepatic heme oxygenase-1 induction by isoflurane. Anesthesiology. 2006;104:101-109. [PubMed] |
| 22. | Massip-Salcedo M, Casillas-Ramirez A, Franco-Gou R, Bartrons R, Ben Mosbah I, Serafin A, Roselló-Catafau J, Peralta C. Heat shock proteins and mitogen-activated protein kinases in steatotic livers undergoing ischemia-reperfusion: some answers. Am J Pathol. 2006;168:1474-1485. [PubMed] |
| 23. | Yamagami K, Enders G, Schauer RJ, Leiderer R, Hutter J, Yamamoto Y, Yamaoka Y, Hammer C, Messmer K. Heat-shock preconditioning protects fatty livers in genetically obese Zucker rats from microvascular perfusion failure after ischemia reperfusion. Transpl Int. 2003;16:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;293:H2543-H2549. [PubMed] |
| 25. | Buñag RD, Barringer DL. Obese Zucker rats, though still normotensive, already have impaired chronotropic baroreflexes. Clin Exp Hypertens A. 1988;10 Suppl 1:257-262. [PubMed] |
| 26. | Pamidimukkala J, Jandhyala BS. Evaluation of hemodynamics, vascular reactivity and baroreceptor compensation in the insulin resistant Zucker obese rats. Clin Exp Hypertens. 1996;18:1089-1104. [PubMed] |
| 27. | Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D'Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044. [PubMed] |
| 28. | Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415-423. [PubMed] |
| 29. | McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923-930. [PubMed] |
| 30. | de Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009;15:1172-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |